Telomeric function and regulation during male meiosis in mice and humans
Abstract
Background
Telomeres are unique structures situated at the ends of chromosomes. Preserving the structure and function of telomeres is essential for maintaining genomic stability and promoting genetic diversity during male meiosis in mammals.
Material-Methods
This review compiled recent literature on the function and regulation of telomeres during male meiosis in both mice and humans, and also highlighted the critical roles of telomeres in reproductive biology and medicine.
Results-Discussion
Various structures, consisting of the LINC complex (SUN-KASH), SPDYA-CDK2, TTM trimer (TERB1-TERB2-MAJIN), and shelterin, are critical in controlling telomeric activities, such as nuclear envelope attachment and bouquet formation. Other than telomere-related proteins, cohesins and genes responsible for regulating telomere function are also highlighted, though the exact mechanism remains unclear. The gene-mutant mouse models with meiotic defects directly reveal the essential roles of telomeres in male meiosis. Recently reported mutant genes associated with telomere activity in clinical practice have also been illustrated in detail.
Conclusions
Proper regulation of telomere activities is essential for male meiosis progression in mice and humans.
1 INTRODUCTION
Telomeres, situated at the ends of chromosomes, are nucleoprotein structures crucial in male meiosis, particularly during meiosis prophase I. Mammalian telomeres comprise repetitive TTAGGG/AATCCC DNA sequences, with 3′ overhangs of G-rich strands at the end.1 Telomere length significantly influences cell fate determination. A study revealed that subtelomeric sequences influence chromosome movement and positioning, thereby affecting telomere length.2 Telomeres protect chromosome ends during the initiation of DNA damage response (DDR) and the repair of double-strand breaks (DSB).3 In mitotic cells, dysfunctional telomeres are identified as genotoxic DSB, triggering the activation of DDR. Consequently, telomeres fuse or degrade, disrupting telomeric and genomic integrity.4 During meiotic prophase I, which occupies an extended duration in meiosis, bouquet formation is a crucial phenomenon. Here, telomeres cluster to one pole of the nucleus, moving at an average speed of 109 nm/s during the zygotene stage in mice. Subsequently, they rearrange along the nuclear periphery as chromosomes pair with their counterparts.5,6 Telomeric movements in mouse meiosis are characterized by rapid and heterogeneous motion. Lee et al.7 proposed a concept of “telomere-led rapid prophase movement (RPMs),” which involves a combination of nuclear rotation and specific microtubule-dynein-directed activities. The proximity of telomeres, referred to as the distance to the distal telomere (the telomere farthest from the centromere), influences the formation of recombination initiating the DSB site. Chromosomal recombination hotspots experience a five-fold increase from the centromere to the distal telomere; therefore, sites near the distal telomere exhibit a greater potential for crossover formation.8 Defective telomeric movement has been observed in recombination and synaptic mutants such as Dmc1−/−, Sycp3−/−, and Hfm1−/− mice.7 Moreover, deletion or deficiency of several telomere-related genes, such as Terb2,9 Trf1,10 Rec8,11 Smc1β,12,13 and more, can lead to impaired recombination. Several complexes are directly involved in telomere function, including the LINC complex (SUN1-KASH5),14 SPDYA-CDK2,15 TTM trimer (TERB1-TERB2-MAJIN),16 and six shelterins (TRF1, TRF2, TPP1, TIN2, RAP1, and POT1).17
Cohesin comprised of SMC1, SMC3, and α-kleisin, forms a ring-like structure crucial for regulating meiotic chromosome architecture. SMC1β and SMC3 converge at the hinge region, while the α-kleisin (RAD21, RAD21L, and REC8) bind tightly to STAG3 at the head region, ultimately forming meiosis-specific cohesin. The complex comprising SMC1α, SMC3, RAD21, and STAG1/2 is present in mitotic and meiotic cells18,19 (Figure 2B). During meiosis prophase I, as spermatocytes transition from zygotene to pachytene, cohesin remains closed due to the action of phosphorated-PP1γ and WAPL. Subsequently, at diplotene, the cohesin gate opens at the head region through phosphorylation, and SORORIN is removed, highlighting a transition from a SORORIN-dominant to a WAPL-dominant state.20 Research on cohesins extensively covers chromosome length determination and assembly; however, associations between cohesins and telomeres remain unknown. Previous articles have identified REC8, RAD21L, STAG3, and SMC1β as closely related to telomeres. These connections will be explored further later.
In addition to molecular studies primarily conducted in mice, investigations involving semen analysis, chromosome spread assays, and sequencing of samples from patients with nonobstructive azoospermia (NOA) confirmed the conserved meiotic roles of telomeres in humans. Therefore, this review aims to consolidate the features of significant telomere-related structures in mammals, emphasize the clinical relevance of meiotic telomeres in medicine, and highlight areas requiring further investigation. This review could enhance our understanding of telomere biology in male meiosis and facilitate reproductive medicine research and practice.
1.1 Overview of male meiosis
Meiosis is a complex and conserved cell division process unique to germ cells. It involves two successive cell divisions following a single round of DNA replication, ultimately resulting in the production of haploid gametes. During the first wave of spermatogenesis in mice, meiotic prophase I progress continuously from postnatal day 8 (P8) to P19. This phase encompasses preleptonema, leptonema, zygonema, pachynema, diplonema, and diakinesis spermatocytes. At the initiation of meiosis, the pre-DSB machinery, comprised of the RMMAI complex (REC114-MEI1-MEI4-ANKRD31-IHO1), facilitates the formation of hundreds of PRDM9-dependent SPO11-directed DSBs.21,22 Following the catalytic DNA breakage directed by SPO11/TOPOBVIL and the resection of SPO11-oligo facilitated by the MRN (MRE11-RAD50-NBS1) complex, ssDNA-binding proteins (MEIOB, RPA2, and SPATA22), and recombinases (RAD51 and DMC1) are recruited to the resected sites for DSB repair.23,24 In pachytene spermatocytes, all autosomes undergo complete synapsis, whereas sex chromosomes exhibit a restricted synapsed region, referred to as the pseudoautosomal region (PAR). Pachytene spermatocytes possess the capability to ensure the fidelity of crossover formation on each synapsed chromosome for further accurate chromosome segregation.25 Usually, 20−30 crossovers are within one spermatocyte, with at least one occurring per homolog pair.25 In meiosis I, homologous chromosomes align at the equatorial plate and then segregate, while in meiosis II, sister chromatids separate into two daughter cells, forming haploid spermatids. Meiotic disorders in mammals can result in male infertility, aneuploid embryos, and various other diseases.
1.2 Essential complexes involved in telomeric activities
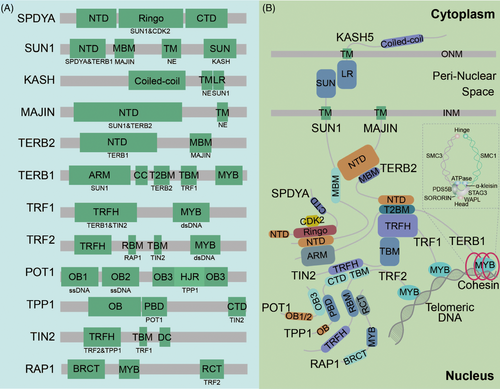
During meiosis, the attachment of the NE telomere engages various distinct complexes. Owing to the complexity of telomere activity, this part is divided into several subgroups to introduce them individually. These include the LINC (SUN-KASH), SPDYA-CDK2, TTM (TERB1-TERB2-MAJIN), and shelterin complexes. The internal structure and indispensable functions of each component of these complexes are described in detail below. Table 1 summarizes the information. Figure 1A and B show the dynamic activity of telomeres, and Figure 2A and B illustrate the interaction domains.
| Genes | Structure | Functions | Phenotypes in mouse mutants | References |
|---|---|---|---|---|
| Sun1 | LINC complex | Nuclear lamina and cytoskeleton connection, NE attachment | Pachytene arrest | 10, 14, 26 |
| Kash5 | Nuclear lamina and cytoskeleton connection, NE attachment | Zygotene arrest | 28, 88 | |
| SpdyA | SPDYA-CDK2 complex | CDK2 activation, T-loop formation regulation | Pachytene31/Zygotene15 arrest | 15, 31 |
| Cdk2 | Cell cycle, homologous repair, BRCA2 phosphorylation, T-loop formation | Pachytene arrest | 15, 31, 32, 35 | |
| Terb1 | TTM complex | NE, telomere cap exchange, STAG3 recruitment, TRF1 binding | Pachytene arrest | 36 |
| Terb2 | NE, telomere cap exchange | Zygotene36/Pachytene9 arrest | 9, 29, 36, 37 | |
| Majin | NE, telomere cap exchange, inner membrane anchoring | Zygotene arrest | 36, 90 | |
| Trf1 | Shelterin complex | Telomeric double-stranded 5′-TTAGGG-3′ repeat binding, telomere length regulation | Pachytene/meiotic division arrest | 10, 17, 96-98 |
| Trf2 | Telomeric double-strand repeat binding, end-to-end fusion prevention | Not found | 30, 43, 44, 47 | |
| Pot1 | Telomeric DNA binding, telomere length regulation | Not found | 44, 49 | |
| Tpp1 | Telomerase recruitment, telomere repeat synthesis | Not found | 44, 49, 50 | |
| Rap1 | Telomere structure regulation | No abnormity | 51 | |
| Tin2 | Telomere length regulation, T-loop regulation | Not found | 30, 44, 45, 47 |
- Abbreviations: CDK2, cyclin-dependent kinase 2; KASH, protein KASH5; MAJIN, membrane-anchored junction protein; POT1, protection of telomeres protein 1; RAP1, DNA-binding protein RAP1; SPDYA, speedy protein A;SUN1, SUN domain-containing protein 1; TERB1, telomere repeats-binding bouquet formation protein 1; TERB2, telomere repeats-binding bouquet formation protein 2; TIN2, TERF1-interacting nuclear factor 2.; TPP1, tripeptidyl-peptidase 1; TRF1, telomeric repeat-binding factor 1; TRF2, telomeric repeat-binding factor 2.
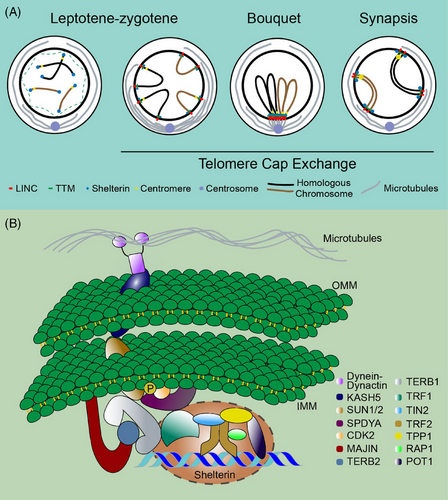
1.2.1 LINC complex
The LINC complex serves as a transmembrane linker between the nucleoskeleton and cytoskeleton, primarily comprising SUN-KASH proteins. This structure facilitates telomere-led chromosome movement and formation of telomere bouquet, thereby facilitating accurate homologous search and pairing.14 SUN1, a component of SUN domain proteins, interacts with TERB1 to pull telomeres toward the inner nuclear membrane, while KASH (also known as CCDC155) is situated at the outer nuclear membrane (ONM), where it binds to dynein–dynactin motor proteins.14 Disruption of Sun1 in mouse meiotic cells results in impaired telomere attachment to the NE, homolog pairing, and synapsis.26 While SUN1 affects chromatin mobility and telomere fusion in somatic cells,27 no telomere fusion has been observed in Sun1−/− spermatocytes.10 Kash5-null mice exhibited compromised homology pairing, persistent DSBs, impaired dynein recruitment, and SUN1 foci lacking structured ring-like formation.28
1.2.2 SPDYA-CDK2 complex
The crystal structure of the SUN1-SPDYA-CDK2 ternary is linked with the connections between telomere and LINC complex during meiotic prophase I.14 During the middle stage of prophase I, a process known as telomere cap exchange occurs; this involves shedding of the shelterin complex (further discussed below) from the telomeres attached to the NE, a process dependent on CDK activity.29,30 SPDYA-CDK2 is the first complex recruited to NE for the formation of the meiotic telomere complex; however, NE attachment occurs independently of CDK2 activation.15 SPDYA/RINGOA serves as a non-canonical meiosis-specific activator of CDK2, facilitating the recruitment of CDK2 to target telomeric DNA.15,31 In SpdyA-null mice (deletion of exon 3), abnormalities in telomere tethering and distribution of SUN1 have been observed in spermatocytes.31 In SPDYA-binding-deficient Sun1 mutant mice (Sun1Trp150 mice), the disruption of the ring-shaped telomere supramolecular architecture at the NE leads to compromised homologous pairing and synapsis.14 Mikolcevic et al.31 reported that both SpdyA and Cdk2 KO mice were arrested at the pachytene stage, exhibiting nonhomologous chromosome pairing, deficiency in DSB repair, and absence of detectable sex bodies. However, Tu et al.32 demonstrated that deletion of exon 2 of SpdyA results in arrest at the zygotene stage of meiotic prophase I. In mutant spermatocytes of mice, SUN1, KASH5, and lamin C2 exhibited a cap-like distribution facing centrosomes due to changes in SUN1 phosphorylation levels, and some telomeres remained within the nuclear interior with NE-detached vesicles. In addition, CDK2 undergoes phosphorylation by CAK (the CDK-activating kinase) at the Thr160 site.33,34 In Cdk2T160A mutant mice, markers for DSB repair (γH2AX) and synapsis (SYCP1), along with the number of pachytene spermatocytes, appeared unaffected. Nevertheless, a decrease in diplotene and the absence of diakinesis spermatocytes were observed.35 Nonetheless, with a mutation in the Asp145 site of CDK2, which is responsible for coordinating Mg2+ ions to orientate ATP for catalysis, the phenotype becomes more severe, with a complete absence of pachytene spermatocytes observed.35
1.2.3 TTM complex
TTM complex, also known as the meiotic telomere complex, interacts with shelterin to tether DNA chains to the nuclear membrane.16 Structural analysis revealed that MAJIN interacts with the MBM domain (MAJIN-binding motif, aa 169–202) of TERB2 through its NTD domain (N-terminal domain, aa 1–120). Additionally, TERB2 interacts with the T2B domain (TERB2 binding domain, aa 593–622) of TERB1 through its NTD domain (aa 2–194). Therefore, these three components combine to form a compact trimer known as the TTM complex.9,16 The TTM complex attaches to NE through the transmembrane motif (aa 232–251) of MAJIN and interacts with the TRFH domain (TRF homology domain, aa 54–251) of TRF1 (which directly binds to telomeric DNA on chromosomes) through the TBM domain (TRF1-binding motif, aa 523–699) of TERB1.9,16 Furthermore, the ARM repeat within the N-terminus of TERB1 (aa 16–384) interacts with the NTD domain of SUN1/2.16 A detailed diagram illustrating the domains of these proteins is shown in Figure 2.
All Terb1/Terb2/Majin mutant mice exhibited defects in chromosome movement and bouquet formation.29,36 TERB1 recruits cohesin STAG3 to telomeres and plays a role in telomere complex assembly to uphold structural rigidity.29 Various mutations in Terb1 resulted in different consequences. The results of Terb1▴MYB mice showed that the MYB domain of TERB1 was dispensable for TTM complex localization; however, it influences the enrichment of SMC3 and the remodeling of axial elements in telomeric regions. This process helps in suppressing telomere erosion, thereby maintaining the stability of telomeric DNA and genomic integrity.29,37 Mice carrying the Terb1AEA/AEA point mutant exhibited disrupted interaction between TERB1 and TRF1, which impairs the homologous pairing of telomere-adjacent PAR on sex chromosomes. This led to an arrest at the zygotene-early pachytene stage.38 Moreover, deletion of Terb2 in mice disrupts telomere attachment, chromosome morphology, DSB repair, synapsis, and chiasma formation. Mutant mice carrying either Terb2Y56E or Terb2F188R exhibited pachytene arrest, characterized by the presence of multiple pachytene-like cells and an absence of normal pachytene or cells beyond this stage.9
1.2.4 Shelterin complex
Telomeric shelterin, comprising six proteins—TRF1, TRF2, TPP1, TIN2, RAP1, and POT1—safeguards telomeres from being mistakenly identified as DSBs.17 Levels of TRF1, TRF2, TIN2, and RAP1 were higher than those of POT1 and TPP1.39 The main difference between TRF1 and TRF2 lies in their N-terminus domain, with TRF1 possessing an acidic domain and TRF2 containing a basic domain.40 TRF1 and TRF2 directly attach to double-stranded telomeric DNA through their MYB/SANT domains,41 while POT1 binds to single-stranded DNA through the OB domain.42 Each component plays a unique role; TRF1 collaborates with the SPDYA-CDK2 complex to promote telomeric DNA pairing and inhibit telomeric fusion.10 Trf1-null mice undergo arrest at two stages; meiocytes showing compromised homolog synapsis and recombination are arrested at a pachytene-like stage, while those with inadequate recruitment of meiosis-specific telomeric proteins and aberrant chromosome end-to-end fusion experienced arrest during meiotic division.10 Similar to TRF1, TRF2 safeguards telomeric ends against fusion.41 The relationship and connections among these proteins deserve attention. TRF1, TRF2, and TIN2 combine to form a trimer.39 TRF2 recruits RAP1 to telomeres, thereby enhancing the binding of TRF2 to telomeric DNA.43 TIN2 serves as the central hub, facilitating the localization and stabilization of TRF1, TRF2, and POT1. It collaborates with TRF2 to regulate the formation and stability of the telomeric T-loop structure.44,45 The interaction between TERB1 and TRF1 is regulated by TIN2.30 Tin2-KO mice do not survive beyond the embryonic stage46; however, studies exploring the role of TIN2 in meiosis remain limited. TIN2 is implicated in the DDR pathway by modulating the TRF2-dependent inhibition of ATM kinase signaling in somatic cells.44,47 Direct evidence, such as a knockout mouse model, confirming the role of TIN2 in meiosis is lacking, given the significance of the DDR pathway in meiosis, TIN2 may play a significant role in meiocytes. Given that several studies highlighted the crucial role of TIN2 in telomere length and processivity,17,48 a study showed that TPP1-POT1 aids in recruiting telomerase to telomeres and synthesizing the telomere repeat sequence. This occurs through the formation of a structured interface with TERT-special TEN-TRAP, the telomerase essential N-terminal (TEN) domain, and the telomerase RAP motif (TRAP).49,50 RAP1 inhibits the improper binding of TERB1 to TRF2.30 In Rap1-KO spermatocytes in mice, no abnormal telomere structure or function has been observed.51
1.3 Cohesins and telomeres
During meiosis, cohesins are essential for chromosomal organization. In metaphase I, they maintain sister chromatid cohesion, aiding homologous chromosome segregation. In contrast, in metaphase II, cohesin REC8 is removed to facilitate sister chromatid segregation. Cohesins also regulate interactions between telomeres.52 TERB1, a part of the TTM complex, has been confirmed to interact with STAG3 and recruit STAG3 to telomeres through its MYB-like domain.53 In addition, analysis of telomere length/subtelomere sequence revealed that two selected sites of cohesin recruitment through TERB1 are situated within cohesin-binding regions,2,29 further confirming the intimate association between TERB1 and cohesins. The recruited cohesins form a rigid architecture resembling that of centromeres in telomeric regions.53
Several studies have employed knockout mouse models to investigate the association between cohesins and telomeres. In Rad21L-deficient mice, meiosis arrests at the zygotene stage are characterized by telomere abnormalities such as aberrant bouquet formation.54 The authors argued that in both budding yeast and mice, there exists a conserved mechanism known as “bouquet exit checkpoint,” which monitors chromosome pairing rather than relying on DSB-dependent recombination. Meiotic cohesins play a crucial role in creating a distinct chromosomal structure necessary for bouquet release.54 In Smc1β null mice, spermatocytes experiencing impaired sister chromatid cohesion underwent apoptosis during early/mid-pachytene in stage IV tubules. Additionally, about one-fifth of meiotic telomeres exhibited failure in NE-anchoring.12,13,52 In addition, most impaired functions in Smc1β−/− mice can be completely or partially restored by supplementing with SMC1α, except for the maintenance of telomere integrity.12 In Smc1β−/− and Smc1β−/−1α (Smc1β KO mice with increased SMC1α expression) spermatocytes, telomere abnormities such as absent, elongated, and telomere bridges and fusions were observed, further confirming the crucial role of SMC1β in telomere maintenance.12,52 Moreover, during late pachytene in Smc1β−/−1α mice, end-joining protein 53BP1, DNA damage kinase CHK2, and DDR protein BRCA1 accumulate at telomeres, highlighting the occurrence of telomeric DDR in late meiotic prophase I spermatocytes.12 To explore the molecular mechanism, the authors propose that the absence of basic amino acids on the C-terminus of SMC1β results in persistent telomeric abnormalities. This region could interact with telomere-associated factors, aiding in the organization of telomeric DNA and the formation of a T-loop structure.12,55 Moreover, another study highlighted an elevated occurrence of telomere fragments and shortened length in Rad21L−/− spermatocytes in mice, while in Smc1β−/−Rec8−/− and Smc1β−/−Rad21L−/− double knockout mice, telomeres were significantly shorter, and disruptions in the telomeric structure were more pronounced in spermatocytes during late prophase I stages.56 However, Rec8−/− spermatocytes exhibited less telomeric abnormalities among these knockout mouse models despite having significantly shorter telomere length than Smc1β−/− and Smc1β−/−Rec8−/−spermatocytes.56 STAG3 is another crucial meiosis-specific cohesin that is associated with SMC1β.57 In contrast to Smc1β−/− spermatocytes, Stag3 deficient spermatocytes in mice exhibited impaired centromeric and telomeric sister chromatid cohesion while maintaining normal telomere structure.58 PDS5, a regulator of SMC1β, plays a crucial role in cohesin dissociation (by interacting with WAPL) and stabilization (by cooperating with SORORIN).55,59,60 In Pds5 mutant mice, both telomere integrity and NE attachment were significantly compromised.55
1.4 Genes affecting meiotic telomere activity in mice
In addition to the previously described classical complexes, several other genes indirectly regulate telomere activity. SYCP3, an axis element of the synaptonemal complex, contributes to telomere clustering and attachment to the NE by forming a flat, disk-shaped plate between the telomere and NE. In Sycp3 KO mice, the number of clustered telomeres increased by 2.8-fold, and the duration of the bouquet stage in spermatocytes was extended than in the controls.61 FBXO47, an F-box protein 47, plays a role in regulating synaptonemal complex (SC) formation by interacting with and stabilizing TRF1/2. Disruption of Fbxo47 in mice results in weakened NE attachment and abnormal bouquet formation.62 YTHDC2, an RNA helicase predominantly expressed in pachytene spermatocytes, functions to prevent microtubule-dependent telomere clustering.63 In Ythdc2 deficient mice, telomere clustering occurred in over 20% of pachytene spermatocytes. In contrast, transient telomere clustering was only seen at the zygotene stage in control mice.63,64 Zhou et al.65 validated the involvement of POLD3 in telomere maintenance in mouse spermatocytes. In Pold3+/− spermatocytes, the proportion of telomere shortening or loss was significantly higher than that of the controls. Based on the characterized relationship between POLD3 and kinase ATR/ATM in previous studies,66–68 Zhou et al.65 proposed that POLD3 mediates telomere integrity by inhibiting ATR/ATM-activated DNA damage signaling pathway. CEP63, a centrosomal protein crucial for centriole duplication, undergoes regulation by DDR factors. In mouse spermatocytes lacking Cep63, chromosome entanglement, centrosome aberrance, and telomere clustering reduction were observed. This directly contributes to the failure in chromosome movement.69 The E-type cyclins, E1 and E2, play roles in maintaining telomere integrity. In mice, the absence of cyclin E2 leads to structural defects in telomeres and the inability to tether them properly. Additionally, the deficiency of cyclin E1 further exacerbates these abnormalities. In pachytene spermatocytes lacking cyclin E (cyclin E1/E2 double knockout mice), reduced shelterin (marked by TRF2 and RAP1) and persistent DSBs (marked by γH2AX) were detected primarily at telomeres, particularly in proximal telomeres (the telomeres next to centromeres) as opposed to distal ones (the telomeres far away from centromeres).70 The authors attributed the increased susceptibility of proximal telomeres to the distinct microenvironment established by the proximity to centromeres. This influences telomere metabolism during male meiosis.70 DGCR8 (a constituent of the endonuclease microprocessor complex) and DICER (another endonuclease) both participate in miRNA processing. When either Dgcr8 or Dicer is disrupted in mice, it impairs the localization of proteins crucial for telomere maintenance, leading to telomere fusion.71 ZBTB40, the zinc finger and BTB domain 40, is a protein associated with telomeres. A recent study highlighted its affinity for binding to telomeric dsDNA in cells utilizing alternative lengthening of telomeres (ALT).72 Another study discovered that Zbtb40+/− mice exhibit longer telomeres in spermatocytes.73 HOP2-MND1 heterodimer serves as a critical partner for the meiosis-specific recombinase DMC1. It forms a higher-order structure with RAD51 to activate telomeric damage repair through the ALT mechanism in somatic cells.74,75 In male mice, deletion of Hop2/Mnd1 led to the unsuccessful DSB repair and homologous recombination in spermatocytes. Nonetheless, it remains unclear whether telomere abnormalities occurred.76,77 Brwd1 (Bromodomain and WD repeat domain containing 1) has been demonstrated to regulate the structure of oocyte telomeres during meiosis78; however, its involvement in telomeres in mouse spermatocytes has not yet undergone comprehensive investigation. Table 2 lists genes influencing telomeric function during meiosis and the resulting meiotic phenotypes in mouse mutants.
| Genes | Functions | Meiotic phenotypes in mouse mutants | References |
|---|---|---|---|
| Sycp3 | Component of the synaptonemal complex, centromere pairing, telomere clustering | Zygotene arrest, telomere loss | 61 |
| Rec8 | Homologous chromosome and sister chromatid segregation, telomere clustering | Zygotene arrest, shorter telomeres | 11 |
| Smc1β | Meiotic cohesion, axis-loop formation, sister chromatid segregation, telomere clustering, T-loop formation | Early/mid pachytene arrest, aberrant telomeres, failed NE attachment | 12, 13, 20, 52, 55, 56 |
| Stag3 | Sister chromatid cohesion and chromosome segregation, telomere structure | Zygotene arrest, compacted axis structure | 57, 58 |
| Rad21L | Sister chromatid cohesion and chromosome segregation, telomere structure | Zygotene arrest, shorter telomeres | 54, 56 |
| Pds5 | Sister chromatid cohesion and chromosome segregation, DNA repair, telomere structure regulation, NE attachment | Shortened axis, impaired telomere integrity, and NE attachment | 55, 59, 60 |
| Fbxo47 | ubiquitination and degradation, TRF1/2 stabilization, NE attachment, bouquet formation | Zygotene arrest, impaired NE attachment | 62 |
| Ythdc2 | M6A-containing RNA regulation, RNA degradation, microtubule-dependent telomere clustering regulation | Telomere clustering at pachytene | 63 |
| Pold3 | Telomere synthesis and maintenance, telomere length regulation | Telomere shortening and loss | 65, 66, 99 |
| Cep63 | Spindle assembly, telomere clustering | Defective telomere clustering | 69 |
| Cyclin E1/E2 | Telomere integrity, NE attachment, homologous pairing and synapsis | Early pachytene arrest, telomere structure defects | 70 |
| Dgcr8 | miRNA biogenesis and processing, telomere protection | Impaired telomere maintenance and integrity | 71 |
| Dicer | miRNA processing, telomere protection, gene silencing | Impaired telomere maintenance and integrity | 71 |
| Zbtb40 | Transcriptional regulation, telomere length regulation | Increased telomere length | 73 |
- Abbreviations: CEP63, centrosomal protein of 63 kDa; Cyclin E1/E2, E-type cyclins; DGCR8, microprocessor complex subunit DGCR8; DICER, endoribonuclease dicer; FBXO47, F-box only protein 47; PDS5, sister chromatid cohesion protein PDS5 homolog; POLD3, DNA polymerase delta subunit 3; RAD21L, double-strand-break repair protein rad21-like protein 1; REC8, meiotic recombination protein REC8 homolog; SMC1β, structural maintenance of chromosomes protein 1B; STAG3, cohesin subunit SA-3;SYCP3, synaptonemal complex protein 3; YTHDC2, 3′−5′ RNA helicase YTHDC2; ZBTB40, zinc finger and BTB domain 40.
1.5 Telomeres and male reproduction in humans
The above studies primarily explore the molecular structure and functions of telomeres in mice. Studies on telomeres in male reproduction have predominantly concentrated on DNA fragmentation and ROS production.79 In contrast to the shorter telomere length observed in the somatic cells of older individuals, older men tend to have longer telomeres in their sperm, potentially linked to the repression of retro transposition.80–82 During spermatogenesis, telomerase undergoes gradual inactivation and becomes undetectable in mature spermatozoa. In contrast to the expected shortest telomeres in spermatozoa (according to telomerase theory), mature spermatozoa possess even longer telomeres,83 a phenomenon potentially explained by the ALT theory.84 Currently, telomerase activity and telomere length serve as biomarkers for evaluating sperm quality and male fertility. However, these findings have not emphasized the fundamental role of telomeres in male meiosis in humans.
With the widespread application of sequencing technology, mutations in telomere-related genes have recently been identified. In 2021, a variant of human KASH5 (L535Q) was discovered, demonstrating mislocalization of KASH5 on the ONM and mistargeting to the mitochondrial membrane, resulting in azoospermia.85 Recently, novel homozygous missense mutations were identified in Kash5 (Ccdc155) among patients with NOA. These mutations include [c.590T > C (p.Leu197Pro)], (NM_144688: c.979_980del: p.R327Sfs*21), and (c.1270_1273del, p.Arg424Thrfs*20). While the exact stages of spermatogenesis arrest varied among patients, it was consistently halted during meiosis. Disruptive NE distribution and anchoring have been observed, with a significant weakening of the interaction between KASH5 and SUN1 observed in mutant spermatocytes.86–88 In addition, spermatocytes from patients with NOA harboring a homozygous variant in Sun1 [c. 663C > A: p.Tyr221X] were also arrested during meiosis. This condition exhibited significant reductions in KASH5 levels and defective NE attachment.89 These findings offer new insights into the involvement of the LINC complex in male fertility among humans. In addition to the LINC complex, mutations in human meiotic telomere complex genes Terb1, Terb2, and Majin were discovered in patients with NOA, resulting in meiosis arrest, consistent with phenotypes observed in mouse mutants.90 Mutations identified in patients with NOA could broaden the genetic spectrum and enhance genetic diagnosis and treatment. While mutations in the shelterin complex have not been discovered, existing research supports the notion that meiotic telomeres play a crucial role in male reproduction in humans.
1.6 Concluding remarks and prospects
This review revealed the crucial role of telomeres in male meiosis in mice and humans. We discussed various telomere-related structures, including SUN1-KASH, SPDYA-CDK2, TERB1-TERB2-MAJIN, and Shelterin. Meiotic functions and phenotypes of the mouse mutants have been comprehensively described. They are depicted in the text description, images (Figures 1 and 2), and tables (Tables 1 and 2). Through an analysis of the phenotypes observed in Kash5/Sun1/Terb1/Terb2/Majin mutant spermatocytes from patients with nonobstructive azoospermia, we deduced that the functions of these telomere-related proteins are conserved in mammals and restoring the normal expression of these proteins can potentially rectify meiotic progression in both mice and humans. This suggests that genetic-level diagnosis and treatment hold promising prospects for reproductive medicine in the future.
This review highlights the significance of cohesins in telomere activity, while a definitive relationship remains unclear. Based on various evidence from previous studies using mouse models, it is reasonable to conclude that cohesins, particularly STAG3, SMC1β, and RAD21L, play a role in regulating telomere structure and activities. While the telomeric T-loop structure is extensively studied in mitosis, there is limited research on its role in meiosis. The formation of the mitotic T-loop structure involves collaboration between cohesins, TRF2, and TIN2. Whether the T-loop regulates chromosomal activity during meiosis necessitates further investigation. In addition, the interaction between STAG3 and the MYB domain of TERB1 validates the role of TERB1 in recruiting STAG3 to telomeres,2,29,53 while other cohesin components did not show such interaction. However, in the Smc1β/Rad21l/Rec8/Pds5-knockout model, telomeres exhibited varying degrees of abnormality. Therefore, further investigation is needed to understand the mechanisms through which these components regulate telomere function. In addition to cohesins and T-loops, the microenvironment shaped by centromeres and proximal telomeres deserves more attention as it is closely linked to the axis-loop structure and telomere arrangement.
A study conducted on yeast suggested that certain factors influencing mitotic telomere activity might be involved in regulating telomere dynamics during meiosis II.91 However, due to the transition to meiosis II in mammals, research on this process is challenging and restricted. Leveraging research conducted on mitotic telomeres for insights into meiosis II could significantly streamline efforts and enhance our understanding of this phase of meiosis. In clinical medicine, the majority of mutations identified are point mutations; however, most mechanistic investigations have relied on knockout models (cell lines or mice). Therefore, future studies integrating point mutation models with mutations observed in patients were warranted.
AUTHOR CONTRIBUTIONS
Shuiqiao Yuan and Lisha Yin reviewed the literature. Lisha Yin, Nan Jiang, and Tao Li wrote the manuscript. Shuiqiao Yuan and Youzhi Zhang conceived and revised the manuscript.
ACKNOWLEDGEMENTS
The authors thank members of the Yuan laboratory for their constructive critique of this manuscript. The authors apologize to any authors whose work we have unfortunately omitted due to space constraints. This work was supported by grants from the National Natural Science Foundation of China (82171605 and 81971444).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




