Non-tuberculous mycobacterial bone and joint infections – a case series from a tertiary referral centre in Australia
Abstract
Background
Non-tuberculous mycobacteria (NTM) are rare causes of bone and joint infection (BJI) and there is limited evidence on which to base management decisions. This study describes 1 year of experience from a multi-disciplinary BJI team which collects data on all cases reviewed at a tertiary referral centre in Queensland, Australia.
Methods
The database was interrogated for all cases in which NTM were recovered from operative samples. Individual chart review was performed to collect the details of each case.
Results
A total of seven cases were managed between 1st February 2021 and 28th February 2022, comprising one patient with chronic osteomyelitis, three with fracture-related infections, two with prosthetic joint infections, and one with infection of a synthetic ligament graft. In contrast to pulmonary NTM infections, most patients were clinically well and immunocompetent, and most infections were propagated by direct inoculation. Time to diagnosis was unknown in three patients, with 1, 2, 2, and 5 months for the remaining four. Rapid growing NTM were diagnosed on routine cultures and specific mycobacterial cultures were confirmatory. Management was characterized by multiple stage surgical procedures and prolonged antimicrobial regimens.
Conclusions
Antimicrobial complications were common; however, all patients were infection free at their latest follow up. Despite the inherent limitations, these results suggest that routinely ordering mycobacterial culture is of low yield. There is potential for shorter-term oral antimicrobial treatments. Prospective research is required to optimize treatment regimens and durations.
Introduction
Non-tuberculous mycobacteria (NTM), a group of more than 190 mycobacteria other than those in the Mycobacteria tuberculosis complex and Mycobacterium leprae, are an increasing cause of disease worldwide.1, 2 NTM are found in water, soil, and dust, and are opportunistic pathogens that can cause disease following environmental exposure, typically through inhalation, ingestion, or direct inoculation.2, 3 Bone and joint infections (BJIs) are a cause of significant morbidity and mortality and impose a high economic burden on health services.4 NTM BJIs are rare, and unlike in pulmonary disease where NTM infection is commonly a complication of a host disease process (cystic fibrosis, bronchiectasis, immunosuppression), NTM BJIs may occur in immunocompetent individuals.1, 5-8
NTM BJIs usually arise following direct inoculation or traumatic injury; however, they can also occur through haematogenous spread in disseminated disease.6, 9, 10 Infection is indolent and may be clinically inapparent for prolonged periods,8 and due to complexities in microbial culture testing diagnosis is often significantly delayed.7 There is a paucity of data on which to guide treatment decisions. Recommendations in published guidelines are based on expert opinion and extrapolation from pulmonary disease.3
This case series describes the characteristics and outcomes of NTM BJI treated by a multi-disciplinary management team at the Royal Brisbane and Women's Hospital (RBWH). The team, which commenced in August 2020 in order to address the complexity of service delivery to patients with orthopaedic device-related infection, includes a dedicated nurse, infectious disease physicians, orthopaedic and trauma surgeons, plastic and reconstructive surgeons, and allied health professionals.11
Methods
A multi-disciplinary device-related infection team (mDRIFT) was commenced at the RBWH in Queensland, Australia in August 2020. Patient demographics, infection characteristics, microbiology, surgical procedures, and antimicrobial therapy data are prospectively collected. This database was interrogated for cases of NTM managed between 1st February 2021 and 28th February 2022. Institutional human research ethics approval was granted by the Metro North Human Research Ethics Committee A (HREC/2023/MNHA/91897) for individual case notes to be reviewed and data extracted. Cases were included if NTM were recovered from at least two tissue or fluid samples collected during surgical procedures. Samples were inoculated onto Horse Blood Agar, Chocolate agar, and Thioglycollate broth for conventional testing, while mycobacterial specific cultures included a liquid broth medium (BD Mycobacterial Growth Indicator Tube) with ODAC growth supplements, and PANTA antibiotics in addition to Lowenstein-Jensen slopes supplemented with pyruvate. NTM were classified as rapidly growing Mycobacterium (RGM) (formation of colonies on subculture within 7 days) or slowly growing Mycobacterium (SGM) (growth after 7 days) according to convention.3 Descriptive statistics, means, and ranges, were used to summarize continuous variables.
Results
A total of 198 cases of BJI were reviewed by the multi-disciplinary BJI team over the study period, of which seven cases involved NTM infection, as seen in Table 1. They comprised one patient with device-related infection (case 1), three with fracture-related infections (FRIs; cases 2, 4, and 5), two with prosthetic joint infections (PJIs; cases 3 and 7), and one with chronic osteomyelitis (case 6). Five of the seven patients were male with a mean age of 53 years (range 25–74). The full cohort had a mean Charlson Comorbidity Index of 2.1 (range 0–7), and a mean American Society of Anaesthesiologists score of 2.3 (ranges 2–3). Only one case was immunosuppressed (case 3, multiple myeloma, receiving lenalidomide). Time to diagnosis was unknown in three patients, with 1, 2, 2, and 5 months in the remaining four patients. Six of seven patients had RGM, including three cases of Mycobacterium abscessus and three of Mycobacterium fortuitum complex (Table 2). One patient had SGM. The mean duration of follow up from diagnosis with NTM infection for cases 2–7 was 26 months (range 13–43) (Table 3). Although lost to clinical follow-up one month after completion of intravenous therapy, this study confirmed that case 1 had no further surgery, no ongoing infection and mobilized independently 33 months post-treatment. Choice and duration of systemic antimicrobial therapy, and local antimicrobials agents and carriers occurred through multidisciplinary discussion, incorporating antimicrobial susceptibilities according to international consensus.1, 5, 12-14 Local antimicrobials were administered with a carrier, as either a polymethyl methacrylate spacer, bead or in calcium sulphate (STIMULAN, Keele, UK).
| Case | Age /sex | Diagnosis | Site | Inciting or predisposing factors | Presenting features | CCI | ASA Score | Time from symptom onset to diagnosis, months |
|---|---|---|---|---|---|---|---|---|
| 1 | 44/M | Synthetic ligament graft infection | Achilles tendon | Penetrating injury, previous LARS | Infected wound, failed to heal despite medical and surgical management | 1 | 2 | 1 |
| 2 | 52/F | FRI | Distal femur | Open fracture (GA1) | Surgical site infection/wound breakdown 7 weeks post fixation | 1 | 2 | 2 |
| 3 | 74/F | PJI | Hip | Immunosuppression and environmental exposure (gardening) | Bursitis, swelling at hip | 7 | 3 | N/A |
| 4 | 25/M | FRI | Distal femur | Open fracture (GA3B) | Asymptomatic – isolated on culture of multiple tissue samples at reconstructive surgery | 0 | 2 | N/A |
| 5 | 58/M | FRI | Distal tibia | Open fracture (GA2) | Draining sinus, non-union | 3 | 3 | 2 |
| 6 | 73/M | OM | Distal tibia | Penetrating injury (agricultural) | Infection at site on injury, persistent despite months of antimicrobials, formation of sinus | 3 | 2 | 5 |
| 7 | 47/M | PJI | Hip | Haematogenous seeding, central line | Central line infection, with blood stream infection | 0 | 2 | N/A |
- Abbreviations: ASA, American Society of Anaesthesiologists; CCI, Charlson Comorbidity Index; FRI, fracture-related infection; GA, Gustilo-Anderson; LARS, ligament augmentation and reconstruction system; N/A, data not available; OM, osteomyelitis; PJI, prosthetic joint infection.
| Case | Species | Diagnostics | Antimicrobial susceptibilities | Intravenous treatment (duration, months) | Oral treatment (duration, months) | Duration of antimicrobial treatment, months | Antimicrobial related complications |
|---|---|---|---|---|---|---|---|
| 1 | M. abscessus ssp. massiliense | Conventional culture negative, MGIT positive after 5 days | S – Amikacin & Clarithromycin I – Cefoxitin & Imipenem | Amikacin (1), Cefoxitin (1) | Clofazimine (0.25), Azithromycin (0.25), Bedaquiline (0.25) | 1.25 | Nil |
| 2 | M. fortuitum complex | Conventional culture positive after 3 days, confirmatory AFB culture | S – Cefoxitin, TMP/SXT, Moxifloxacin, Doxycycline, Imipenem, Linezolid, Amikacin, Ciprofloxacin & Clarithromycin I – Tobramycin | Cefoxitin (1), Imipenem (0.5), Amikacin (1), Tigecycline (0) | Ciprofloxacin (9), TMP/SXT (8.5), Minocycline (1), Azithromycin (6) | 9 | Nausea, diarrhoea |
| 3 | M. heckeshorne-nse & M. avium | Conventional culture negative, mycobacterial culture positive | S – TMP/SXT, Amikacin, Clarithromycin & Ethambutol I –Moxifloxacin (for M. avium) | Nil | Nil | 0 | Nil |
| 4 | M. fortuitum | Conventional culture positive after 5 days, confirmatory mycobacterial culture | S – TMP/SXT, Moxifloxacin, Linezolid, Amikacin & Ciprofloxacin I – Cefoxitin & Imipenem | Nil | TMP/SXT (6), Ciprofloxacin (6), Linezolid (1) | 6 | Nausea, linezolid associated leukopenia |
| 5 | M. fortuitum complex | Conventional culture positive after 3 days, confirmatory mycobacterial culture | S – TMP/SXT, Moxifloxacin, Imipenem, Amikacin & Ciprofloxacin I – Cefoxitin & Linezolid | Cefoxitin (0.5), Imipenem (1) | TMP/SXT (6), Moxifloxacin (6) | 6 | Nausea |
| 6 | M. abscessus ssp. abscessus/bolletti | Conventional culture positive after 4 days, confirmatory mycobacterial culture | S – Amikacin & Clarithromycin I – Cefoxitin & Imipenem | Amikacin (2), Cefoxitin (1), Meropenem (0.5), Tigecycline (2) | Rifabutin (3), Azithromycin (4), Bedaquiline (15), Clofazimine (18.5) | 19.5 | Eosinophilia with meropenem, tinnitus and vertigo on amikacin, nausea with tigecycline |
| 7 | M. abscessus ssp. abscessus/bolletti | Blood culture positive after 5 days, confirmatory AFB smear of tissue | S – Amikacin & Clarithromycin I – Cefoxitin & Imipenem | Amikacin (1), Imipenem (3.5), Tigecycline (0.25) | Rifabutin (9), Azithromycin (9), Bedaquiline (11), Clofazimine (11) | 12 | Tigecycline induced pancreatitis, persistent tinnitus with amikacin |
- Abbreviations: AFB, acid-fast bacilli; I, susceptible, increased exposure; MGIT, mycobacteria growth indicator tube; S, susceptible; TMP/SXT, trimethoprim/sulfamethoxazole.
| Case | Surgical management | Local antimicrobials | Number of procedures | Surgical complications | Duration of follow up since diagnosis, months | Outcomes |
|---|---|---|---|---|---|---|
| 1 | Debridement and implant removal, ALT free flap closure | Nil | 6 | Nil | 33 | Clinically well and infection free, wounds healed, good functional outcome at last review |
| 2 | Multi-stage debridement, ORIF with Masquelet and 3D printed cage reconstruction | Vancomycin, flucloxacillin, amikacin, and tigecycline in PMMA beads | 8 | Infection relapse (source control), superimposed surgical site infection (Candida parapsilosis) | 26 | Clinically well and infection free, wounds healed, good functional outcome |
| 3 | Excision and irrigation | Nil | 1 | Nil | 14 | Observation only, clinically well and infection free, walking independently |
| 4 | Multi-stage debridement, reconstruction with Masquelet procedure and muscle rotational flap | Vancomycin as powder in bone defect | 7 | Nil | 31 | Clinically well and infection free, wounds healed, varus deformity requiring operative revision |
| 5 | Multi-stage debridement and external fixation, followed by ORIF | Vancomycin, flucloxacillin, and amikacin in PMMA cement | 6 | Nil | 13 | Clinically well and infection free, chronic pain, good functional outcome |
| 6 | Multi-stage debridement and insertion of amikacin beads, transposition flap closure | Vancomycin and tobramycin in PMMA beads, vancomycin and flucloxacillin in PMMA beads, amikacin in calcium sulphate, vancomycin and amikacin in PMMA cement | 9 | Surgical site infection (Serratia marcesens) | 42 | Clinically well and infection free, good functional outcome |
| 7 | 2 stage revision arthroplasty | Vancomycin, amikacin and cefoxitin in PMMA cement, gentamicin in calcium sulphate | 5 | Hip dislocations requiring revision (aseptic) | 33 | Clinically well and infection free, good functional outcome |
- Abbreviations: ALT, anterolateral thigh; ORIF, open reduction internal fixation; PMMA, polymethyl methacrylate.
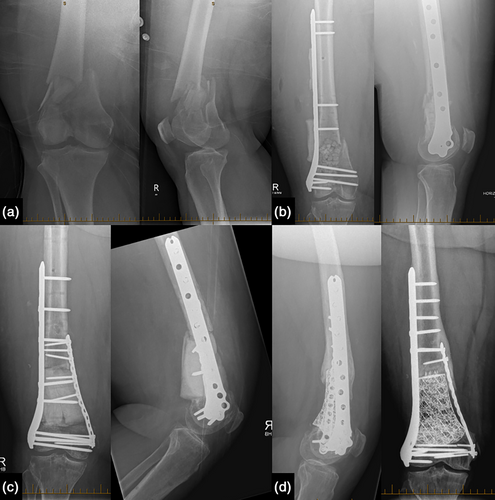
All M. fortuitum cases (cases 2, 4, and 5) were recovered through conventional laboratory culture methods on day 3, 3, and 5, without specific mycobacterial culture procedures (Table 2). Reflex confirmatory testing was performed when mycobacteria were identified on conventional media. All three cases were FRIs following compound fractures (Gustilo-Anderson type 1, 2, and 3b) of the lower limb managed with internal fixation (case 2 and 4) and intermedullary nail (case 5). Clinical presentation was varied, including a late surgical site infection at 7 weeks (case 2), asymptomatic infection identified when returning for an elective second stage of induced membrane (Masquelet) procedure (case 4), and one case of non-union with NTM infection identified at debridement and revision of fixation (case 5, Table 1). Surgical management involved meticulous debridement of the infected area and reconstructive procedures as indicated, with an example for case 2 seen in Figure 1. Local antimicrobials were used in two cases (cases 2 and 5, Table 2). Systemic antimicrobials were administered for 6–8 months, comprising an initial intravenous phase in cases 2 and 5, followed by an oral phase of at least two agents (Table 2). Case 2 had seven procedures (Table 3). Initial open reduction internal fixation (ORIF) was complicated by wound breakdown and infection recurrence requiring multiple debridements, and ultimately two-stage reconstruction using the Masquelet technique with bone grafts. Case 4 had seven procedures including wound debridements due to recurrence of infection following ORIF, two-stage reconstruction using the Masquelet technique, and flap closure. Case 5 required six procedures. Initially, an intermedullary nail was complicated by compartment syndrome requiring fasciotomy as well as formation of a draining sinus and non-union requiring further procedures with insertion of an antibiotic nail and external fixation, and ultimately ORIF. At end of follow up, all wounds were healed, antimicrobials had ceased. All cases were mobilizing freely, although case 4 developed varus deformity and required further surgical correction.
The three M. abscessus cases included one of M. abscessus ssp. massiliense (case 1, Table 2), and two of M. abscessus ssp. abscessus/bolletti (cases 6 and 7, Table 2). Clinical presentation was of nonhealing wounds after penetrating injury in cases 1 and 6. The third case was a total hip replacement PJI following central line associated blood stream infection (case 7, Table 1). Microbial diagnosis was achieved by conventional tissue culture in case 1, by dedicated mycobacterial culture in case 6, and by recovery from blood culture using the BACT/ALERT (bioMérieux) blood culture system in case 7 (with mycobacterial tissue culture at revision). Surgical management was complex, comprising multiple procedures (six, nine and five) involving radical debridement followed by reconstructive procedures (Table 3). Case 1 had six procedures including multiple debridements due to ongoing infection, resection of a synthetic ligament, and flap closure. Case 6 had nine procedures. Initial management of osteomyelitis from a penetrating injury was characterized by debridement, insertion of antibiotic beads, a cement spacer, and flap closure. Complicated by surgical site infection, they required further debridements, and insertion of antibiotic beads/spacer leading to bone graft and flap closure. Case 7 needed five procedures including two-stage revision arthroplasty with antibiotic cement. Due to recurrent hip dislocations, they required two closed reductions and operative revision. Local antimicrobials were administered in cases 6 and 7 including amikacin and cefoxitin (Table 3). All three patients were treated with an initial induction phase comprising intravenous agents (amikacin, cefoxitin, tigecycline) and oral agents, followed by three to four oral agents for another 1–19 months (Table 2). Adverse events included amikacin-induced tinnitus in cases 6 and 7, nausea in case 6, and tigecycline-induced pancreatitis in case 7. At the end of follow up, all wounds were healed, antibiotics were completed, and all were mobilizing independently.
The only infection caused by an SGM was a sub-acute, late poly-mycobacterial total hip replacement PJI (case 3). Mycobacterium avium and Mycobacterium heckeshornense were recovered from multiple tissue samples collected at debridement and implant retention of a prosthetic hip joint (Table 1). This was the only patient receiving immunosuppression (lenalidomide for multiple myeloma). They presented with swelling around the hip joint. Conventional culture as well as culture for Mycobacterium and fungi was performed. While conventional and fungal cultures were negative, M. avium and M. heckeshornense were recovered from 3 tissue samples at Mycobacterium culture after 22 days. Histology revealed granulomatous inflammation, consistent with Mycobacterium infection. As the patient's symptoms were completely resolved following operative debridement, and due to significant co-morbidities, preserved function, and the low virulence of the organism, revision arthroplasty was considered an unacceptable risk. Without surgical revision, prolonged combination antimicrobial therapy was thought unlikely to be effective and carries significant morbidity. It was, therefore, withheld, and they were then observed for a further 14 months with no symptom relapse, no reduction in function, or radiological lucency identified, and remain well.
Discussion
This study provides a detailed report of the detection, management, and outcomes of BJI involving NTM in a series of patients treated by a multidisciplinary BJI team. The most important observation from this investigation is that adverse events related to antimicrobials in these patients are common, and they warrant close observation during treatment.
RGM infection occurred in patients with open fractures (3), inoculating injuries (2), or a complication of central venous line infection. Four of the six RGM cases in this series presented with delayed healing or late surgical site infection. This is consistent with a case series of PJI caused by NTM, where the clinical presentation is described as subacute, with pain and swelling, low C-reactive protein (less than 4.0 mg/dL), and white blood cell count on arthrocentesis under 20 000.15 Although the majority of this series' RGM cases were associated with trauma and penetrating injuries, presentation was similarly subacute, often weeks after the injury, and delay to diagnosis was common.
RGM BJI is rare and delays in diagnosis are characteristic, as illustrated by the case series in Park et al.,7 with an average time to diagnosis of 20 months. In this series, most cases were identified by conventional tissue culture rather than dedicated mycobacterial studies. This supports the findings that routine mycobacterial culture is of poor diagnostic yield and should not be performed as routine practice, but reserved for specific situations including subacute, culture negative infections, or immunocompromised patients.10
Management was complex, requiring multidisciplinary co-ordination from orthopaedics, plastics and reconstructive surgery, infectious disease and microbiology, and rehabilitation and allied health services. Patients underwent multiple surgical procedures with meticulous debridement and local antimicrobial administration. Three of the seven cases required muscle flap reconstruction of soft tissue defects. Compared with infection caused by typical organisms, with average antimicrobial treatment durations between 1 and 3 months,16 treatment durations for BJI caused by NTM were prolonged to between 1 and 19 months in this cohort, and between 2 and 39 months in Goldstein et al.8 Adverse drug reactions were common, highlighting the need for close adverse event monitoring during prolonged oral antimicrobial treatments, as described by Seaton et al.17 for all BJIs.
There is a paucity of data on which to base treatment decisions for mycobacterial BJI. Therapeutic recommendations have generally been extrapolated from experience and guidelines for management of pulmonary infections, that currently recommend treatment for a minimum of 12 months.1, 18 However, it is possible that shorter, more conventional treatment approaches may be effective in NTM BJI. Importantly, the opportunity to surgically control infection through operative debridement distinguish BJI from pulmonary disease. A case series of NTM osteoarticular infection by Marchevsky et al.19 provides a compelling anecdote that surgical management alone may be successful. This is supported in this series by case 3, who has remained well without mycobacterial treatment, and case 1 who sustained cure after debridement and only 5 weeks of antimicrobial treatment (due to non-compliance).
Despite little evidence supporting local antimicrobial administration, it is widespread in bone and joint infection, supported by international consensus,13, 20, 21 and supported by compelling clinical examples.22 Local antimicrobial administration may improve efficacy through delivering high concentrations at the site of infection, while limiting toxicity associated with systemic exposure.23 This is particularly attractive for difficult to treat infections, like NTM, in which treatment toxicity impacts on morbidity, as illustrated here. The results of SOLARIO, a randomized clinical trial evaluating the efficacy of locally applied antimicrobials are eagerly awaited.24
This report is subject to the limitations inherent in any small observational cohort study of an uncommon pathogen. Retrospective studies ultimately rely on data from the medical records and are subject to both selection bias and recall bias. Another major limitation is the lack of a comparison group; absence of a control group makes it difficult to meaningfully compare results with other treatment protocols in other centres. The study cohort was necessarily small, reflecting the uncommon nature of the pathogen, although the cohort is comparable in size to other similar reports. Furthermore, the inherent heterogeneity of the study cohort resulted in a broad range of presentations, that renders them difficult to analyse and compare objectively. Finally, because all care was provided at a single, specialized centre it is difficult to extrapolate these results to those achieved at less-experienced units.
Despite the outcomes described in this series, there is still considerable therapeutic uncertainty in the treatment of NTM BJI. Because NTM BJIs are rare, with diverse microbiology and presenting in heterogenous populations, their study is not readily amenable to controlled trials. It is imperative that clinical experience is published and that large multi-centre, prospective observational studies are performed.
Most importantly, antimicrobial complications were common in this cohort, and these patients benefit from close monitoring while undergoing treatment; nevertheless, all patients were deemed to be infection free at their latest follow up. Despite the inherent limitations, these results suggest that routinely ordering mycobacterial culture is of low yield and perhaps shorter-term antimicrobial treatments may be considered in the setting of thorough surgical debridement.
Author contributions
Cameron Holscher: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; visualization; writing – original draft; writing – review and editing. Silvia Manzanero: Formal analysis; supervision; visualization; writing – review and editing. Anna Hume: Formal analysis; writing – review and editing. Andrew L. Foster: Formal analysis; visualization; writing – review and editing. Kevin Tetsworth: Formal analysis; writing – review and editing. Paul R. Chapman: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; supervision; writing – original draft; writing – review and editing.
Conflict of interest
None declared.