Preconception carrier screening and preimplantation genetic testing in the infertility management
Abstract
Background
Genetic testing serves as a valuable element of reproductive care, applicable at various stages of the reproductive journey: (i) before pregnancy, when a couple's genetic reproductive risk can be evaluated; (ii) before embryo implantation, as part of in vitro fertilization (IVF) treatment, to ascertain several inherited or de novo genetic/chromosomal diseases of the embryo before transfer; (iii) during the prenatal period, to assess the genetic costitution of the fetus. Preconception carrier screening (CS) is a genetic test typically performed on couples planning a pregnancy. The primary purpose of CS is to identify couples at-risk of conceiving a child affected by a severe genetic disorder with autosomal recessive or X-linked inheritance. Detection of high reproductive risk through CS allows prospective parents to be informed of their predisposition and improve reproductive decision-making. These include undergoing IVF with preimplantation genetic testing (PGT) or donor gametes, prenatal diagnosis, adoption, remaining childless, taking no actions. Both the presence of the affected gene (PGT-M) and chromosomal status (PGT-A) of the embryo can be comprehensively assessed through modern approaches.
Objectives
We provide a review of CS and PGT applications to equip healthcare providers with up-to-date information regarding their opportunities and complexities.
Results and Discussion
The use of CS and PGT is currently considered the most effective intervention for avoiding both an affected pregnancy whilst using autologous gametes in couples with known increased risk, and chromosomal abnormalities. As our understanding in the genetic component in pathological conditions increases, the number of tested disorders will expand, offering a more thorough assessment of one's genetic inheritance. Nevertheless, implementation and development in this field must be accompanied by scientific and ethical considerations to ensure this approach serves the best long-term interests of individuals and society, promoting justice and autonomy and preserving parenthood and the healthcare system.
Conclusion
The combination of CS and PGT aligns with principles of personalized medicine by offering reproductive care tailored to the individual's genetic makeup.
1 INTRODUCTION
Preconception carrier screening (CS) is a type of genetic testing performed on couples or individuals who are considering parenthood but are not yet pregnant. The primary purpose of CS is to assess the risk of an individual or a couple of passing genetic disorders to their offspring.1 Over 7000 diseases are expected to follow Mendelian inheritance, of which 40%–50% are estimated to follow recessive inheritance.2, 3 Different from genes with dominant inheritance where a single copy of the mutated gene results in the manifestation of the condition, disorders deriving from aberrant genes with recessive inheritance can manifest only when both copies of the gene are abnormal (whether in homozygosis or compound heterozygosis) or when they are located on the X chromosome of a male individual (X-linked, XL). A “carrier” is generally defined as asymptomatic individual who is heterozygous for a pathogenic variant in a gene with a recessive mode of inheritance. A couple is defined at risk (ARC) when both partners carry an autosomal recessive (AR) mutation in the same gene resulting in a 25% chance of having an affected pregnancy. Alternatively, if the pathogenic condition is located on one of the X chromosomes of the female partner (i.e., XL), the male offspring has a 50% chance of being affected, while the female offspring has 50% chance of being carriers. As defined, ARCs are approximately 2% of the general population.4, 5
CS can be particularly valuable when there is no known family history of a genetic disorder, as carriers are often unaware of their status due to the lack of symptoms. By identifying carrier status before pregnancy, CS informs couples about their reproductive risk, thus increasing their genetic awareness and allowing them to make an informed decision about reproductive strategies and family planning, including natural conception with prenatal diagnosis, the use of assisted reproductive technologies (ART) with preimplantation genetic for monogenic disorders (PGT-M), the use of donor gametes, adoption, or remain childless. A recent survey on ARCs’ reproductive decision making showed that most ARCs (59%−100%) decide to mitigate their risk through in vitro fertilization (IVF) combined with PGT rather than taking no actions.5-8 For infertile couples that become aware of their ARC status while already undergoing ART, minimal modifications to their reproductive treatment are required.9 The fact that, when offered risk-mitigating strategies, most ARCs choose to undergoing IVF and PGT, it shows that how crucial CS is in the management of reproductive choices (Figure 1).
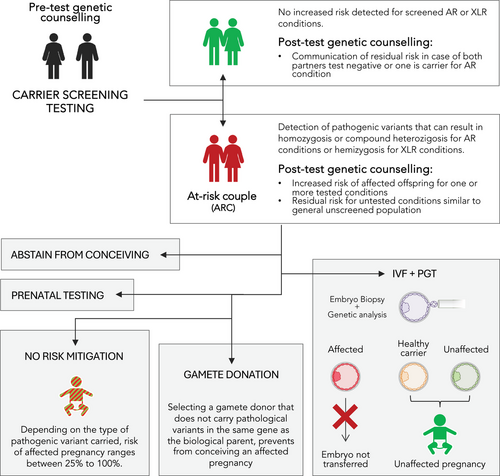
Because of its complex diagnosis and downstream ramifications, CS should be accompanied by pre- and post-test genetic counseling where a professional geneticist can provide detailed information about the scope and reach of the test, the pathogenic characteristics of the specific condition identified, discuss the potential risks to offspring, and review the reproductive options available to the couple based on their carrier status.
It should be noted that preconception CS differs from postnatal screening because the latter is designed to identify a potentially lethal or severely debilitating conditions in newborns to provide an early intervention that can improve long-term health outcomes.10, 11 Therefore, it is aimed at urgent secondary prevention for decreasing pediatric morbidity and mortality. However, the impact of having a child with a genetic disease, in terms of quality of life and both of family and societal costs should not be underestimated.12
1.1 Historical context of carrier screening programs
For over 50 years, CS has been employed for carriership assessment of recessive single-gene disorders and was first adopted in populations with increased risk for certain conditions due to geographical isolation and socio-cultural characteristics that escalated local prevalence of the defective allele. These pioneering programs involved community-wide education and premarital screening and were based on rapid and cost-effective biochemical assays. For example, Cyprus implemented a national screening program for Beta-thalassemia in the 1970s.13 This program led to a significant decline in the birth rate of affected children and inspired similar initiatives in other Mediterranean countries with high thalassemia prevalence.14, 15 Carriers for Beta-thalassemia were characterized by haematological (e.g., determination of the mean corpuscular volume—MCV, and mean corpuscular Hb concentration—MCH) and/or biochemical methods (e.g., electrophoresis, high-pressure liquid chromatography, HPLC, and mass spectroscopy).16 In the same years, Tay-Sachs Disease screening was initiated on the Ashkenazi Jewish population,17 dramatically reducing the incidence of the condition. Carrier detection for Tay-Sachs disease was carried out by monitoring the activity of the enzyme hydrolase beta-hexosaminidase-A.18 Over the past several years, the identification of more causative genes and associated pathogenic variations, and advances in DNA analysis techniques19, 20 made the molecular-based screening the standard. In 1990s, the development of polymerase chain reaction (PCR) offered the possibility to screen for cystic fibrosis (CF) using PCR primers targeting DNA sequences flanking main mutations sites.21, 22 Over the years, CF CS was implemented in regions of the USA, the UK, and Italy.23-25 Over time, these CS programs demonstrated their efficacy in reducing the incidence of genetic disorders in the population, increasing awareness and education about genetic risks, and empowering individuals and couples to make informed reproductive choices.
In the last decades, the advent of next-generation sequencing (NGS) and the consequent reduction of analytical costs26 bolstered the expansion of CS testing, enabling simultaneous screening of multiple genes with greater accuracy, with current methodologies encompassing whole exome and genome targets.
1.2 Diagnostic reach of current carrier screening platforms
Nowadays, the number of laboratories offering CS, as well as the list of tested disorders, continues to increase. There is considerable variability in the conditions included in CS panels, with some focusing on a limited number of conditions and others offering a more expanded selection.27 This lack of standardization complicates direct comparison, confusing referring professionals and receiving individuals about the scope of the screening. To reduce this risk, several scientific societies have developed professional recommendations to maximize tests’ clinical utility,28, 29 providing criteria for inclusion of conditions in CS panels. These involve (i) a clear gene–disease association, (ii) an onset in infancy or childhood, (iii) a cognitive and/or physical impairment determining a detrimental effect on the quality and/or duration of life, (iv) the requirement of medical intervention, and (v) the possibility of prenatal/preimplantation testing. Notably, the use of carrier frequency as a criterion for panel inclusion is largely debated as it can vary significantly across ethnic groups due to historical genetic bottlenecks, founder effects, genetic drift or historical migration patterns, generating inaccurate estimations and potentially impacting on universality of the test. Similarly, challenges in gene selection include evolving knowledge, variable expressivity, and incomplete penetrance of certain variants, which complicates the estimation of carrier frequency. Indeed, shortcomings in the basic thresholds recommended for the inclusion of variants in CS panels have been recently exposed. For example, it was recently discussed the matter of ciliopathies, included in the ACMG-recommended “Tier 329” but characterized by prominent phenotypic variability and allelic/genetic heterogeneity. This makes that the proportion of individuals carrying a P/LP variant can be substantially higher than expected based on estimated disease prevalence,30 demonstrating that the proposal of carrier frequency cut-off could be misguided. However, this scenario is limited to a subset of genetic disorders and the majority of screened conditions in CS panels are well-known genotype-phenotype association and their causing variant interpretation is reliable. Thus, a carrier frequency threshold ranging from 1/100 to 1/200 in any ethnic group is usually suggested.31, 32 Moreover, this pan-ethnic setting improves the diagnostic yield and test proportionality and reduces residual risk by testing all pathogenic variants irrespective of specific ethnic carrier frequencies. On the other hand, the use of ethnic specific CS panels has been shown to be significantly less effective when employed based on self-reported ethnicity compared to pan-ethnic expanded panels.33 This divergence is due to the inadvertent misreporting of one's ethnicity, hence directing testing to CS panels customized for different genetic backgrounds. Based on this evidence, pan-ethnic CS approaches should be employed.29
Moreover, the clinical interpretation of genetic test results is crucial. Indeed, genetic variants can be classified as pathogenic or likely pathogenic (P/LP) when their association with a disease is extremely likely. Alternatively, they can be categorized as benign or likely benign (B/LB) when their presence has been confirmed in individuals without a specific disease or trait and is very unlikely to cause a pathogenic effect. When insufficient data on the variant's impact on the onset of a condition are available, its classification falls under “variant of unknown significance” (VUS).34 The American College of Medical Genetics and Genomics (ACMG) recommends the reporting of only P/LP variants (class 4 and 5), whereas variants classified as VUS (class 3) at the time of analysis should not be reported, except in specific clinical circumstances.29 Over time, VUSs undergo continuous re-classification, and their status is redefined when enough evidence on their association with a condition is reached. Recent data suggested that a minority of VUS variants (6%–7%) is reclassified.35, 36 Particularly, 80%−90% of reclassified VUSs are ultimately downgraded to B/LB and the remaining 10%−20% is upgraded to P/LP.35-37 However, VUS re-classification can occur over a period of years35, 36 and require additional resources for counseling, testing, and variant interpretation. Improvements in the performance of tools for predicting variant effects and for measuring variant function, and in data-sharing could result in more timely re-classification till largely eliminating VUSs by 2030.38
Based on reliable estimation of carrier frequencies and robust variant interpretation, CS panels provide high positive and negative predictive values (PPV and NPV, respectively),1 that truly predict individuals as carriers or non-carriers. This maximizes the clinical validity of CS and is essential for the work of genetic counselors in helping patients understand the significance of the detected variants and the risks associated with their presence, as well as clarifying the residual risk of conditions not covered or detected by the test. To maintain and further upgrade a tests’ efficacy, up-to-date knowledge of allele frequency patterns is crucial for CS panels development and effective genetic counseling. In fact, CS guidelines and population genetics repositories should be constantly curated and used by providers to update testing panels. Moreover, on top of carrier frequency, structural protein information, and accurate knowledge of gene sequence may assist in the interpretation and prioritization of genetic variants, further improving test's clinical validity.30
Finally, it is currently debated whether P/LP variants associated with dominant inheritance should be included in CS panels. The risk of dominant conditions is likely to be detected through the collection of a careful personal and familial history. However, some adult-onset diseases might not yet have appeared in the testee, while many individuals lack accurate and current information about their family health history. Hence, the inclusion of dominant conditions could be considered for further increasing test clinical utility.39
1.3 Technologies and methodologies for genetic diagnostics
CS can be carried out using different genetic testing technologies and methodologies, each with its own strengths in terms of accuracy, sensitivity, and specificity. Compared to initially employed biochemical assays, DNA analysis supports definitive carrier detection and since the 1990s, PCR/DNA sequencing established itself as the standard method for carriership analysis. This approach enabled the detection of specific genetic variants and mutations that cause inherited diseases, thus providing more detailed and reliable results for CS. With the development of genetic technologies, microarray-based genetic tests became more popular as they could cover structural and sequence variants in selected regions of the genome in a single high throughput, low cost, and robust assay.40 However, the design of microarray panels is fixed and focused to a limited number of variants, not comprehensively addressing the molecular pathogenesis of recessive monogenic disorders included, resulting in a limited analytical and clinical validity.41 Conversely, most recent genomic technologies (i.e., next generation sequencing—NGS) can enrich and sequence not only pre-selected variants but the whole gene sequence,26 by simultaneously processing millions of DNA fragments. Nonetheless, although NGS sensitivity and specificity are extremely high, they can still be influenced by the complexity of the genomic region and the type of mutation investigated. NGS is particularly effective for detecting single-nucleotide variants and small insertions or deletions but may be less reliable for detecting large structural variants or repeat expansions without supplementary methodologies. Indeed, for several common gene pathogenic variants like exon 7 deletion on SMN1 gene and CGG repetitions in FMR1 gene, separated tests including Multiplex Ligation-dependent Probe Amplification (MLPA) or qPCR-based assays are usually performed.5, 42, 43 NGS offers the possibility to expand the scope of the analysis to whole-exome and whole-genome sequencing (WES, WGS, respectively).44 Briefly, WES targets exons, which are the genome's coding regions that directly contribute to protein production and represent around 1%−2% of the whole DNA within a cell.45 On the other hand, WGS encompasses the entire genome, providing the most comprehensive genetic analysis available, including non-coding regions with potential regulatory effects and involvement in the manifestation of certain genetic disorders.46, 47 However, the vast amount of data generated can lead to challenges in data analysis and results interpretation.44
Currently, CS is mostly conducted using alternative NGS-based analytical strategies: a targeted gene panel focused on specific genes known to be associated with pathological conditions,5, 48 or WES, followed by in silico filtered panel, where pathological variants are detected, filtered according to diagnostic criteria and then prioritized based on their clinical significance.49, 50 Targeted panels tend to have higher specificity for the tested conditions, while broader panels offer a more comprehensive assessment.51
Irrespective of the screening methodology employed, it is essential that the requirements on analytical and clinical validity of the test are met.52 To date, the NGS technology has reached an optimal level of analytical (e.g., sensitivity and specificity > 99%)53 and clinical validity (e.g., detection rate > 85%),54 heavily dependent on rigorous quality control measures, calibration of equipment, and validation of testing protocols, proven by the individual providers. Moreover, to ensure the reliability of test results and the competency of the professionals interpreting them, the laboratories performing genetic testing should be often licensed and accredited, adhering to high standards of quality and service, according to national legal specifications and international requirements (i.e., ISO15189, CLIA, CAP).55
In summary, the choice of the technology and the methodology employed for CS depends on various factors, including the specific conditions the individual is being screened for and the clinical resources available for interpreting complex genetic data.
2 NON-TECHNICAL CONSIDERATIONS OF CARRIER SCREENING
2.1 Ethical, legal, and social implications
When implementing CS in the society, non-technical issues are likely to arise and deserve careful consideration. These questions are multifaceted, intersecting technical/scientific capabilities with culture, ethics, family dynamics, public health policy, and individual psychology. Navigating these implications requires a thoughtful and sensitive approach, balancing the benefits of genetic screening with respect for individual values and societal diversity. Scientific societies expressed suggestions on how to ensure that CS is offered ethically, responsibly, and respectfully.9, 28, 29 Moreover, a complex interplay of regulations and laws is designed to protect individuals’ rights, ensure the accuracy and reliability of genetic tests, and govern the use and disclosure of genetic information, across all genetic diagnostics applications including CS.
CS should be voluntary and accompanied by informed consent, ensuring individuals understand the potential outcomes, benefits, and limitations of screening. Therefore, healthcare providers should offer information and counseling without coercing or directing personal decisions, by supporting autonomous decisions regarding CS and subsequent reproductive choices.
The confidentiality and the protection of genetic information are paramount, particularly given the sensitive nature of genetic information and its implications for family members. In particular, CS can sometimes reveal unexpected information, such as non-paternity or undisclosed adoptions, which can have significant social and emotional implications. Moreover, there is the risk of genetic discrimination/stigmatization based on carrier status, which could affect insurance, employment, and social relationships. Ethical frameworks should protect individuals and families from harm and discrimination and ensure that CS results are used solely for informed reproductive decision-making and healthcare related interventions. Alternatively, if the genetic information obtained from CS is used in research, de-identification of personal data, informed consent for participation in research, and adherence to protocols approved by institutional review boards (IRBs) or ethics committees are required.
Ethical considerations also include ensuring equitable access to CS across different socio-economic, racial, and ethnic groups. Disparities in access to CS can exacerbate existing health inequities. Overall, CS should be sensitive to cultural, religious, and personal values that can influence perceptions of genetics and reproductive decisions, by respecting diverse viewpoints and traditions in the context of genetic screening.
2.2 Economic and accessibility issues
Economic and accessibility issues surrounding CS are critical factors that influence the implementation and utilization of this screening approach. The cost of CS can vary widely depending on the complexity of the screening panel and the technologies used. For many individuals and couples, the out-of-pocket expense for CS can be a significant barrier, particularly in countries or regions without public healthcare or health insurance coverage for genetic testing. In particular, the integration of CS into these initiatives requires careful policy development, balancing the benefits of decreasing the incidence of certain genetic disorders, the need for equitable access and cost-effective implementation.56-59 A recent Italian study showed that, in a universal healthcare system, the implementation of preconception CS is always more cost effective compared to no screening whether the test includes ACMG Tier 1 conditions (e.g., CF, spinal muscular atrophy (SMA), and risk based conditions), the 15 most common severe, recessive, highly penetrant, childhood conditions in the Mediterranean population, or ACMG Tier 3 conditions (including diseases with ≥1/200 carrier frequency).59
2.3 Psychological and emotional considerations
The psychological and emotional considerations surrounding CS are significant, as the process involves not only medical and technical aspects but also deeply personal and challenging emotional responses on health and parenting. For this reason, as well as the complexity of data interpretation, CS requires comprehensive genetic counseling, that can provide a space to explore emotional responses and offer support.
Prior to testing, the prospect of undergoing CS can evoke anxiety, particularly for individuals or couples with a known family history of genetic disorders. The waiting period for results and the potential for unexpected findings can exacerbate uneasiness and apprehension at different levels. After testing, discovering being a carrier for a serious genetic condition can cause feelings of guilt and lead to a sense of responsibility for the health of future generations. Moreover, positive CS results can place stress and pressure on individuals and couples to make significant and sometimes immediate reproductive decisions. On other hand, although the lack of detection does not eliminate all risk, negative CS results can provide some relief and reassurance about reproductive plans. The uncertainty regarding the residual risk should be discussed and comprehended during genetic counseling.
3 FUTURE DIRECTIONS OF CARRIER SCREENING
The future directions of CS are being shaped by emerging trends in genomics and healthcare, supported by advances in genomic technologies and a strong ethical framework. CS is becoming an integral part of personalized medicine, where genetic information guides individualized healthcare decisions as well as treatment and prognosis. In the future, CS could be combined with more extensive genomic profiling to provide advanced tools for infertility diagnosis and associated consequences on personal and long-term health.60 Furthermore, CS could expand its scope to the genetic screening of recessive causes of pregnancy losses caused by lethal genes.61, 62
The integration of CS data with electronic health records (EHRs) and the use of artificial intelligence (AI) and machine learning could enhance the interpretation of genetic data, improve risk prediction models, and personalize reproductive advice based on an extremely wide range of health data.63, 64 These advancements promise to enhance the scope, accuracy, and utility of CS, potentially transforming how genetic risks are assessed and managed in the context of family planning.
Efforts should focus on overcoming obstacles to CS, including ensuring coverage and affordability for reproductive partners, securing comprehensive informed consent, meeting educational needs for providers and patients, and improving access to genetics professionals during both preconception and prenatal stages.
4 PREIMPLANTATION GENETIC TESTING
In the reproductive context, the presence of a genetic risk, whether known to be passed on in the family or discovered through a positive PCS test, can be directly addressed using IVF and PGT by identifying embryos carrying pathological variants prior to their transfer and exclude from treatment. Of note, PGT can equally detect the presence of a dominant monogenic disorder where the offspring has a 50% risk to be affected. Similarly, PGT can be employed to determine the presence of chromosomal abnormalities (PGT-A) which are not specific of a family but accumulate in gametes as part of their biological development and maturation.
4.1 In vitro fertilization and embryo biopsy
Briefly, the embryos are generated through ART techniques involving ovarian stimulation, oocyte retrieval, sperm preparation, and insemination through intracytoplasmic sperm injection (ICSI). The embryos generated are generally cultured for 5/6 days before being subjected to trophectoderm (TE) biopsy,65 followed by cryopreservation66, 67 to allow the genetic assessment. In particular, TE biopsy consists in the sampling of 5−10 cells from the part of the embryo that gives rise to extraembryonic membranes, such as the placenta. During early development, the embryo is enclosed within a glycoprotein structure called the zona pellucida. This thin layer is stretched by the growing embryo until it breaks, allowing the embryo to hatch and make direct contact with the endometrial cells at the implantation site. Hatching can start in vitro around Day 5/6 post fertilization. However, not all embryos reach this stage during IVF culture, hence the zona pellucida must be opened to biopsy the TE. Different approaches are currently being used for this procedure. A small opening in the zona can be lasered on Day 3 when the embryo has 6–10 cells.68 When the blastocoel forms around Day 4 and its internal pressure starts growing, TE cells will herniate through the opening, allowing the collection of a cellular specimen. However, this approach can lead to inner cell mass (ICM) herniation, which is inconvenient as it cannot be biopsied. Some authors have suggested to delay laser-assisted zona opening to Day 5, when the location of the ICM is clearly visible and the lasering can be performed at a more convenient site.65 Moreover, the technique used to separate the biopsied cells from the main embryo also include two main approaches. One involves stretching the embryo and exposing intercellular gap junctions, followed by their lasering until full detachment. The second entails a mechanical maneuver (i.e., flicking) that recedes the target cells from the main embryo through a quick friction between the two pipettes used for micromanipulation. A combination of the two techniques is also very popular among operators. Biopsied cells are used as substrates for genetic testing, while the embryos are cryopreserved using vitrification protocols to allow for PGT testing and reporting. Following genetic diagnostic evaluation, the embryos not affected by the conditions tested (e.g., depending on the type of test employed) are warmed and transferred into the patient's uterus.69
4.2 Preimplantation genetic testing for monogenic conditions
From a technical standpoint, PGT analysis for monogenic disorders (PGT-M) is carried out mainly with three molecular methods: targeted multiplex PCR, Karyomapping, and NGS.70-72 Targeted multiplex PCR allows the amplification of the gene region of interest, which can be later analyzed by Sanger sequencing, SNP-genotyping or mini-sequencing to determine the presence of the target mutation/pathological variant. This diagnostic approach is usually strengthened with the analysis of linked informative markers. These markers are polymorphic (i.e., multiple allelic variants at locus) and close to the target mutation (1–2 Mb), and therefore segregate with the tested locus, allowing identifying the corresponding haplotype (linkage analysis). The most used flanking markers are the short tandem repeats (STR) or single nucleotide polymorphism (SNPs). Karyomapping is a methodology based on the SNP-array technology. Through previous characterization of the SNP landscape present in the maternal and paternal genomes, this approach allows the identification of the haplotype inherited by the embryo using linkage analysis, thus determining the presence or absence of the affected gene. NGS offers various protocols for PGT-M application, all characterized by the targeted amplification of the gene region of interest, which enables the combined downstream analysis of the target mutation and flanking markers. Overall, the accuracy of the methods used exceeds 99%, with a misdiagnosis rate of less than 0.1%, confirming the reliability of PGT-M technologies.73, 74
Historically, PGT-M was applied for the first time on humans in 1990,75 when the genetic analysis of embryos from patients carrying X-linked recessive genetic diseases allowed the identification and selective transfer of female embryos (avoiding hemizygous males with 50% risk of manifesting the condition). Since then, there has been a rapid and continuous evolution in the use of PGT-M. The PGT Consortium of the European Society of Human Reproduction and Embryology (ESHRE) has collected data regarding PGT treatments performed. The latest collection of data published by ESHRE reported that 35% (3098/8803) of the PGT analyses performed so far have been for monogenic diseases.76 To date, there are specific diagnostic protocols for over 200 monogenic conditions and the list is constantly updated.77 For autosomal dominant diseases (64%), the most frequent are Huntington's disease (HD, #143100), Neurofibromatosis 1 (NF1, #162200), Myotonic Dystrophy type 1 (DM1, #160900), and Marfan syndrome (MFS, #154700); while for autosomal recessive diseases (21%), CF and Beta-thalassemia are the most common. Among X-linked conditions (15%), Fragile X Syndrome and Duchenne muscular dystrophy (DMD, #310200) are the most frequent. Furthermore, mitochondrial diseases, HLA-typing and tumor predisposition analysis also fall within the possible applications of PGT-M. In particular, susceptibility to familial breast and ovarian cancer (e.g., BRCA1/2, #604370/#612555) has become the fifth most frequent indication for PGT-M at the European level.76
From a clinical point of view, most of the embryos analyzed (88%) obtained a conclusive diagnosis and specifically 47% of them were not affected by the monogenetic disease evaluated and therefore deemed transferable. Following the transfer procedure, the live birth rate was 31.5%, comparable to the efficiency generally considered for the natural human reproduction.76
4.3 Preimplantation genetic testing for aneuploidies
Although monogenic conditions have a high impact on affected individuals, they are relatively rare compared to chromosomal abnormalities. Human reproduction is characterized by a high incidence of embryonic chromosomal aneuploidies which is largely due to segregation errors during oogenesis, and represents the main cause of implantation failures, miscarriages, and chromosomal syndromes in live-born offspring (Trisomies 13, 18, and 21, and aneuploidies of sex chromosomes).78-81 The clear fertility decline in women of advanced maternal age (AMA; > 35 years) is attributable to the dual impact (quantitative and qualitative) of aging, involving the depletion of the ovarian reserve and the contemporary exponential increase in the incidence of embryonic aneuploidies, from a baseline of 30% in young women under 35, to 60% in women aged 38, 60%–70% in women aged 40, 80% in women aged 42, and over 90% in women close to menopause.82, 83 Preimplantation genetic testing for aneuploidy (PGT-A) represents the only safe and effective strategy to identify the presence of meiotic aneuploidies in embryos following TE biopsy.84, 85 Particularly, PGT-A provides a higher rate of full-term pregnancy following embryo transfer, lower time to pregnancy, and lower miscarriage rate per clinical pregnancy,86, 87 without impacting a priori chances of ART treatment effectiveness, as evidenced by comparable cumulative pregnancy rates with or without PGT-A.88, 89 Furthermore, it is possible to combine the evaluation of chromosomal anomalies (including both whole chromosomes and microdeletions and duplications) and monogenic diseases in a single analysis, allowing patients affected or carrying a genetic disease to be informed about the overall genetic status of the embryo.
The main methodologies for PGT-A developed over time are based on comprehensive chromosome testing (CCT) techniques (array-CGH, SNP-array, q-PCR, or NGS) which allow the assessment of all 24 chromosomes in a single test. All these techniques require amplification of the limited embryonic DNA collected through biopsy. This can be achieved using different strategies (i.e., whole genome amplification (WGA) or targeted PCR amplification). In particular, there are two main WGA methods that vary in their amplification accuracy and applicability90: (i) multiple displacement amplification under isothermal conditions (MDA)-based WGA, employed in CTT techniques that rely on genotyping (e.g., array-CGH, SNP-array); and (ii) PCR-based WGA (e.g., degenerate oligonucleotide PCR (DOP-PCR) and primer extension preamplification (PEP)) used in read-count approaches (e.g., NGS). Array-CGH technology is based on fixed probes corresponding to specific loci of each individual chromosome. After amplification and fluorescent-labeling of the embryonic DNA sample, its co-hybridization with a reference DNA on the microarray allows the detection of chromosomal abnormalities through variations of fluorometric signals.91 Using SNP-arrays, embryonic DNA is also amplified and labeled with fluorescent probes and then hybridized with a microarray carrying thousands of SNPs probes corresponding to loci across the whole genome. Similar to array-CGH, this approach also allows the chromosomal state (copy number variation analysis) of the embryos to be determined, identifying trisomies and monosomies, while also providing genotyping information for additional downstream analysis.92 Quantitative PCR (qPCR) is a rapid method based on the simultaneous amplification and quantification of a specific DNA sequence. For PGT-A purposes, the sequences amplified are located in several regions of each chromosome, which, based on their amplification curves allow inference of chromosome copy number present in the embryonic specimen.93-95 Finally, NGS provides a reliable high-throughput methodology for chromosome screening which, due to increasing affordability and scalability, has become the most common approach. First, multiple genomic regions are enriched by PCR-based WGA or by targeted PCR amplification methodologies. To note, PCR-based WGA can give incomplete genome coverage and produce nonspecific amplification artefacts. For example, amplification bias can occur when one sequence is preferentially amplified over the other due to more favorable binding of the primers to specific regions; while the low-fidelity of the DNA polymerase used can result in the introduction of several errors (e.g., single base-pair mutations, and repeat size variations) into the sequence (i.e., error-prone amplification). However, these methods provide a distribution of reads uniform enough to be able to accurately assign chromosome copy number values (CNVs).96 Next, the amplified DNA fragments are tagged and sequenced. Subsequent bioinformatic analysis determines the chromosomal origin of each sequenced fragment (i.e., read) allowing an estimation of their representation compared to other fragments, thus inferring the presence of copy number variations and therefore aneuploidies of the entire chromosomes or part of them.97 Specifically, NGS platforms have properly defined and validated QC parameters to ensure the accuracy of chromosome CNVs calling. These criteria usually include measurements including: (i) the number of total reads per sample; (ii) genome coverage, required to be 70%−80% of total reads aligned to the genome; (iii) the dispersion/noise measured as the mean absolute percent deviation (MAPD) required to be < 0.3. For every NGS run, QC parameters are assessed through their direct extrapolation by a specific software and standards must be met to consider the analysis informative.98 Therefore, uniform euploid/aneuploid classifications provide very high reliability and reproducibility with over 95% concordance.99, 100 To establish the PPV and NPV of PGT-A defined as the likelihood for embryos to truly carry an aneuploidy or not, experimental studies and follow-up of clinical practice are respectively required. Evidence shows that a uniform aneuploidy result successfully predicts implantation or developmental failure with over 98% accuracy84, 85, 101-104 while a euploidy result represents the strongest predictor of embryo implantation.105
Over the last decade, and since multicellular biopsy (i.e., TE) and NGS have been employed for PGT-A, chromosome CNVs have also become accessible for analysis. The detection of intermediate CNVs has often been explained as chromosomal mosaicism, a biological phenomenon in which karyotypically distinct cell lines are present within the embryo. However, interpretation of mosaicism is challenged by both biological and technical confounding factors (e.g., DNA contamination, polyploidy, cellularity/quality, experimental conditions) and has generated much controversy over its management in the ART/PGT field. The current debate on chromosomal mosaicism detection can be here summarized in terms of analytical validity, clinical validity, and clinical utility.106 With regards to analytical validity, the calling of embryonic mosaicism is often inaccurate and unreliable. Its clinical validity is affected by low concordance and predictivity in detecting true mosaicism in the corresponding ICM and ensuing fetus/newborn. Similarly, clinical utility (defined as the possibility to enhance clinical outcomes of pregnancies and children) is also unproven as a recent prospective, non-selection clinical trial reported similar clinical results between embryos displaying low grade intermediate CNV (< 50%) and uniformly euploid ones.100 The likely hypothesis behind these outcomes is that low grade intermediate CNVs may be the result of artefacts or transient mitotic errors in euploid embryos which are somehow resolved or isolated to extra-embryonic tissues without affecting the reproductive competence of the embryo. On the other hand, embryos displaying high-grade intermediate CNVs (> 50%) have shown reduced clinical outcomes compared to uniformly euploid ones. In these cases, putative mosaicism may derive from technical artefacts of opposite nature, where some cells of uniformly aneuploid embryos are affected. In conclusion, it is not possible to report a consensual approach for managing the detection of intermediate CNVs; however, this should be critically addressed by PGT centers, and refined based on emerging data. As a future prospect, since true chromosomal mosaicism is the product of a mitotic error in a cell of a post-fertilization embryo, the ability to discern the segregation origin of the chromosomal abnormality could significantly improve the diagnostic accuracy in cases showing putative mosaicism.
Using the latest NGS technology, it is possible to genotype the embryo,107-109 thus acquiring information on the parental origin of chromosomes110 (hence, in case of trisomy, determine whether the additional chromosome is the product of a segregation error of mitotic or meiotic origin). Embryo genotyping is also useful for detecting chromosomal abnormalities beyond copy-number aneuploidies, also resulting in poor outcomes. Briefly, different genome-wide ploidy level (e.g., haploidy/triploidy) can be discriminated using the distribution of allele frequency observed at biallelic variants across the whole genome109, 111; small deletions/duplications (< 5Mb) associated with genomic syndromes can be revealed evaluating allele frequencies within the region of interest112 and finally uniparental disomy (i.e., inheritance of both alleles from one parent) can be assessed based on the level of heterozygosity for a given chromosome.113
5 FUTURE DIRECTIONS OF PREIMPLANTATION GENETIC TESTING
Embryo genotyping opens the possibility of testing not only chromosomal abnormalities and single-gene disorders but also common human diseases (e.g., hypertension, diabetes, or schizophrenia) to enhance screening. Indeed, most common diseases have a genetic component, with a growing proportion showing a polygenic causal pattern. These conditions entail the involvement of multiple common genetic variants whose combined suboptimal performance results into an increased risk of disease onset. Indeed, while considered individually, these common variants produce a small effect on the fitness of the organism, cumulatively they can confer a significant predisposition to disease, comparable to levels caused by rare monogenic variants. This effect has been described using genome-wide association studies (GWAS)114, 115 and represented as polygenic risk score (PRS).116-119 Briefly, a PRS for a given disease is an estimation of the risk carried by an individual to manifest a disease, as a result of genotyping analysis based on the detection of common disease-associated variants and the strength of their association with the disease. PRS for specific diseases can also be calculated on preimplantation embryos, by performing what is called polygenic embryo screening (PES) or PGT for polygenic disorders (PGT-P).120, 121 The aim of PES/PGT-P is to stratify embryos’ suitability for transfer based on the estimated risk of the ensuing individual to develop the condition over time. Despite its technical feasibility, multiple factors limit the practical benefits of PES/PGT-P,122 including: (i) the ethnical bias of the population included in GWAS studies, (ii) the role of non-genetic risk factors for complex diseases in future generations, (iii) the low genetic variability between sibling embryos, (iv) gene pleiotropy effects. Finally, ethical implications122 that require serious consideration include (i) insufficient genetic counseling and choice overload, (ii) discarding of embryos with uncertain prognosis, (iii) genetic determinism possibly resulting in eugenics and alteration of population demographics, (iv) unequal access and discrimination/stigmatization against individuals affected by common diseases.
6 CONCLUSIONS
In conclusion, PCS and PGT represent crucial components of modern infertility management, providing prospective parents with vital information on their reproductive risks and options. PCS helps identify carriers of genetic disorders before pregnancy, enabling informed decision-making. PGT, in conjunction with IVF, allows a reliable approach for the selection of embryos without specific genetic abnormalities, ultimately reducing the risk of inherited conditions. Although these technologies empower individuals and couples with choices about their health options, their integration into reproductive care also poses ethical, legal, and social considerations that must be navigated carefully. This aspect will become ever more important as genetic testing technologies evolve and become more routinely and comprehensive. Similarly, the scope of PCS and PGT will likely expand, further enhancing personalized reproductive strategies and contributing to the field of personalized medicine.
AUTHOR CONTRIBUTIONS
Antonio Capalbo conceived the paper and provided a critical manuscript review. Silvia Caroselli and Maurizio Poli were involved in data curation and drafted the manuscript. Liborio Stuppia and Valentina Gatta provided a critical review of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this paper as no new data were created or analyzed in this study.




