Diagnostic accuracy of Charcot's triad: a systematic review
Abstract
Background
Charcot's triad is a well-established diagnostic tool for acute cholangitis (AC). It has been recognized as an inaccurate test in clinical practice; however, its exact sensitivity and specificity remain unclear. This knowledge is key to informing the value of its continued clinical application. The objectives of this study are to calculate an estimate of the sensitivity and specificity of Charcot's triad based on published research and consider its applicability to clinical practice and medical education.
Methods
Electronic database search for relevant literature and review of reference lists of the subsequent articles for additional resources. Two independent researchers located articles which were qualitatively and quantitatively reviewed. The overall sensitivity and specificity values across all studies were subsequently calculated.
Results
The 16 articles included in the review varied widely in study design and the sensitivity of Charcot's triad was reported for AC. Across the included articles, there were 4288 patients studied. The overall sensitivity for Charcot's triad was calculated as 36.3%. The specificity was only analysed in three studies and had an overall value of 93.2%. Nine of the articles also investigated the sensitivity of Reynold's pentad which was consistently low with an overall value of 4.82%. The specificity of Reynold's pentad was not studied.
Conclusion
Charcot's triad has limited clinical utility as a diagnostic algorithm for AC. It is an effective rule-in test but a poor rule-out test and should be applied and taught accordingly. A more sensitive diagnostic tool is required to achieve superior outcomes for AC patients.
Introduction
Acute cholangitis (AC) is a potentially serious cause of abdominal pain in the hospital setting which requires a high index of suspicion.1 Historically, the condition has been diagnosed clinically using Charcot's triad, the combination of right upper quadrant (RUQ) pain, fever and jaundice.2, 3 Reynold's pentad – which includes the addition of shock and lethargy or mental confusion – has also been used as a diagnostic tool to help identify acute obstructive cholangitis.4 Over time, it has been acknowledged that presentations of AC do not consistently fulfil the features of the triad;1, 5, 6 however, the exact diagnostic accuracy of Charcot's triad still remains unclear. Prompt diagnosis of AC is essential given the high mortality associated with severe disease, which is made more favourable when optimal treatment is implemented.7-9
Despite advances in medicine, there is no universally recognized gold standard for the diagnosis of AC.1, 5, 6, 10, 11 In addition, treatment pathways differ significantly depending on the severity of the disease, which is also difficult to assess clinically.1, 6, 10, 12 In view of this, other diagnostic algorithms have been developed, most notably the Tokyo Guidelines which were first introduced in 2007 and then revised in 2013.6, 13
In studies assessing the accuracy of the Tokyo Guidelines, it is clear that further improvements are required to achieve the desired effect of this diagnostic tool in practice.5, 14-17 In contrast to the Tokyo Guidelines, Charcot's triad is widely recognized, simple and does not rely on laboratory data, so may still have an important role in the clinical workup of AC patients. The purposes of this review are to establish the sensitivity and specificity of Charcot's triad in the diagnosis of AC, and better characterize the utility of the triad as a diagnostic tool in practice.
Methods
Search
To perform our review, we conducted an electronic search of the following databases: Medline (1946–2015), Embase (1980–present), Embase Archives (1947–1979), Cochrane Library, Scopus, Web of Science, ProQuest Dissertations & Theses (1861–present), Trove and EThOS. Our search terms included ‘cholangitis’, ‘cholangitides’, ‘bile duct inflam*’, ‘biliary tract inflam*’, ‘biliary tract diseas*’, ‘bile duct diseas*’, ‘charcot*’, ‘sensitivity’, ‘specificity’, ‘diagnosis’, ‘accuracy’ and ‘reliability’. We also reviewed the citations of the papers generated by our search for any additional articles. Search limits were placed to include only the English language and published literature.
Inclusion criteria
Articles were included in the review if they met the following criteria: (i) it was a clinical study publication; (ii) the sensitivity or specificity of Charcot's triad was reported or calculable from the study results; and (iii) the full text article was available. Any study design was included and there was no restriction on the date of publication. Papers were excluded if the study size was less than 10 participants or the cause of AC was due to a subset of rare conditions.
Review
The search was conducted according to the PRISMA (preferred reporting items for sytematic reviews and meta-analyses) guidelines.18 Two independent reviewers each selected eligible articles based on the inclusion criteria. Any inconsistencies were first discussed between the reviewers, with the senior author being consulted if a consensus could not be reached. We performed a descriptive narrative. The points of interest from each paper were the study type, aim, participant characteristics, definition of Charcot's triad, the gold standard method used to diagnose AC and the reported sensitivity and specificity of Charcot's triad. In addition, data from all studies were collated to give an overall estimate of the sensitivity and specificity of Charcot's triad based on each study's ‘Gold Standard’ criteria for cholangitis. This was also performed for Reynold's pentad data when data were available.
Results
Search
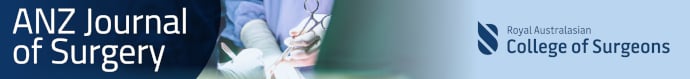
The numbers of articles yielded by our electronic search are as follows: Medline 20, Embase 33, Embase Archives 3, Cochrane Library 8, Scopus 30, Web of Science 27, ProQuest Dissertations & Theses 0, Trove 0 and EThOS 0. A further eight articles were identified from the reference lists of papers generated by the electronic search. Sixty-five articles remained after all duplicates were removed, of which the full texts were available for 46. These were screened for eligibility which produced 16 papers for the review, encompassing a total of 4288 participants (Fig. 1). Two of these papers15, 19 appeared to use data from the same pool of participants so only the more recent article was selected for analysis19 (Table 1).
| Reference number | Study type | Study aim | Participant characteristics | Measurement and definition of Charcot's triad | Gold standard test for AC | Sensitivity and specificity of Charcot's triad and Reynold's pentad |
|---|---|---|---|---|---|---|
| 2 | Retrospective cohort |
Retrospective analysis of the presentation, diagnosis, findings, cause, treatment and outcome of AC cases
|
99 consecutive cases of acute AC at UCSF medical centre from 1966 to 1976 | Not defined | Evidence of infection and biliary tract obstruction confirmed by radiological or surgical findings (ERCP only became available late in study) |
Charcot's triad: Sensitivity 69.7% Reynold's pentad: Sensitivity 5.05% |
| 13 | Retrospective cohort |
To assess diagnostic accuracy of 2007 Tokyo Guidelines for AC compared with Charcot's triad
|
1432 suspected cases of AC of whom 794 had AC across multiple centres in Japan from January 2007 to July 2011 | Not defined | Purulent bile observed on ERCP, or clinical remission following bile duct drainage or remission achieved by antibiotic therapy alone when only possible source was biliary tree |
Charcot's triad: Sensitivity 26.4% Specificity 95.6% |
| 16 | Retrospective cohort from prospectively established database | To analyse outcomes and identify predictors of poor outcome in patients with AC managed using Tokyo guidelines 2007
|
215 consecutive cases of AC admitted from February 2007 to July 2011 at Chiba University hospital |
Abdominal pain Fever and/or chills Jaundice |
Tokyo guidelines 2007 (severe, moderate or mild) |
Charcot's triad: Sensitivity 33.5% overall, 7.7% for mild AC, 39.8% for moderate, 50% for severe |
| 17 | Retrospective cohort |
To assess the validity of 2007 Tokyo guidelines for management of AC and cholecystitis
|
74 consecutive cases of suspected AC with 61 confirmed cases (also 81 suspected cases of cholecystitis in separate part of study) from November 2004 to November 2005 admitted to Nagoya Daini Red Cross Hospital |
RUQ or upper abdominal pain Fever and/or chills Jaundice |
Final diagnosis made by the physician taking care of the patient based on all available clinical information. If unsure after that, there was an expert panel who decided |
Charcot's triad: Sensitivity 11.5% Specificity 84.6% |
| 19 | Retrospective cohort from prospectively established database |
To compare the 2007 and 2013 Tokyo Guidelines for management of AC and cholecystitis
|
120 cases of AC in ERCP database from January 2000 to December 2009. 18-year old and older first episode of AC requiring biliary drainage |
RUQ or upper abdominal pain Fever and/or chills Jaundice |
Identification of purulent bile during endoscopic biliary drainage |
Charcot's triad: Sensitivity 50.8% overall, 53.7% for benign obstruction, 44.7% for malignant Reynold's pentad: Sensitivity 10.0% overall |
| 20 | Prospective cohort |
Prediction of AC and severity from clinical and laboratory features of patients with CBD stones
|
1282 cases with acute suppurative cholangitis due to extrahepatic CBD stones confirmed intraoperatively from 1980 to 1988 |
Pain Fever or chills Jaundice |
Intraoperative CBD bile aspirate showing fluid with turbid or frank pus |
Charcot's triad: Sensitivity 22% Specificity 91% |
| 21 | Retrospective cohort | Prediction of suppurative cholangitis in patients with malignant biliary tract obstruction
|
107 cases with malignant bile duct obstruction undergoing surgery from 1992 to 2003, 16 of whom had suppurative cholangitis | Not defined | Suppurative cholangitis found at laparotomy |
Charcot's triad: Sensitivity 43.8% Reynold's pentad: Sensitivity 6.25% |
| 22 | Retrospective cohort |
Retrospective analysis of diagnosis, cause, treatment and outcome for cases of AC
|
15 consecutive cases of acute suppurative cholangitis from 1962 to 1965 at the Lutheran Hospital, Milwaukee |
RUQ pain Chills and fever Jaundice |
Acute illness + evidence of CBD obstruction + frank pus found in CBD at time of exploration or post-mortem |
Charcot's triad: Sensitivity 13.3% Reynold's pentad: Sensitivity 6.7% |
| 23 | Retrospective cohort |
To assess whether diagnosis of AC is delayed in elderly (>75) compared to younger and why
|
122 cases of AC. 45 young patients (<75 years), 77 old patients (>75 years) |
Abdominal pain Fever Jaundice |
(1) Fever >38°C and/or rigours and/or WCC >12 × 103/dL and (2) Biliary tract dilation on USS or ERCP and biochemical features of cholestasis or CBD calculus on ERCP |
Charcot's triad: Sensitivity 17.6% overall, no significant difference between young and old patients (15.6% in young, 18.8% in old) |
| 24 | Retrospective cohort | To explore diagnostic factors for AC in the elderly
|
17 cases of AC in people aged over 65 who underwent ERCP with CBD stones from April 2008 to June 2011. 36 diagnosed with AC from 71 patients with 101 total ERCPs. Unsure why all 36 not recruited – perhaps age |
Abdominal pain Fever Jaundice |
Guidelines from Miura et al.10 |
Charcot's triad: Sensitivity 23.5% Reynold's pentad: Sensitivity 5.9% |
| 25 | Retrospective cohort | Assess success of endoscopic sphincterotomy for cure of AC
|
159 cases diagnosed with AC out of 1061 who underwent ERCP and EST from 1981 to 1989 | Not defined | Infected bile (green or pyobile) on ERCP |
Charcot's triad: Sensitivity 43.4% Reynold's pentad: Sensitivity 4.4% |
| 26 | Retrospective cohort | Prediction of mortality from AC based on clinical, biochemical and imaging features
|
449 cases of AC in 412 different patients from 1963 to 1983 at Paul Brousse Hospital, France. All patients in that time frame |
Abdominal pain Fever Jaundice |
Clinical picture of cholestasis and infection + positive blood and/or bile culture + anomaly of the biliary tract |
Charcot's triad: Sensitivity 72% Reynold's pentad: Sensitivity 3.5% |
| 27 | Retrospective cohort | To predict mortality risk from serious AC based on clinical and biochemical features
|
86 consecutive cases of severe AC secondary to choledocholithiasis requiring emergency surgery from January 1984 to September 1988 managed by department of surgery University of Hong Kong , Queen Mary Hospital |
Abdominal pain Fever (maximum >38.2°C) Jaundice (total bilirubin >25 mmol/L) |
AC: hyperbilirubinaemia + fever or abdominal pain Severe AC: Progression of biliary sepsis evident despite adequate trial of conservative treatment |
Charcot's triad: Sensitivity 55.8% |
| 28 | Retrospective cohort | To identify admission factors predictive of major complications and mortality in AC
|
117 cases of cholangitis from 137 with discharge diagnosis of cholangitis from 1995 to 2005 at from UCLA medical centre |
RUQ pain Fever Jaundice |
Biliary obstruction (CBD ≥1 cm on USS or CT, or serum total bilirubin ≥2 mg/dL and ALP ≥150 mg/dL) + systemic infection, two or more out of (1) body temperature ≥38 or ≤36°C, (2) SBP ≤100 mmHg, (3) WCC ≥12000/mm3, ≤4000/mm3 or ≥10% band cells and (4) positive blood or capillary cultures |
Charcot's triad: Sensitivity 41.9% Reynold's pentad: Sensitivity 2.6% |
| 29 | Retrospective cohort | To analyse the course and outcome of patients with acute suppurative cholangitis with or without obstruction
|
20 patients with acute suppurative cholangitis (15 obstructive, 5 not obstructive) treated from 1962 to 1970 at Massachusetts General Hospital |
RUQ pain Fever Chills |
ASC: abdominal pain, fever, chills and leucocytosis; obstructive jaundice and purulence in the bile duct at surgery or autopsy AOSC: As described above with added central nervous system depression with bacteraemia and hypotension, and complete biliary obstruction, common duct pus under pressure and possible liver abscesses at surgery or autopsy |
Charcot's triad: Reported sensitivity of 50% for each group does not equate to even numbers of participants |
| 30 | Retrospective cohort | To distinguish acute suppurative cholangitis and acute non-suppurative cholangitis
|
65 cases of cholangitis (46 NSC, 19 SC) from review of 400 suspected cases of cholangitis, cholecystitis, CBD exploration or pancreatic cancer from 1957 to 1980 |
Not clearly defined: Abdominal pain Fever Jaundice or Pain Fever Chills Jaundice |
Roentgenographic or operative or post-mortem evidence of biliary tract obstruction and biliary sepsis (fever and chills, jaundice or abdominal pain) + presence of purulence in the CBD (NSC) or no evidence or purulence (SC) |
Charcot's triad: Sensitivity 53% for suppurative cholangitis 63% for non-suppurative Reynold's pentad: Sensitivity 5% suppurative 9% non-suppurative |
- AC, acute cholangitis; ALP, alkaline phosphatase; AOSC, acute obstructive suppurative cholangitis; ASC, acute suppurative cholangitis; CBD, common bile duct; CT, computed tomography; ERCP, endoscopic retrograde cholangiopancreatography; NSC, non-suppurative cholangitis; RUQ, right upper quadrant; SBP, systolic blood pressure; SC, suppurative cholangitis; UCLA, University of California Los Angeles; UCSF, University of California San Francisco; USS, ultrasound scan; WCC, white cell count.
Study type
Fifteen of the 16 articles reviewed were retrospective cohort studies reviewing clinical records. Two of these 15 articles retrospectively assessed data from a prospectively generated database intended for use in research.16, 19 The only prospective study identified in this search was by Csendes et al., where participants having surgery for common bile duct (CBD) stones were divided into those with and without AC based on aspirated fluid from the CBD.20
Study aim
The studies in our review had a range of primary objectives. Charcot's triad was a key focus in only three of the 16 articles.5, 17, 19 Nine papers aimed to identify factors predictive of a diagnosis of AC,2, 13, 17, 19-24 four assessed management strategies for AC,2, 19, 22, 25 nine papers analysed factors predicting prognosis in AC patients2, 16, 19, 20, 22, 26-29 and two articles focused on drawing comparisons between different subset groups of people with AC.23, 30
Participant characteristics
The size of the studies we reviewed varied widely, with the largest study having 1282 participants with AC20 and the smallest having 15 participants.22 The mean number of participants with AC in each study was 228 and the median was 108.
Nine articles identified the location of participant recruitment which included three from the USA,2, 22, 29 three from Japan13, 16, 17 and one each from Chile,20 France,26 Hong Kong27 and China.19 One study recruited participants from multiple centres, all of which were in Japan.13 Six studies failed to give clear information about where their participants were recruited from. The authors of these articles were from the USA,28, 30 Japan,24, 25 Turkey21 and the UK.23
The causes of AC were not specified in 12 of the 16 reviewed studies, while four studies focused on specific AC aetiology. Csendes et al. and Lai et al. only looked at AC caused by extrahepatic CBD stones, Doganay et al. investigated malignant causes of bile duct obstruction, and Tomizawa et al. only selected people over 65 years with AC due to CBD stones.20, 21, 24, 27
Measurement and definition of Charcot's triad
Although the definition of Charcot's triad varied subtly between articles, each definition had a pain element, a fever and/or chills element and a jaundice element. The one exception to this was by Welch et al. who did not include jaundice in their definition of Charcot's triad (RUQ pain, fever and chills).29 The pain element was identified as abdominal pain in six articles,16, 23, 24, 26, 27, 30 RUQ pain in three articles,22, 28, 29 RUQ or upper abdominal pain in two articles17, 19 and simply as pain in two articles.20, 30 Four articles failed to explicitly define Charcot's triad,2, 13, 22, 25 while O'Connor et al. referred to two separate classifications of Charcot's triad, neither of which were described as part of their methods.30 None of the articles clearly identified which point in time Charcot's triad was present during the hospital stay.
Gold standard test for AC
A number of articles commented on the lack of an agreed gold standard for AC diagnosis,13, 19, 28 which resulted in a wide range of diagnostic methods. The most common means of definitive diagnosis was through surgical exploration, which was at least partially used by six articles,2, 20-22, 29, 30 followed by endoscopic retrograde cholangiopancreatography (ERCP) in four articles,13, 19, 23, 25 post-mortem findings in three articles22, 29, 30 and the 2007 Tokyo Guidelines in two articles.16, 24 Among the other methods used, expert opinion17 and remission of symptoms post-treatment were included.13 In order to not miss any diagnoses, a number of articles included multiple modalities (often in combination with clinical and biochemical findings) to achieve their definitive diagnostic criteria of AC. For example, Welch and Donaldson used surgical exploration or post-mortem examination findings.29
Sensitivity and specificity
When data from all studies were collated to estimate an overall sensitivity and specificity for Charcot's triad, these figures were calculated as 36.3% and 93.2%, respectively. Sensitivity data were obtained from all 16 studies, while the specificity data were obtained from just three articles.13, 17, 20
There was a large variation in the reported sensitivity of Charcot's triad between studies. We have included all figures in our analysis where the prevalence of Charcot's triad was analysed for different subgroups within the same study. The values ranged from 7.7% in a subgroup of mild AC cases from the study by Tsuyuguchi et al.,16 to 72% in the study by Gigot et al.26 Six studies reported a sensitivity of <25%,16, 17, 20, 22-24 while six reported figures of 50% or above2, 16, 19, 26, 27, 29, 30 and the remaining six values were between these figures.13, 16, 19, 21, 25, 28 The median sensitivity was 43.6%. Of note is the study by Welch and Donaldson where the sample of 20 AC patients was split into five who had acute suppurative cholangitis and 15 who had acute obstructive suppurative cholangitis. The article reported that 50% of each group demonstrated the features of Charcot's triad, which does not correspond appropriately to the odd numbers in each group.29 The true figure was not calculable from the information given in the article so we have used the reported values for our analysis.
A number of studies also completed analysis on the sensitivity of the Charcot's triad in relation to the severity of AC with varying results. Kiriyama et al. found no association between Charcot's triad and the severity of AC, and Gigot et al. found no association between Charcot's triad and mortality outcomes.13, 26 In contrast, Tsuyuguchi et al. reported an increased sensitivity of Charcot's triad with disease severity, and O'Connor et al. found that the prevalence of Charcot's triad was higher in participants who survived.16, 30
Only three of the studies in our review reported figures for the specificity of Charcot's triad in diagnosing AC, which were 91%,20 95.6%13 and 84.6%.17 This was based on different comparison groups for each of the studies which were: people with CBD stones without AC;20 people with other biliary diseases including acute cholecystitis, choledocholithiasis or biliary stricture13 and people with suspected AC with a non-specified final diagnosis.17 All other studies in the review were conducted on AC patients alone, so the specificity of Charcot's triad was not able to be deduced. Of note is that each of the studies reporting specificity values also had a relatively low corresponding sensitivities of 22%,20 26.4%13 and 11.4%,17 with overall sensitivity calculated to be 19.5% (compared with 36.3% overall for all studies).
The varied definition of Charcot's triad across different articles did not seem to affect its sensitivity. There was no difference found between less precise pain definitions (such as ‘pain’ or ‘abdominal pain’)16, 20, 23, 24, 26, 27 and the more specific definition of ‘RUQ pain’17, 19, 22, 28, 29 (M = 37.4%, SD = 21.8% and M = 33.5%, SD = 19.6% respectively, P = 0.76). The wide range in sensitivity values persisted between the larger (>200 AC patients)13, 16, 20, 26 and smaller studies (<200 AC patients)2, 17, 19, 21-25, 27-30 (M = 38.5%, SD = 22.8% and M = 40.1%, SD = 19.3% respectively, P = 0.89). Sensitivity also did not differ significantly between the articles with different study objectives (AC diagnostic predictors: M = 30.9%,2, 13, 17, 19-23 SD = 19.6%, AC prognostic factors: M = 45.4%,2, 16, 19, 20, 22, 26-29 SD = 20.0%, P = 0.14) or gold standard tests for AC (surgical exploration: M = 43.1%,2, 20-22, 29, 30 SD = 21.8%, ERCP: M = 34.6%,13, 19, 23, 25 SD = 15.2%, P = 0.52). There was also no difference between studies carried out in North American2, 22, 28-30 and European countries23, 26 compared with Asian countries13, 16, 17, 19, 24, 25, 27 (M = 46.4%, SD = 23.6% and M = 35.0%, SD = 15.9%, respectively, P = 0.31).
Sensitivity data regarding Reynold's pentad were found in nine of the 16 articles with values ranging from 2.6% to 10%.2, 19, 21, 22, 24-26, 28, 30 Across all studies, the overall sensitivity value for Reynold's pentad was calculated to be 4.82% from 1057 patients. There was unfortunately insufficient data to assess the specificity of Reynold's pentad in any of the articles.
Discussion
This review was performed to assess the available literature on the sensitivity and specificity of Charcot's triad to gauge its applicability in clinical practice. Our results have shown that the sensitivity of Charcot's triad as reported in published literature has an enormous variance ranging from 7.7% to 72% across 16 separate studies, and an overall value calculated as 36.3%. The specificity of Charcot's triad, however, was found to have a much narrower range, between 84.6% and 95.6% with an overall value of 93.2%, although this was only reported across three studies. Charcot's triad is an attractive diagnostic tool because it requires no laboratory results or investigations, but its low and variable sensitivity suggests that it is not a reliable rule-out test. The relatively high specificity of Charcot's triad suggests that it may be a more useful rule-in test, although this is unfortunately only based on the results of three studies.13, 17, 20
To our knowledge, this is the only systematic or literature review investigating Charcot's triad's sensitivity and specificity. In saying this, the development of the Tokyo Guidelines for the management of AC and cholecystitis highlights the diagnostic limitations of Charcot's triad.15, 31
We were surprised to see such variation in the definition of Charcot's triad and feel this may negatively influence its clinical and research applicability. We expected precise Charcot's triad definitions to have lower sensitivity, yet we found no difference between less and the more specific definitions of pain in Charcot's triad. We were also surprised that despite the variation in study designs there were no differences in the range of sensitivity values between the larger (>200 AC patients) and smaller studies (<200 AC patients) or the sensitivities for different study objectives, gold standard tests for AC or study locations.
In addition, we were surprised to note that the studies assessing specificity found Charcot's triad to be less sensitive than many of the other studies in our review. It is difficult to draw conclusions from this finding given that only three studies included specificity data, all of which were based on retrospective data with the exception of Csendes et al.20
The degree of heterogeneity between the studies in this review limited our ability to compare and draw conclusions from their results. The definitive diagnostic testing for AC across these articles varied significantly, as did the definition of Charcot's triad. There were four articles which looked at specific cases of AC20, 21, 24, 27 whose data must be viewed carefully as they cannot be applied to all causes of AC. In addition, the study by Welch et al. provided an inaccurate report of the sensitivity of Charcot's triad based on its study population.29 The combination of all these inconsistencies may explain why a review like this has not yet been performed, as well as the need for continued development of the Tokyo Guidelines.
It was unfortunate that all but one of the articles we reviewed were based on retrospective data. Two articles, however, did extract their data from prospectively established database16, 19 which should increase the quality of their data. This may affect the quality and consistency of data collection when compared with a review of exclusively prospective studies. Something of particular importance in this review was the methodology behind the timing of Charcot's triad assessment. All of the articles failed to specify whether Charcot's triad was detected at first presentation, at any time during the hospital stay or whether all three signs and symptoms occurred at the same time. This limits the clinical utility of our analysis. Ideally, a diagnostic tool for AC would be used at the time of presentation of a patient. Knowing the accuracy of Charcot's triad at this specific time would provide useful information to inform practice and help prevent adverse outcomes of the disease.
In summary, this review has found Charcot's triad to have high specificity but poor sensitivity in the diagnosis of AC. Additionally, Reynold's pentad appears to have even weaker sensitivity for the disease, and its specificity had not been analysed in the studies we reviewed. This is despite these two historical diagnostic tools continuing to be commonplace in medical education. In comparison, even though the Tokyo Guidelines are still being improved, the framework appears to have far greater diagnostic yield. Yet the teaching and utilization of this framework outside of Japan is minimal.
We would argue that Charcot's triad is still a useful tool when considering differential diagnoses for abdominal pain. It is a simple framework that is easy to remember and apply in a clinical context. Our review suggests that Charcot's triad is an effective rule-in test, but has little clinical value as a rule-out test. Additionally, the promise of the Tokyo Guidelines has been highlighted in the literature which suggests that its introduction into clinical practice and medical training internationally may lead to improved diagnosis of AC. However, it is a far more complicated diagnostic criteria which may lead to resistance in both its learning and teaching.