Effects of dietary betaine on cholesterol metabolism and hepatopancreas function in gibel carp (Carassius gibelio) fed with a high-fat diet
Abstract
This research aimed to investigate the role of dietary betaine in high-fat diet in cholesterol metabolism in gibel carp. Fish were randomly allocated to five groups and fed with basic diet, high-fat diet and high-fat diet with 1 g/kg, 4 g/kg and 16 g/kg betaine, respectively. The feeding trial lasted 10 weeks. The results showed that though betaine addition decreased fish final body weight, it alleviated lipid metabolism disorder caused by high-fat diet according to serum TC, TG, LDLC and HDLC levels. More importantly, betaine supplementation enhanced cholesterol synthesis as well as conversion of cholesterol to bile acid by promoting expression of HMGCR and CYP7A1 genes. Meanwhile, betaine supplementation not only promoted bile acid efflux and increased total bile acid level in the intestine but also improved intestinal lipase activity. Moreover, high-fat diet with betaine addition improved the hepatopancreas’ antioxidant capacity, lessened hepatopancreas injury and increased fish survival during the feeding trial and under bacterial challenge. In summary, dietary betaine supplementation was beneficial to alleviate high-fat feeding-induced lipid metabolism disorder, promote cholesterol conversion to bile acid and enhance hepatopancreas function in gibel carp.
1 INTRODUCTION
Betaine (N,N,N-trimethylglycine) is a trimethyl derivative of the amino acid glycine. Researches in mammals have demonstrated that betaine is mainly absorbed from food and can also be synthesized of choline in body. Its content in tissues can be transiently increased by dietary supplementation (Clow et al., 2008). Betaine not only functions as methyl donor and an osmotic regulator but also plays important roles in lipid metabolism (Martins et al., 2012; Pekkinen et al., 2013; Wang et al., 2013; Xu et al., 2015; Zhang et al., 2013). Studies in human also showed that dietary betaine can decrease body fat, prevent the deterioration of steatosis and ameliorate nonalcoholic steatohepatitis (Abdelmalek et al., 2009; Gao et al., 2019). In aquaculture, betaine is used as a feed additive to promote fish and shrimp feeding, growth performance and enhance innate immune system (Dong et al., 2020; Lim et al., 2016). Researches in different aquaculture species, however, have led to conflicting results. Our previous study showed that appropriate betaine supplementation in basic diet not only improved growth but also reduced lipid deposition in gibel carp (Dong et al., 2018), but the underlying mechanism is still unclear.
Cholesterol is one of the most important lipoids with a wide range of physiological functions in animals, while the liver plays a central role in maintaining cholesterol balance in different animal species, including humans (Dietschy et al., 1993). A balanced cholesterol metabolism is critical for animal growth and health. Dietary cholesterol is transported out of the liver with low-density lipoprotein (LDL) and LDL receptor (LDLR) involved (Goldstein and Brown, 2009). Organisms also synthesize cholesterol from acetyl-CoA catalysed by 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR) (DeBose-Boyd, 2008). A portion of cholesterol in body is catalysed by 7α-hydroxylase (CYP7A1), the rate-limited enzyme, to produce bile acids (Vaz and Ferdinandusse, 2017), which plays critical roles in lipid digestion, absorption and metabolism through enterohepatic circulation (Chiang, 2009). Earlier studies have found that dietary betaine affects the levels of cholesterol in plasma and different tissues in animals, but the results have been inconsistent (Li et al., 2020). For instance, betaine increased the concentration of cholesterol in adipose and muscle; however, the plasma cholesterol was not changed by betaine supplementation in pig (Albuquerque et al., 2017; Fernández-Fígares et al., 2012; Martins et al., 2010). Researches in rodent showed that dietary betaine decrease plasma cholesterol in mice, but increase plasma cholesterol in rats (Ahn et al., 2015). These inconsistent results indicated that the specific mechanism of cholesterol metabolism by diet betaine requires further research.
High-fat diet is widely used to promote fish growth, while brings out health problems such as excessive lipid deposition and lipid metabolism disorder (Lu et al., 2017; Zhang et al., 2014). Our previous study has shown that dietary betaine not only enhances growth performance but also helps alleviate lipid deposition in gibel carp (Dong et al., 2018). The effects of dietary betaine added in high-fat diet, however, has not been determined. The objective of the present study was to investigate the effects of dietary betaine added to high-fat diet on growth performance and especially cholesterol metabolism and hepatopancreas function in gibel carp.
2 MATERIALS AND METHODS
2.1 Ethical statement
Our study on gibel carp was approved by the Animal Care and Use Committee of the Yangzhou University (ethical protocol code: YZUDWSY 2017-09-06), and all efforts were made to minimize suffering. For instance, experimental fish was anaesthetized with MS-222 (150 mg/L, Sigma) before sacrificing and handling.
2.2 Experimental diets and procedure
Four high-fat experimental diets with similar proximate compositions (42% crude protein and 10% lipid) were formulated, differing only in betaine content (0 g/kg, 1 g/kg, 4 g/kg and 16 g/kg dry weight), and named as HFB0, HFB0.1, HFB0.4 and HFB1.6, respectively. Another basic diet, formulated with 5% lipid, was used as the control diet (Diet CG). All ingredients were ground into fine powder before passing through a 200 µm mesh and were thoroughly mixed with soybean oil mixture and water to produce a stiff dough. The dough was then pelletized using an experimental feed mill, dried for approximately 12 h in a ventilated oven at 45°C and stored at −20°C until use. The experiment diet and proximate composition are presented in Table 1.
| Ingredients | CG | HF | HFB0.1 | HFB0.4 | HFB1.6 |
|---|---|---|---|---|---|
| Fish meal | 100 | 100 | 100 | 100 | 100 |
| Soybean meal | 200 | 200 | 200 | 200 | 200 |
| Rapeseed meal | 160 | 160 | 160 | 160 | 160 |
| Cottonseed meal | 140 | 140 | 140 | 140 | 140 |
| Wheat meal | 250 | 250 | 250 | 250 | 250 |
| Vitamin Premixa | 1 | 1 | 1 | 1 | 1 |
| Mineral Premixa | 4 | 4 | 4 | 4 | 4 |
| Choline chloride | 2 | 2 | 2 | 2 | 2 |
| Soybean oil | 40 | 100 | 100 | 100 | 100 |
| DL-Met | 5 | 5 | 5 | 5 | 5 |
| Ca(H2PO4)2 | 15 | 15 | 15 | 15 | 15 |
| Betaine | 0 | 0 | 1 | 4 | 16 |
| Microcrystalline cellulose | 83 | 23 | 22 | 19 | 7 |
| Total | 1000 | 1000 | 1000 | 1000 | 1000 |
| Proximate composition | |||||
| Crude protein | 343.51 | 344.43 | 343.82 | 344.24 | 345.01 |
| Crude lipid | 64.30 | 136.12 | 136.72 | 134.08 | 135.60 |
| Crude ash | 62.22 | 63.03 | 61.96 | 63.30 | 62.37 |
- a The Vitamin Premix (FV88) and Mineral Premix (FM88) are purchased from ZHENGCHANG FEED SCI&TECH.
2.3 Feeding experiments
The gibel carp, mean initial weight of 1.65 ± 0.40 g, were purchased from a commercial farm in Jiangsu, China. At the beginning of the experiment, fish were fasted for 24 h, weighed and randomly sorted into 15 tanks (300 L, 35 fish per tank). Ten weeks of feeding experiment was then conducted in consistence with our previous study (Dong et al., 2018). Briefly, experimental fish was fed twice daily, with the feeding amount adjusted according to the weight of fish. The residual feed, if any, was collected, dried and weighed.
2.4 Sample collection
At the end of the feeding trial, fish were starved for 24 h before sampling. After fasting and anaesthetization, the number of live fish in each tank was counted and each fish was weighed. Then, three fish from each tank were dissected, and the back muscle, intestinal and hepatopancreas were collected and stored at −20°C for composition and biochemistry analysis. Another three fish from each tank were selected for whole body composition analysis, stored at −20°C before use. For gene expression analysis, the hepatopancreas was removed from another three fish per tank, immediately frozen in liquid nitrogen and stored at −80°C until use. Blood of ten fish from each tank was taken via a caudal vein (27-gauge needle and 1-ml syringe) and straightway centrifuged at 12,000 g, 4℃ for 10 min for the serum biochemistry analyses. Serum was immediately frozen in liquid nitrogen and then stored at −80℃ until use.
2.5 Chemical analysis
The proximate analysis of the samples was carried out by AOAC methods (AOAC, 1995). Samples of experimental diets, whole body and muscle samples were dried to a constant weight at 105°C to determine moisture content. Crude protein was determined as Kjeldahl nitrogen ×6.25 using Kjeltec 8400 auto-analyzer (FOSS). The crude lipid content of muscle was measured according to the Folch method and quantified gravimetrically (Folch, 1957).
Amino acid contents in the samples were analysed by automatic amino acid analyzer S-433D (Sykam). Before analysis, triplicate freeze-dried samples were hydrolysed with hydrochloric acid (6 M) under N2 at 110°C for 13 h. The hydrolysates were rotary evaporated to dryness under vacuum at 40°C, redissolved in a sodium citrate buffer at PH 2.2 and filtered through 0.22 μm membrane. In regard to the automatic amino acid analyser analysis, an LCA K06/Na chromatographic column was used. The following analyser setting was used: buffer flow rate of 0.45 ml/min, reagent flow rate of 0.25 ml/min, reactor heater temperature of 58–74°C, sample injection volume of 0.05 ml and detection wavelength of 570 nm or 440 nm. An external standard was used to calculate the concentration of each amino acid.
The biochemistry parameters in serum, intestinal and hepatopancreas were evaluated using commercial kits (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer's instructions. As of intestinal and hepatopancreas analysis, precooled 0.85% NaCl was added to the collected samples before snipping. Then, the ultrasonic cell crushing apparatus was used for crushing. After centrifugation, the supernatant was taken for enzyme activity detection.
2.6 Quantitative real-time PCR analysis
Total RNA was isolated from the hepatopancreas of gibel carp using TRIzol (Invitrogen) and was treated with TransScriptTM One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen China) in a 20 μl volume. All primers for target genes were designed using the Primer Premier 5 software according to the available sequences (Table 2) and synthesized by Biosune. Real-time PCR was carried out in a quantitative thermal cycler (LightCycler480II) in a final volume of 20 μl containing cDNA, primer pairs and 1× SYBR Green Real-time PCR Master Mix (TaKaRa, Bio Inc.). The β-actin gene and EF-1α gene were used as an internal control. The 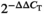 method was used to calculate the relative expression levels of each target gene.
method was used to calculate the relative expression levels of each target gene.
| CG | HF | HFB0.1 | HFB0.4 | HFB1.6 | |
|---|---|---|---|---|---|
| IBW1 (g) | 1.44 ± 0.02 | 1.41 ± 0.04 | 1.44 ± 0.01 | 1.43 ± 0.03 | 1.45 ± 0.02 |
| FBW2 (g) | 11.06 ± 0.25 | 11.78 ± 0.30*a | 11.41 ± 0.11ab | 11.12 ± 0.08b | 11.17 ± 0.22b |
| WGR3 (%) | 645.34 ± 35.37 | 654.39 ± 16.20a | 657.81 ± 8.75a | 606.66 ± 18.41b | 600.17 ± 21.97b |
| SGR4 (% day−1) | 3.14 ± 0.08 | 3.26 ± 0.03*a | 3.23 ± 0.04a | 3.06 ± 0.04b | 3.04 ± 0.05b |
| FCR5 | 2.33 ± 0.05 | 2.47 ± 0.13 | 2.42 ± 0.12 | 2.41 ± 0.13 | 2.53 ± 0.08 |
| CF6 (%) | 1.57 ± 0.04 | 1.54 ± 0.03 | 1.52 ± 0.02 | 1.56 ± 0.03 | 1.52 ± 0.02 |
| SR7 (%) | 100.00 ± 0.00 | 93.33 ± 3.34*b | 97.78 ± 1.92ab | 100.00 ± 0.00a | 100.00 ± 0.00a |
| Survival under challenge (%) | 63.33 ± 2.89 | 26.67 ± 2.89*c | 41.67 ± 2.89b | 66.67 ± 2.89a | 68.33 ± 2.89a |
Note
- Values presented are the mean ± SD. Different letters indicated the presence of a significant difference between the high-fat diet groups (p < .05). The same as below.
- 1 IBW: Initial body weight.
- 2 FBW: Final body weight.
- 3 WGR: 100 × (W0−Wt)/W0.
- 4 SGR = 100 × (ln Wt–ln W0)/t.
- 5 FCR = feed intake/(Wt−W0).
- 6 CF: condition factor = 100 × body weight/(body length)3.
- 7 SR: survival rate.
- * Significantly different from the CG group (p < .05).
2.7 Bacterial challenge
After the feeding trial, 20 fish from each tank were transferred to 80-L aquaria containing 60 L of water. The bacterium used for the challenge was a previously isolated strain of Aeromonas veronii from diseased gibel carp in China (Sun et al., 2016). Procedures for bacteria preparation were according to methods described previously (Dong et al., 2020). Briefly, the bacterial strain was streaked onto LB agar plates and grew at 28°C for 18 h. A single colony was suspended in 50 ml of LB broth medium at 28°C for 18 h and enumerated by 10-fold serial dilution in sterile PBS. All groups were infected by exposure to 1.8 × 106 CFU/ml (LD50) of bacteria. The experimental fish was fed as before challenge. Mortality was monitored every day for 14 days. Dead fish were sampled to re-isolate and confirm specific mortality by the challenge isolate. The surviving fish were euthanized to death in the end of the experiment.
2.8 Calculations and statistical analysis

Wt and W0 are final and initial fish weight, respectively, while t was the duration of the experiment in days.
Results were presented as means ± standard deviation (STD). Statistical analysis was performed by one-way ANOVA using SPSS 19.0 for Window, and differences between the means were tested by Tukey's multiple range test. In all analyses, the level of significance was chosen as p < .05.
3 RESULTS
3.1 Growth performance and feed utilization
The FBW and SGR were significantly higher in the high-fat model group (HF) than in the control group (CG) (p < .05), while 4 g/kg and 16 g/kg betaine addition led to significantly decreased FBW, WGR and SGR compared with the HF group (p < .05). However, SR in betaine addition group was significantly higher than the HF group (p < .05). As of FCR and CF, none significant difference was detected between different groups (p > .05) (Table 2).
3.2 Body composition
The protein content of the whole body and muscle decreased when fed high-fat diet, but increased when 4 g/kg betaine was added (HFB0.4) (p < .05). In addition, though high-fat diet treatment led to increased lipid content in whole body and muscle, betaine supplementation significantly reduced the level of lipid deposition (p < .05). Whole body moisture content was not significantly affected by high-fat or betaine addition treatments (p > .05). As of muscle moisture, however, high-fat diet feeding significantly decreased its content compared with CG group (p < .05), while 4 g/kg and 16 g/kg betaine addition significantly increased its content (p < .05) (Table 3). More importantly, both total essential amino acids and total amino acids content in muscle were significantly higher in HFB0.4 and HFB1.6 groups compared with HF group (p < .05) (Table 4).
| CG | HF | HFB0.1 | HFB0.4 | HFB1.6 | |
|---|---|---|---|---|---|
| Whole body | |||||
| Moisture | 687.7 ± 11.54 | 679.46 ± 5.50 | 697.15 ± 10.79 | 695.58 ± 12.15 | 685.24 ± 19.52 |
| Crude protein | 161.90 ± 1.00 | 153.84 ± 1.53*b | 157.74 ± 1.82ab | 160.62 ± 3.32a | 158.31 ± 2.23ab |
| Crude lipid | 73.53 ± 2.20 | 89.90 ± 2.24*a | 85.14 ± 2.51a | 74.63 ± 3.03b | 72.73 ± 3.33b |
| Muscle | |||||
| Moisture | 742.82 ± 4.63 | 731.25 ± 0.91*b | 751.90 ± 17.22ab | 777.21 ± 13.40a | 770.55 ± 5.56a |
| Crude protein | 203.01 ± 2.12 | 198.4 ± 0.83*ab | 199.23 ± 1.05ab | 201.76 ± 1.75a | 196.54 ± 2.91b |
| Crude lipid | 27.54 ± 1.73 | 35.83 ± 4.01*a | 25.03 ± 5.42b | 19.94 ± 3.52b | 16.02 ± 1.90b |
| CG | HF | HFB0.1 | HFB0.4 | HFB1.6 | |
|---|---|---|---|---|---|
| Asp | 59.35 ± 0.25 | 58.62 ± 0.43*b | 60.62 ± 2.32b | 65.32 ± 1.61a | 65.04 ± 1.71a |
| Thr | 24.63 ± 0.43 | 24.17 ± 0.41b | 24.90 ± 1.03ab | 26.44 ± 0.63a | 26.12 ± 0.71a |
| Ser | 21.13 ± 0.32 | 20.73 ± 0.25b | 21.74 ± 0.83ab | 23.15 ± 0.56a | 22.80 ± 0.62a |
| Glu | 94.23 ± 1.33 | 91.17 ± 0.85*c | 93.86 ± 0.72b | 102.28 ± 1.62a | 101.53 ± 0.21a |
| Gly | 29.82 ± 0.20 | 28.32 ± 0.35 | 28.42 ± 0.73 | 30.42 ± 1.04 | 30.61 ± 1.54 |
| Ala | 36.18 ± 0.15 | 35.39 ± 0.34*b | 35.74 ± 0.21b | 40.01 ± 0.53a | 40.24 ± 0.93a |
| Cys | 6.83 ± 0.24 | 6.52 ± 0.24b | 7.22 ± 0.21a | 6.91 ± 0.13a | 6.53 ± 0.24b |
| Val | 25.92 ± 0.17 | 26.03 ± 0.41c | 26.82 ± 0.75bc | 28.49 ± 0.90a | 28.21 ± 0.74ab |
| Met | 15.78 ± 0.13 | 15.42 ± 0.35c | 16.64 ± 0.53b | 18.12 ± 0.51a | 17.84 ± 0.62a |
| Ile | 24.72 ± 0.15 | 24.11 ± 0.24*b | 25.15 ± 0.26ab | 26.73 ± 1.45a | 26.82 ± 0.63a |
| Leu | 45.72± 0.09 | 45.8 ± 0.05b | 47.50 ± 1.61b | 50.84 ± 1.55a | 50.60 ± 1.00a |
| Tyr | 17.82 ± 0.12 | 17.61 ± 0.12b | 18.01 ± 0.30b | 19.89 ± 0.44a | 19.93 ± 0.55a |
| Phe | 25.52 ± 0.21 | 25.16 ± 0.10b | 25.35 ± 1.30b | 27.81 ± 0.91a | 28.02 ± 0.62a |
| His | 33.51 ± 0.13 | 33.50 ± 0.13d | 33.75 ± 0.06c | 36.48 ± 0.10b | 36.86 ± 0.12a |
| Lys | 53.82 ± 0.14 | 53.31 ± 0.21*b | 54.04 ± 0.18b | 59.65 ± 0.56a | 59.70 ± 0.61a |
| Arg | 33.84 ± 0.59 | 33.46 ± 0.22*b | 34.21 ± 1.41b | 37.23 ± 0.51a | 36.88 ± 0.86a |
| Pro | 13.78 ± 0.55 | 12.50 ± 0.38*c | 13.35 ± 0.13b | 13.32 ± 0.11b | 14.23 ± 0.21a |
| TEAAa | 283.68 ± 0.48 | 280.98 ± 1.45*b | 288.26 ± 6.42b | 311.57 ± 6.07a | 310.84 ± 4.86a |
| TAAb | 562.69 ± 2.05 | 551.79 ± 3.10*b | 567.05 ± 10.85b | 612.78 ± 9.72a | 611.45 ± 9.41a |
- a TEAA: Total essential amino acids, the same as below.
- b TAA: Total amino acids, the same as below.
3.3 Biochemistry in serum, intestinal and hepatopancreas
In contrast with the CG group, the HF group owned much higher total cholesterol (TC), triglyceride (TG) and low-density lipoprotein cholesterol (LDLC) concentrations but significantly lower high-density lipoprotein cholesterol (HDLC) in serum (p < .05) (Table 5). Similarly, in hepatopancreas, TC, TG and non-esterified fatty acid (NEFA) concentrations were significantly increased in HF group compared with CG group (p < .05) (Table 6). Supplementing betaine, however, brought out substantial changes. First of all, betaine addition of 4 g/kg significantly reduced serum TC, TG and LDLC concentrations compared with the HF group (p < .05). Secondly, in all the betaine added groups, both TG and NEFA in hepatopancreas were significantly decreased in contrast with HF group (p < .05). In addition, high-fat feeding significantly reduced lipase activity in the hepatopancreas (p < .05), but adding betaine in high-fat diet substantially improved enzyme activity (p < .05). In intestinal, however, high-fat diet increased lipase activity, and betaine supplementation showed no effect on it (p > .05) (Table 7). As of total bile acid (TBA) content in intestinal, feeding high-fat diet significantly decreased its level compared with the CG group (p < .05), but adding betaine in high-fat diet significantly improved its level (p < .05) (Table 7). In hepatopancreas, however, opposite results were observed. Taken together, these results indicated that dietary betaine could alleviate lipid metabolism disorders caused by high-fat feeding.
| CG | HF | HFB0.1 | HFB0.4 | HFB1.6 | |
|---|---|---|---|---|---|
| TC, mmol/L | 3.20 ± 0.12 | 3.86 ± 0.14*a | 3.74 ± 0.16ab | 3.59 ± 0.09 b | 3.67 ± 0.12b |
| TG, mmol/L | 1.83 ± 0.23 | 2.10 ± 0.09*a | 2.00 ± 0.08a | 1.64 ± 0.06b | 2.02 ± 0.10a |
| HDLC, mmol/L | 1.55 ± 0.03 | 1.34 ± 0.10*b | 1.71 ± 0.01a | 1.55 ± 0.05a | 1.63 ± 0.10a |
| LDLC, mmol/L | 1.41 ± 0.11 | 1.88 ± 0.13*a | 1.95 ± 0.16a | 1.56 ± 0.08b | 1.69 ± 0.08ab |
| INS, mIU/L | 10.99 ± 0.13 | 12.02 ± 0.29*a | 10.40 ± 0.28b | 9.73 ± 0.56b | 10.36 ± 0.27b |
| IgM, mg/ml | 1.91 ± 0.12 | 1.54 ± 0.08*b | 1.52 ± 0.09b | 2.08 ± 0.18a | 2.09 ± 0.13a |
| C3, μg/ml | 306.94 ± 7.65 | 289.76 ± 5.82*c | 327.92 ± 7.99b | 342.10 ± 10.10a | 327.03 ± 11.20b |
| C4, μg/ml | 194.79 ± 5.01 | 182.90 ± 6.68*c | 220.64 ± 7.37a | 200.32 ± 8.65b | 217.99 ± 3.53a |
| CG | HF | HFB0.1 | HFB0.4 | HFB1.6 | |
|---|---|---|---|---|---|
| Protein concentration (mg/L) | 1.74 ± 0.11 | 1.72 ± 0.02c | 1.79 ± 0.02bc | 2.04 ± 0.12a | 1.92 ± 0.10ab |
| SOD (U/mgprot) | 423.19 ± 13.01 | 381.06 ± 5.23*b | 414.28 ± 16.17a | 406.65 ± 11.53ab | 374.51 ± 19.37b |
| AKP (King U/gprot) | 756.38 ± 16.36 | 594.65 ± 13.43*c | 1068.78 ± 24.55a | 873.27 ± 17.73b | 899.95 ± 23.07b |
| ACP (King U/gprot) | 402.19 ± 14.83 | 417.13 ± 13.30 | 386.26 ± 13.30 | 388.63 ± 12.61 | 373.41 ± 16.48 |
| Trypsin (U/mgprot) | 3585.25 ± 545.76 | 3534.92 ± 311.90 | 3451.27 ± 388.86 | 3408.28 ± 394.39 | 3108.62 ± 423.01 |
| Lipase (U/gprot) | 3.23 ± 0.41 | 4.38 ± 0.53* | 4.49 ± 0.28 | 4.20 ± 0.28 | 4.09 ± 0.66 |
| TBA (μmol/gprot) | 9.12 ± 1.71 | 4.03 ± 1.12*c | 6.89 ± 0.13b | 8.92 ± 0.44a | 8.45 ± 0.22ab |
| CG | HF | HFB0.1 | HFB0.4 | HFB1.6 | |
|---|---|---|---|---|---|
| Protein concentration (g/L) | 3.02 ± 0.22 | 2.38 ± 0.19*b | 2.83 ± 0.25a | 2.80 ± 0.14a | 2.88 ± 0.24a |
| SOD (U/mgprot) | 613.03 ± 5.24 | 576.44 ± 5.75*b | 626.34 ± 8.51a | 614.06 ± 5.37a | 599.52 ± 11.00ab |
| MDA (nmol/mgprot) | 1.27 ± 0.26 | 2.77 ± 0.26*a | 1.43 ± 0.65b | 1.71 ± 0.42b | 1.81 ± 0.14b |
| CAT (U/mgprot) | 130.35 ± 2.16 | 125.13 ± 2.19*b | 137.96 ± 6.77a | 128.97 ± 4.29ab | 126.79 ± 4.78b |
| ALT (U/gprot) | 6.41 ± 0.18 | 7.61 ± 1.18*a | 5.86 ± 0.29b | 5.78 ± 0.14b | 7.09 ± 1.66a |
| AST (U/gprot) | 32.42 ± 0.91 | 35.44 ± 2.22*a | 26.35 ± 2.48c | 30.43 ± 1.51b | 34.06 ± 0.24a |
| Trypsin (U/mgprot) | 580.12 ± 78.09 | 775.39 ± 85.45* | 656.26 ± 96.46 | 646.79 ± 75.47 | 798.20 ± 97.14 |
| Lipase (U/gprot) | 1.73 ± 0.29 | 1.18 ± 0.11*c | 2.19 ± 0.08a | 2.18 ± 0.18a | 1.50 ± 0.20b |
| TC (mmol/gprot) | 0.06 ± 0.00 | 0.08 ± 0.01*b | 0.10 ± 0.01a | 0.07 ± 0.00b | 0.09 ± 0.02ab |
| TG (mmol/gprot) | 0.55 ± 0.05 | 0.81 ± 0.14*a | 0.51 ± 0.08b | 0.52 ± 0.10b | 0.53 ± 0.11b |
| NEFA (mmol/gprot) | 0.26 ± 0.02 | 0.36 ± 0.03*a | 0.22 ± 0.06b | 0.17 ± 0.02b | 0.15 ± 0.03b |
| TBA (μmol/gprot) | 12.60 ± 2.30 | 18.82 ± 0.04*a | 15.40 ± 1.06b | 14.75 ± 2.63b | 20.61 ± 1.00a |
Besides, high-fat diet treatment significantly reduced the activity of several antioxidant kinases and immune factors in serum, intestinal and hepatopancreas, including Superoxide dismutase (SOD), Catalase (CAT), immunoglobulin M (IgM), complement of C3 and complement of C4 (p < .05), but betaine addition of different levels significantly improved their activities (p < .05) (Tables 5–7). On the other hand, high-fat feeding led to significant increase of MDA level compared with CG group (p < .05), but it was significantly reduced by betaine addition (p < .05) (Table 6). These results clearly demonstrated that betaine supplementation was beneficial to improve antioxidant and immunity capacity under high-fat diet treatment.
The levels of alanine aminotransferase (ALT) and aspartate transaminase (AST) were significantly higher in HF group compared with CG group (p < .05) (Table 6). On the contrary, 1 g/kg and 4 g/kg betaine supplementation significantly decreased their activities. On the other hand, alkaline phosphatase (AKP) activity was significantly decreased in HF group compared with CG group (p < .05), but betaine addition significantly improved its activity (Table 7). Hence, it was obvious that dietary betaine played a significant protective effect against high-fat diet-induced hepatopancreas and intestinal injury.
3.4 Expression of genes in hepatopancreas
Compared with CG group fish, high-fat feeding up-regulated HMGCR expression but down-regulated expression levels of LDLR and CYP7A1 in hepatopancreas (p < .05) (Figure 1). Betaine addition, however, significantly enhanced fish LDLR mRNA expression compared with HF group. Nevertheless, significantly increased gene expression of HMGCR and CYP7A1 was only observed in HFB0.1 and HFB1.6 group, respectively (Figure 1).
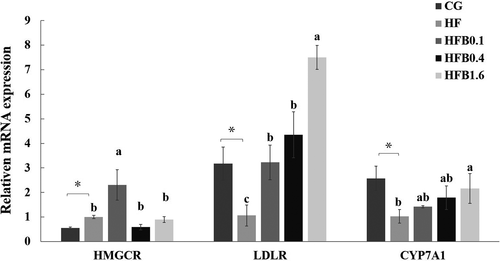
3.5 Survival of fish under bacterial challenge
The HF group showed a significantly lower survival rate under bacterial challenge than the CG group (p < .05). The groups that fed betaine, however, showed a significantly higher survival rate in contrast with HF group (p < .05), and the survival rate in HFB0.4 and HFB1.6 was at the same level as in CG group (Table 2).
4 DISCUSSION
As a common feed supplement, betaine has been widely used to enhance fish growth performance in aquaculture. However, many studies investigating the effect of betaine on growth performance have produced variable results, possibly because they were conducted in different species and used different feed compositions. (Normandes et al., 2006; Ratriyanto et al., 2009). For example, betaine addition does not improve feed intake and growth performance in Coregonus schinzi palea (Dabrowski et al., 1985), Oncorhynchus mykiss (Hughes, 1991) and Leporinus macrocephalus (Normandes et al., 2006). Our previous study showed that basic diet containing 4 g/kg betaine could significantly enhance gibel carp growth performance (Dong et al., 2018). In the present study, high-fat feeding significantly strengthened final body weight. When betaine was supplemented in high-fat diet, however, both final body weight and specific growth rate were significantly decreased compared with high-fat diet group. Thus, it seemed that betaine addition weakened the positive effect of high-fat feeding on gain weight in gibel carp. On the other hand, however, betaine addition significantly increased fish survival rate during the feeding trial and under bacterial challenge and showed no negative effect on feed conversion rate and fish condition rate. Hence, the benefit of betaine supplementation in gibel carp culture was obvious, especially in the context that fish disease has long been a main factor threatening aquaculture sustainable development.
Compared with control group, high-fat feeding significantly decreased whole body and muscle crude protein content, but significantly increased crude lipid content. In betaine supplementation groups, however, lipid deposition was significantly alleviated while crude protein content was significantly improved. Similar results were also observed in our previous study in gibel carp feeding normal level fat diet with betaine (Dong et al., 2018). The content of muscle amino acids was also compared, and a positive effect of dietary betaine on amino acids deposition was determined. Since excessive lipid deposition, especially in the liver (hepatopancreas), is harmful to fish health (Qu et al., 2014), the significantly reduced crude lipid content might explained the much higher fish survival rate in betaine supplementation groups under bacterial challenge. In addition, betaine addition improved hepatopancreas lipase activity and enhanced hepatopancreas bile acid secretion, suggesting dietary betaine was beneficial to dietary lipid digestion and absorption. Taken together, we speculate the existence of a complex network through which dietary betaine regulate fish lipid metabolism.
In medicine, hyperlipidaemia is often attributed to abnormal lipid operation and metabolism, manifesting increased serum TC, TG and LDLC contents and reduced HDLC content (Wang et al., 2021). In the present study, 10 weeks of high-fat feeding significantly increased gibel carp serum TC, TG and LDLC levels, while significantly decreased HDLC content. Simultaneously, hepatopancreas TC and TG also increased significantly in the HF group. These findings are consistent with previous researches in fish (Cao et al., 2019). In contrast, betaine addition significantly decreased serum TC, TG and LDLC levels and increased serum HDLC, suggesting dietary betaine had an effective regulation on serum lipid metabolism. Hence, high-fat feeding brings out potential harms to fish, but adding betaine in the diet helps alleviate this negative effect.
Liver (or hepatopancreas) plays a central role in maintaining cholesterol balance (Dietschy et al., 1993). Metabolisms involved in cholesterol regulation include cholesterol synthesis, uptake, storage and efflux (Millar and Cuchel, 2018). 3-Hydroxy-3-methylglutaryl-CoA reductase (HMGCR) is the rate-liming enzyme in cholesterol synthesis (DeBose-Boyd, 2008). Previous studies in mouse and rat found that high-fat diet could increase the activity of HMGCR with total cholesterol unchanged in liver (Ahn et al., 2015; Cho et al., 2010; Li et al., 2020; Xu et al., 2015). In maternal pigs and chicks, it was reported that betaine supplementation increased the concentration of total cholesterol in the liver (Albuquerque et al., 2017; Zhao et al., 2019). Results of the present study showed that 1 g/kg betaine addition significantly increased the level of total cholesterol and HMGCR expression in the hepatopancreas, but not for 4 g/kg and 16 g/kg betaine addition. Low-density lipoprotein receptor (LDLR) is a cell surface receptor that mediates the uptake of low-density lipoprotein cholesterol (LDLC) into hepatocyte, so its level is associated with serum LDLC concentrations (Vaziri and Liang, 1996). Previous research in mammals reported that maternal betaine supplementation could up-regulate gene expression and protein content of LDLR (Cai et al., 2016; Zhao et al., 2019). Given the results that betaine addition groups had much lower serum LDLC level but higher LDLR gene expression level compared with high-fat diet group, it was suggested that both dietary lipid and betaine could regulate serum LDLC level via targeting LDLR gene. It can speculate that liver cholesterol level is strictly regulated in animals, and dietary betaine to some extent may regulate its metabolism.
The elimination of cholesterol in animals include conversion to bile acids in hepatic cell and secretion of cholesterol with bile acids into the biliary tract, while the former is the major path of excess cholesterol in animals (Li et al., 2020). Cholesterol 7α-hydroxylase (CYP7A1) is the first and rate-limiting enzyme of the classical bile acid synthesis pathway from cholesterol, and much progress has been made in its transcription regulation, bile acid feedback regulation and bile acid synthesis (Chiang et al., 2020; Vaz and Ferdinandusse, 2017). Betaine was found to increase bile acid secretion in rabbits and rats (Li et al., 2020; Zapadniuk and Panteleimonova, 1987). In this study, intestinal total bile acid (TBA) level was significantly reduced by high-fat diet, but betaine addition highly increased its level. Furthermore, hepatopancreas TBA level was increased by high-fat diet, but decreased by 1 g/kg and 4 g/kg betaine addition in high-fat diet. In contrast, high level of betaine addition (16 g/kg) elevated hepatopancreas TBA level, as well as CYP7A1 expression compared with high-fat group. Therefore, our results clearly demonstrated that high-fat diet inhibited TBA efflux from the hepatopancreas into the intestine and that dietary betaine, to a great extent, promoted the process.
Lipid metabolism disorders lead to abnormal lipid accumulation in hepatopancreas (Friedman et al., 2018). Meanwhile, excessive lipid accumulation induces inflammatory reactions, resulting in hepatocyte damage, which often manifests as an increased liver (or hepatopancreas) index. In this study, MDA, ALT and AST were used as the main indicators of hepatopancreas function (Henao-Mejia et al., 2012), while SOD and CAT as pointers of antioxidant ability. The results showed that betaine reduced MDA, ALT and AST levels in the hepatopancreas and up-regulated activities of both SOD and CAT compared with high-fat diet group. Similar results were also reported in broiler chicken and giant freshwater prawn (Alirezaei et al., 2012; Dong et al., 2020). It can speculate that dietary betaine enhanced hepatopancreas antioxidant enzyme activity and further lessened free radical induced organ damage.
Taken together, our study demonstrated that betaine added in high-fat diet not only promotes hepatopancreas cholesterol metabolism and alleviates lipid metabolic disorder induced by high-fat diet but also improves hepatopancreas antioxidant activity and is beneficial to gibel carp health.
ACKNOWLEDGEMENTS
This research was financially supported by the National Natural Science Foundation of China (grant no. 31802310), State Key Laboratory of Developmental Biology of Freshwater Fish (2019KF006) and Ningbo Public Service Platform for High Value Utilization of Marine Biological Resources (NBHY-2017-P2).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.




