Efficacy and safety assessment of traditional Chinese medicine for erectile dysfunction: A meta-analysis and trial sequential analysis
Abstract
Background
Several patients with erectile dysfunction do not accept or benefit from conventional therapy with phosphodiesterase type 5 inhibitors; thus, alternative and complementary therapies are in need. Traditional Chinese medicine has been treating erectile dysfunction in China, but its clinical value is inconclusive.
Objective
To systematically evaluate the efficacy and safety of traditional Chinese medicine in treating erectile dysfunction.
Methods
Randomized controlled trials were retrieved from a comprehensive search in the literature published in the past decade from the Web of Science, PubMed, Embase, Cochrane Library, SinoMed, China National Knowledge Internet, WanFang, and VIP. We performed a meta-analysis of the International Index of Erectile Function 5 questionnaire scores, clinical recovery rates, and testosterone levels using Review Manager 5.4 software. The trial sequential analysis was conducted to check the results.
Results
A total of 45 trials with 5016 patients were included. Meta-analysis results showed that traditional Chinese medicine effectively improved the International Index of Erectile Function 5 questionnaire scores (weighted mean difference = 3.78, 95% confidence interval: 3.12, 4.44; p < 0.001), clinical recovery rates (risk ratio = 1.57, 95% confidence interval: 1.38, 1.79; p < 0.001), testosterone levels (weighted mean difference = 2.42, 95% confidence interval: 1.59, 3.25; p < 0.001) compared with the controls. The single and add-on applications of traditional Chinese medicine could improve the International Index of Erectile Function 5 questionnaire score (p < 0.001). The trial sequential analysis confirmed the robustness of the analysis of the International Index of Erectile Function 5 questionnaire scores. A significant difference in the incidence of adverse effects between the treatment and control groups was not observed (risk ratio = 0.82, 95% confidence interval: 0.65, 1.05; p = 0.12).
Conclusion
Traditional Chinese medicine can gain better responses in improving the International Index of Erectile Function 5 questionnaire scores, clinical recovery rates, and testosterone levels as an alternative and complementary treatment, with no increase in side effects. However, more standardized, long-term, traditional Chinese medicine and integrative therapy clinical trials are needed to support the clinical application of traditional Chinese medicine.
1 INTRODUCTION
Erectile dysfunction (ED) is one of the most frequently reported diseases in men, the estimated prevalence of ED escalated from 40 years old and is closely associated with age.1-3 Additionally, ED is increasingly common in young men.4 The causes of ED consist of a mix of vasculogenic, neurogenic, endocrine/metabolic, and psychogenic factors,5 among which lower urinary tract symptoms,6 diabetes,7 metabolic syndromes,8 cardiovascular diseases,9 obesity,10 depression,11 and smoking12 have gained more attention. Currently, phosphodiesterase type 5 inhibitors (PDE5Is) are the first-line therapy for treating ED.13, 14 Though the efficacy, tolerability, and overall safety of PDE5Is are strongly supported,15 some patients still fail to achieve the clinical benefit or refuse the on-demand therapy.16, 17 The common reasons for the discontinuation or rejection included reluctant medication-dependent intercourse, psychological factors, concerns about the cardiovascular safety of PDE5Is, and adverse effects (such as facial redness and headache). Besides, patients with high cardiovascular risk are more likely to show low clinical response, which may be related to the severity of neuropathy.18, 19
Botanical medicine and natural products have been used for ED worldwide,20 and several of them have been recommended and validated for application.21 Traditional Chinese medicine (TCM) originated in ancient China and developed through long-term medical practice under the influence of ancient Chinese philosophical thinking and natural science. TCM practitioners employ several approaches to promote health and treat disease, complying with the theory of conception of holism and treatment determination based on syndrome differentiation, including Chinese herbal medicine and acupuncture. TCM has been applied for treating ED in China.22, 23 Formal research has assessed the effectiveness and safety of acupuncture for ED24; thus, the current study focused on the oral application of Chinese herbal medicine. Preclinical trials indicated that TCM could improve erectile function by suppressing oxidative stress, curing vascular endothelial injury, inhibiting apoptosis, and anti-atherosclerotic effects.25-28 Further research validates that TCM combined with tadalafil has significant efficacy in treating ED with no increase in side effects.29 Accumulating research focuses on the efficacy of TCM on ED and a systematic evaluation of whether TCM is available as a complementary and alternative therapy is in need. Considering these situations, we selected studies published in the past decade to evaluate the efficacy of TCM on ED through a systematic review and meta-analysis.
2 MATERIALS AND METHODS
2.1 Study registration
This systematic review protocol has been registered on PROSPERO (CRD42022337110) and conducted under the Preferred Reporting Items for Systematic Reviews and Meta-analyses reporting guideline.30
2.2 Eligibility criteria
We included studies that satisfied the following criteria: (a) trials that were designed to be randomized controlled trials comparing Chinese herbal medicine with placebo, other intervention, or other pharmaceuticals as treatments for ED; (b) trials where the participants had a definite diagnosis of ED; (c) trials with sample size more than 60; (d) trials with intervention period over 12 weeks; (e) trials where the only difference between the two groups was whether received Chinese herbal medicine; (f) trials that used the International Erectile Function Index Questionnaire 5 (IIEF-5)31 as primary outcome measurement.
Based on previous studies in China, the response rates were 90.5% and 62.4% using the integration of traditional Chinese and western medicine and sildenafil for ED treatment, respectively.32, 33 The sample size was calculated by assuming a test power of 80% and a confidence level of 95%, Z1-alpha/2 = 1.960, alpha = 5% for the two-tailed hypothesis, Z1-beta = 0.842, sigma = 0.024. A total of 32 patients are required in each group. Considering the different treatment comparisons, we set the minimal sample size as 60.
2.3 Search strategies
Relevant clinical trials were identified by searching for articles published from January 2012 to May 2022 in the following databases: Web of Science, PubMed, Embase, Cochrane Library, SinoMed, China National Knowledge Internet, WanFang, and VIP. Search terms included the following: (“ED”, “impotence”, or “male sexual dysfunction”) and (“randomized controlled trial”, “controlled clinical trial”, “randomly”, “randomized”, or “randomized”) and (“TCM”, “Chinese herbal medicine”, “herbal medicine”, “phytomedicine”, or “ethnobotanical”). The search strategy of PubMed is summarized in Table S1. This search strategy will be modified according to the characteristics of different databases.
The China Food and Drug Administration issued the guideline for a clinical study on angina pectoris of coronary atherosclerotic heart disease treated with traditional Chinese medicines and natural medicines in 2011 and the technical requirements for the research of new drugs from natural medicines in 2013, aiming to guide and regulate clinical trial of Chinese medicine. Therefore, the TCM research quality has been significantly improved in the last decade, as well as the quantity of relevant clinical trials. Accordingly, we focused on the publications in the previous decade.
2.4 Study selection and data collection
The Endnote software (Version 20) was used for literature management and duplication filtration and removal. Then the two reviewers independently selected the eligible studies by reading the title and abstracts. Further detailed screening was conducted by full-text reading. Controversial opinions were resolved by discussion with the third reviewer. Only the most recent study was enrolled if potential overlapping populations were repetitively reported.
Data extraction was performed after the screening process by two independent reviewers. The content of data extraction included the general publication information (including the name of the first author and the publication year), trial design and methodology (including sample size and participants), intervention profiles (including drug name, dosage, duration, and administration method), and outcome measurements (including IIEF, the percentage of participants with IIEF-5 score over 21, testosterone levels, and adverse events).
2.5 Risk of bias assessment
Two reviewers independently assessed the study risk of bias based on the Cochrane risk-of-bias tool for randomized trials (RoB 2).34 Discrepancies between the two review authors were resolved through discussion with a third review author. The assessment outcomes were visualized using Review Manager software (version 5.4; Cochrane Collaboration, Oxford, UK).35 The publication bias would be assessed using a funnel plot if the result of the current meta-analysis contains more than 10 articles. Quantitative methods, including the Begg test and Egger test, would be used to assess publication bias.
2.6 Synthesis methods
The meta-analysis of comparable data was carried out using RevMan. Risk ratios were calculated with 95% confidence intervals (CIs) for dichotomous outcomes. We calculated the weighted mean differences for continuous outcomes accompanied by 95% CIs. Heterogeneity was examined using the I2 statistic, and its significance was calculated with Cochran's Q test. The significance level of the Q test was set at 0.1. Besides, a fixed-effect model was used if I2 < 50% and p > 0.1; the random-effect model was used if 50% < I2 < 85%. If I2 is greater than 85%, predetermined sub-analyses were performed to explore the cause(s) of heterogeneity. If data was available, we intended to explore the potential sources of heterogeneity using subgroup analyses, including duration of treatment, different intervention combinations, and ED types. If data were available, regression analysis would be conducted to find the sources of heterogeneity using the meta R package (v6.1-0). Sensitivity analysis was performed to check whether the results were affected by the inclusion of certain trials, and the differences between the REM and FEM were also observed to test the robustness of the results. In detail, the sensitivity analysis was conducted by assessing the changes in the overall results by eliminating individual studies one-by-one and applying different effect models.
2.7 Trial sequential analysis
The trial sequential analysis (TSA) was conducted to explore whether cumulative data were adequately powered to evaluate the primary outcome. We used the TSA program (TSA software version 0.9.5.10 Beta; Copenhagen Trial Unit, Copenhagen, Denmark)37 to acquire the estimation of required information size (RIS) with an adjusted threshold for statistical significance under an overall 5% risk of type I error and 80% power. The sample size was taken as the RIS and calculated using the means and variances according to the results of the meta-analysis.
3 RESULTS
3.1 Description of included studies
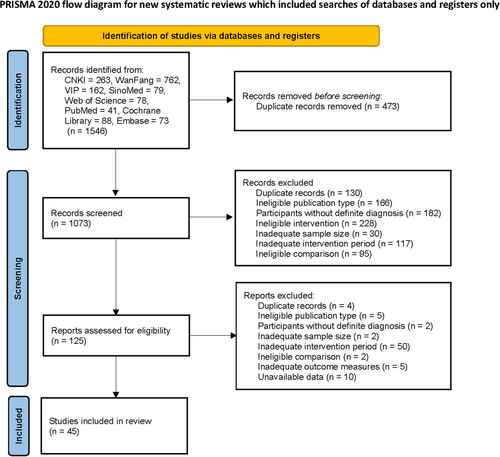
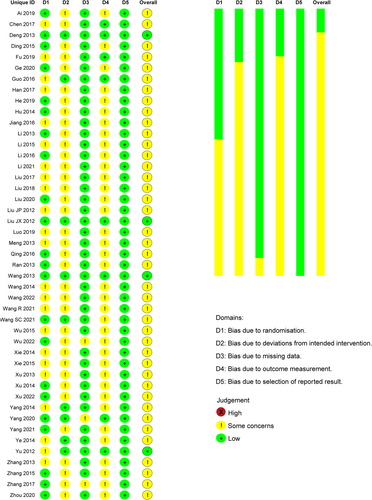
A total of 1546 potential records were identified from the eight databases following the search strategy. After screening and assessment, 45 eligible studies were enrolled, including 5016 participants in total (Figure 1). All the trials were performed in China, with the intervention duration ranging from 12 to 24 weeks. Overall, the methodological quality of the included studies was moderate. The quality evaluation is documented in Figure 2 (evaluated by ROB 2). Most concerns focused on the randomization process, intended intervention, and outcome measurement. The lack of detailed random sequence generation and concealment of allocation sequence raised concerns, though there were no significant baseline differences. Besides, only six studies used the placebo, and fewer reported whether deviations arose because of the trial context, which might lead to bias. As for the outcome measurement, bias might occur since the IIEF-5 acts as a self-reported tool.
The characteristics of enrolled trials are presented in Table 1. Among the included studies, a total of six studies compared TCM with placebo,38-43 four studies compared TCM with PDE5Is,44, 45 17 studies focused on the effect of TCM as an add-on to PDE5Is,46-60 and 19 studies focused on the effect of TCM as add on to pharmaceuticals except for PDE5Is.61-79 Besides, one study compared TCM with bromocriptine.80 It should be noted that two references81, 82 both reported the comparisons between TCM and PDE5Is, TCM with PDE5Is and PDE5Is.
| Study | Sample Size (E/C) | Duration (weeks) | Diagnosis | Age (years, E/C) | TCM syndrome differentiation | Experimental | Control | Outcome measurement |
|---|---|---|---|---|---|---|---|---|
| Ai 2019 | 73/73 | 12 | ED and type 2 diabetes mellitus | 51.87 ± 4.26/51.63 ± 4.15 | kidney deficiency and blood stasis |
a. Yishen Huoxue Decoction, PO, bid b. valsartan (80 mg), 80 mg, PO, qd |
a. valsartan (80 mg), 80 mg, PO, qd | IIEF-5, sex hormone indexes, potency recovery rate |
| Ge 2020 | 45/45 | 12 | ED and hyperuricemia | 43.66 ± 6.96/44.14 ± 7.15 | N/A |
a. Quyu Huatan Erxian Decoction, PO, tid b. febuxostat tablets (40 mg), 40 mg, PO, qd |
a. febuxostat tablets (40 mg), 40 mg, PO, qd | IIEF-5, penile hemodynamics function, endothelin-1 and nitric oxide in serum |
| He 2019 | 34/34 | 12 | ED and post-kidney transplantation | 35.86 ± 6.18/34.72 ± 5.42 | N/A |
a. Compound Xuanju capsule (0.4 g), 1.2 g, PO, tid b. psychotherapy |
a. psychotherapy | IIEF-5, potency recovery rate |
| Hu 2014 | 30/30 | 16 | ED and type 2 diabetes mellitus | 39.69 ± 11.37/37.24 ± 8.33 | qi deficiency and blood stasis |
a. Jiawei Buyang Huanwu Decoction, PO, bid b. Vitamin B1 tablets (10 mg), 10 mg, PO, tid c. routine treatment of diabetes |
a. Vitamin B1 tablets (10 mg), 10 mg, PO, tid b. routine treatment of diabetes |
IIEF-5, sex hormone indexes, World Health Organization Quality of Life Scale, potency recovery rate |
| Jiang 2016 | 45/41 | 24 | ED and metabolic syndrome | 53.41 ± 10.30/54.12 ± 10.23 | phlegm and stasis obstructing the collateral |
a. Xuefu Zhuyu tablets, 6 #, PO, bid b. testosterone undecanoate soft capsules (40 mg), 80 mg (1st month)/ 40 mg (rest months), PO, bid |
a. testosterone undecanoate soft capsules (40 mg), 80 mg (1st month)/ 40 mg (rest months), PO, bid | IIEF-5, endothelin-1, and nitric oxide in serum, sex hormone indexes, potency recovery rate |
| Li 2013 | 48/47 | 12 | ED and late-onset hypogonadism | 54.2/54 | N/A |
a. Compound Xuanju capsule (0.4 g), 1.2 g, PO, tid b. testosterone undecanoate soft capsules (40 mg), 40 mg, PO, bid |
a. testosterone undecanoate soft capsules (40 mg), 80 mg, PO, bid | IIEF-5, testosterone |
| Li 2015 | 67/68 | 12 | ED and chronic nonbacterial prostatitis | 31.5 ± 7.2/32.6 ± 6.8 | N/A |
a. Qiyang Zhuyu Decoction, PO, bid b. tamsulosin, 0.2 mg, PO, qn c. levofloxacin, 0.2 g, PO, bid d. indometacin enteric-coated tablet, 25 mg, PO, tid |
a. tamsulosin, 0.2 mg, PO, qn b. levofloxacin, 0.2 g, PO, bid c. indometacin enteric-coated tablet, 25 mg, PO, tid |
IIEF-5, testosterone |
| Liu 2020 | 61/61 | 12 | ED and diabetic nephropathy (stage III or IV) | 38.97 ± 5.68/39.87 ± 7.94 | N/A |
a. Jinshuibao capsule (0.33 g), 1.65 g, PO, tid b. valsartan (80 mg), 80 mg, PO, qd |
a. valsartan (80 mg), 80 mg, PO, qd | IIEF-5, sex hormone indexes, endothelin-1, potency recovery rate |
| Meng 2013 | 30/30 | 12 | ED and hypertension | 48.5 ± 5.7/51.2 ± 4.1 | N/A |
a. Bushen Yixin Decoction, PO, bid b. nifedipine controlled-release tablets, 30 mg, PO, qd c. metoprolol succinate, 25 mg, PO, qd (sustained-released tablets 47.5 mg) |
a. nifedipine controlled-release tablets, 30 mg, PO, qd b. metoprolol succinate, 25 mg, PO, qd (sustained-released tablets 47.5 mg) |
IIEF-5, potency recovery rate |
| Qing 2016 | 40/40 | 12 | ED and hypertension (stage II) | 45.70 ± 12.30/45.20 ± 11.60 | N/A |
a. Bushen Yixin tablets (0.25 g), 1.0 g, PO, tid b. amlodipine besylate tablets (5 mg), 5 mg, PO, qd c. benazepril hydrochloride (10 mg), 10 mg, PO, qd (valsartan (80 mg), 80 mg, PO, qd, for patients intolerant of benazepril) |
a. amlodipine besylate tablets (5 mg), 5 mg, PO, qd b. benazepril hydrochloride (10 mg), 10 mg, PO, qd (valsartan (80 mg), 80 mg, PO, qd, for patients intolerant of benazepril) |
IIEF-5, endothelin-1, and nitric oxide in serum, potency recovery rate |
| Ran 2013 | 43/43 | 12 | ED and hypertension (stage I or II) | 53.4 ± 8.6/55.8 ± 8.7 | kidney deficiency |
a. Bushen Jiangya Formula, PO, bid b. benazepril hydrochloride, 10 mg, PO, qd (combined with amlodipine besylate tablets, 5 mg, PO, qd, PRN) |
a. benazepril hydrochloride, 10 mg, PO, qd (combined with amlodipine besylate tablets, 5 mg, PO, qd, PRN) | IIEF-5 |
| Wang 2014 | 44/48 | 12 | ED and diabetic nephropathy | 51.9 ± 2.0/53.4 ± 2.6 | N/A |
a. Jinshuibao capsule (0.33 g), 1.65 g, PO, tid b. valsartan, 80 mg, PO, qd |
a. valsartan, 80 mg, PO, qd | IIEF-5, potency recovery rate |
| Wang 2021 | 30/30 | 12 | ED and hypertension (stage I or II) | 54.92 ± 10.21/55.13 ± 11.86 | kidney qi deficiency |
a. Bushen Yixin tablets (0.3 g), 1.5 g, PO, tid b. amlodipine besylate tablets (5 mg), 5 mg, PO, qd |
a. amlodipine besylate tablets (5 mg), 5 mg, PO, qd | IIEF-5, endothelin-1, and nitric oxide in serum |
| Wu 2015 | 48/47 | 12 | ED and late-onset hypogonadism | 45.5–66/45–66 | N/A |
a. Shenrong Qiangshen tablets, 6#, PO, bid b. testosterone undecanoate soft capsules, 40 mg, PO, bid |
a. testosterone undecanoate soft capsules, 80 mg, PO, bid | IIEF-5, sex hormone indexes |
| Xie 2014 | 40/40 | 12 | ED and CHD (underwent off-pump coronary artery bypass) | 51.66 ± 12.5/49.90 ± 13.3 | kidney deficiency and blood stasis |
a. Bushen Huoxue Decoction, PO, bid b. routine treatment of CHD |
a. routine treatment of CHD | IIEF-5, potency recovery rate |
| Xie 2015 | 40/40 | 12 | ED and CHD (underwent percutaneous coronary intervention) | 50.12 ± 14.3/51.9 ± 12.1 | kidney deficiency and blood stasis |
a. Bushen Huoxue Decoction, PO, bid b. routine treatment of CHD |
a. routine treatment of CHD | IIEF-5, potency recovery rate |
| Xu 2013 | 35/30 | 12 | ED | 56.5 ± 0.5 | N/A |
a. Shugan Yiyang capsule, 1 g, PO, tid b. testosterone undecanoate soft capsules, PO, 80 mg qm, 40 mg qn |
a. testosterone undecanoate soft capsules, PO, 80 mg qm, 40 mg qn | IIEF-5 |
| Yang 2021 | 35/35 | 12 | ED and diabetes mellitus | 48.58 ± 11.28/49.48 ± 11.19 | qi deficiency and blood stasis |
a. Buyang Huanwu Decoction, PO, bid b. Vitamin B1 tablets (10 mg), 10 mg, PO, tid c. routine treatment of diabetes |
a. Vitamin B1 tablets (10 mg), 10 mg, PO, tid b. routine treatment of diabetes |
IIEF-5, sex hormone indexes, World Health Organization Quality of Life Scale, potency recovery rate |
| Zhang 2015 | 52/50 | 12 | ED and benign prostatic hyperplasia | 56.8 ± 7.7/54.2 ± 8.6 | kidney yang deficiency |
a. Jinkui Shenqi Pill, 5 g, PO, bid b. tamsulosin (0.1 mg), 0.2 mg, PO, qd |
a. tamsulosin (0.1 mg), 0.2 mg, PO, qd | IIEF-5 |
| Deng 2013 | 53/51 | 12 | ED and diabetes mellitus | 35–50 | N/A |
a. Wuzi Yanzong capsule b. routine insulin treatment for diabetes |
a. placebo b. routine insulin treatment for diabetes |
IIEF-5, Erectile Hardness Grading Scale |
| Guo 2016 | 50/50 | 12 | ED and type 2 diabetes mellitus | 30–60 | N/A |
a. Zhenyang Decoction, PO, bid b. routine treatment of diabetes |
a. placebo b. routine treatment of diabetes |
IIEF-5, endothelin-1, and nitric oxide in serum |
| Liu 2012 | 82/80 | 12 | ED and diabetes mellitus | 35–49 | N/A |
a. Wenshen Huoxue Decoction, PO, bid b. routine insulin treatment for diabetes |
a. placebo, PO, tid b. routine treatment of diabetes |
IIEF-5, Erectile Hardness Grading Scale |
| Wang 2013 | 60/30 | 24 | ED and type 2 diabetes mellitus | 51.21 ± 10.32/50.66 ± 10.67 | N/A | a. Tongxinluo capsule, 4 #, PO, tid | a. placebo, 4 #, PO, tid | IIEF-5 |
| Yang 2020 | 56/53 | 12 | ED and type 2 diabetes mellitus | 51.23 ± 8.05/51.60 ± 8.04 | N/A | a. Qiyao Xiaoke oral liquid (10 ml), 10 ml, PO, tid | a. placebo (10 ml), 10 ml, PO, tid | IIEF-5, Treatment Satisfaction Scale, sex hormone indexes |
| Yu 2012 | 83/44 | 12 | ED | 25–60 | binding constraint of liver qi | a. Yikan capsule, 2#, PO, bid | a. placebo, 2#, PO, tid | IIEF-5, potency recovery rate |
| Chen 2017 | 265/267 | 12 | ED and diabetes mellitus | 41.27 ± 4.37/42.31 ± 5.69 | N/A |
a. Bushen Huanyang Decoction, PO, bid b. tadalafil, 10 mg, PO, qod c. routine treatment of diabetes |
a. tadalafil, 10 mg, PO, qod b. routine treatment of diabetes |
IIEF-5, testosterone |
| Ding 2015 | 40/40 | 12 | ED | 55.1 ± 5.3/53.4 ± 5.8 | kidney yang deficiency |
a. Congrong Yishen granule, 2 g, PO, tid b. tadalafil (10 mg), 5 mg, PO, Biw |
a. tadalafil (10 mg), 5 mg, PO, Biw | IIEF-5, responses to questions 2 and 3 of the Sexual Encounter Profile, potency recovery rate |
| Fu 2019 | 65/65 | 12 | ED | 34.25 ± 6.89/33.21 ± 7.19 | binding constraint of liver qi |
a. Jiajian Xiaoyaosan, PO, bid b. tadalafil (5 mg), 5 mg, PO, qn |
a. tadalafil (5 mg), 5 mg, PO, qn | IIEF-5, potency recovery rate |
| Han 2017 | 41/40 | 12 | ED | 44.53 ± 5.57/44.56 ± 5.57 | N/A |
a. Yougui Pill (9 g), 9 g, PO, tid b. sildenafil citrate (50 mg), 50 mg, PO, Biw |
a. sildenafil citrate (50 mg), 50 mg, PO, Biw | IIEF-5 |
| Li 2016 | 45/45 | 12 | ED | 42.3 ± 5.6/43.1 ± 6.2 | N/A |
a. Zhuangyang Decoction, PO, bid b. sildenafil citrate (25 mg), 50 mg, PO, Biw c. psychotherapy |
a. sildenafil citrate (25 mg), 50 mg, PO, Biw b. psychotherapy |
IIEF-5, potency recovery rate |
| Li 2021 | 60/60 | 12 | ED | 54.28 ± 3.45/52.17 ± 2.16 | N/A |
a. Xianlu Oral Liquid (10 ml), 10 ml, PO, tid b. sildenafil citrate (100 mg), 50 mg, PO, PRN |
a. sildenafil citrate (100 mg), 50 mg, PO, PRN | IIEF-5, potency recovery rate, sex hormone indexes |
| Liu 2017 | 90/90 | 12 | ED and type 2 diabetes mellitus | 41.6 ± 2.5/43.6 ± 2.9 | kidney deficiency and blood stasis |
a. Wenshen Huoxue Tongluo Decoction, PO, bid b. vardenafil hydrochloride tablets (10 mg), 20 mg/10 mg/5 mg, PO, PRN (dose regulated based on response) c. acipimox (0.25 g), 0.25 g, PO, bid d. routine treatment of diabetes |
a. vardenafil hydrochloride tablets (10 mg), 20 mg/10 mg/5 mg, PO, PRN (dose regulated based on response) b. acipimox (0.25 g), 0.25 g, PO, bid c. routine treatment of diabetes |
IIEF-5, potency recovery rate |
| Liu 2018 | 60/60 | 12 | ED | 45.20 ± 4.91/45.89 ± 4.75 | N/A |
a. Canrong Zhutian capsules (0.4 g), 0.8 g, PO, bid b. sildenafil citrate (100 mg), 50 mg, PO, PRN (1-4 times per week) |
a. sildenafil citrate (100 mg), 50 mg, PO, PRN (1–4 times per week) | IIEF-5, potency recovery rate |
| Luo 2019a | 32/32 | 12 | ED and diabetes mellitus | 40.01 ± 8.21 | N/A |
a. Xuefu Zhuyu tablets (0.45 g), 2.7 g, PO, bid b. tadalafil, 5 mg, PO, qn |
a. tadalafil, 5 mg, PO, qn | IIEF-5 |
| Wang 2022 | 31/31 | 12 | ED | 29.27 ± 2.23/29.41 ± 2.17 | N/A |
a. Shugan Qiwei Decoction, PO, bid b. tadalafil, 5 mg, PO, qn |
a. tadalafil, 5 mg, PO, qn | IIEF-5, potency recovery rate |
| Wang 2021 | 130/75 | 12 | ED | 31.65 ± 5.41 | N/A |
a. Shanhaidan granule (10 g), 10 g, PO, tid b. tadalafil (5 mg), 5 mg, PO, qd |
a. tadalafil (5 mg), 5 mg, PO, qd | IIEF-5, Erection Hardness Score, penile hemodynamics function |
| Wu 2022 | 30/31 | 12 | ED | 36.6 ± 8.41/34.1 ± 9.18 | liver constraint and kidney deficiency |
a. Bushen Shugan Xingyang Decoction, PO, bid b. tadalafil (5 mg), 5 mg, PO, qd |
a. tadalafil (5 mg), 5 mg, PO, qd | IIEF-5, responses to questions 4 and 5 of the Sexual Encounter Profile, penile hemodynamics function |
| Xu 2022 | 46/46 | 12 | ED | 42.37 ± 8.98/40.81 ± 9.76 | N/A |
a. Jinjie tablet (0.3 g), 1.2 g, PO, tid b. vardenafil hydrochloride tablets (10 mg), 10 mg, PO, on demand |
a.vardenafil hydrochloride tablets (10 mg), 10 mg, PO, on demand | IIEF-5, Erection Hardness Score, Erection Quality Scale, penile hemodynamics function, potency recovery rate |
| Yang 2014a | 30/30 | 12 | ED | 36.43 ± 9.78/32.60 ± 6.73 | N/A |
a. Shugan Yiyang capsule (0.25 g), 1.0 g, PO, tid b. tadalafil, 5 mg, PO, qn |
a. tadalafil, 5 mg, PO, qn | IIEF-5 |
| Ye 2014 | 47/33 | 24 | ED | 53.5 | N/A |
a. Liuwei Dihuang Pill, 8#, PO, tid b. testosterone undecanoate soft capsules (40 mg), 80 mg, PO, qd c. sildenafil citrate, 50 mg, PO, PRN |
a. testosterone undecanoate soft capsules (40 mg), 80 mg, PO, qd b. sildenafil citrate, 50 mg, PO, PRN |
IIEF-5, potency recovery rate |
| Zhang 2013 | 80/80 | 12 | ED | 38.2 ± 5.2/37.8 ± 6.82 | N/A |
a. Jiawei Sinisan, PO, tid b. sildenafil citrate, 50 mg, PO, Biw |
a. sildenafil citrate, 50 mg, PO, Biw | IIEF-5 |
| Zhang 2017 | 39/37 | 12 | ED | 39.85 ± 9.48/40.32 ± 12.85 | N/A |
a. Hongjing Yihao Formula, PO, bid b. tadalafil (5 mg), 5 mg, PO, qn/qod (dose decreased after 4 weeks) |
a. tadalafil (5 mg), 5 mg, PO, qn | IIEF-5, Erection Hardness Score, potency recovery rate |
| Luo 2019b | 32/32 | 12 | ED and diabetes mellitus | 40.01 ± 8.21 | N/A | a. Xuefu Zhuyu tablets (0.45 g), 2.7 g, PO, bid | a. tadalafil, 5 mg, PO, qn | IIEF-5 |
| Xu 2014 | 68/68 | 12 | ED and diabetes mellitus | 36.5 ± 12.1/37.1 ± 12.3 | N/A |
a. bailing capsule (0.2 g), 1.0 g, PO, tid b. routine treatment of diabetes |
a. tadalafil (20 mg), 5–20 mg, PO, qn b. routine treatment of diabetes |
IIEF-5, testosterone, seminal plasma Zn2+, potency recovery rate |
| Yang 2014b | 30/30 | 12 | ED | 33.10 ± 5.45/32.60 ± 6.73 | N/A | a. Shugan Yiyang capsule (0.25 g), 1.0 g, PO, tid | a. tadalafil, 5 mg, PO, qn | IIEF-5 |
| Zhou 2020 | 56/56 | 12 | ED and chronic prostatitis | 44.2 ± 5.7/43.9 ± 5.1 | kidney yang deficiency |
a. Bushen Tianjing Decoction, PO, bid b. Qianlie Huichun capsule (0.3 g), 1.5 g, PO, bid c. tamsulosin hydrochloride (0.2 mg), 0.2 mg, PO, qd |
a. vardenafil hydrochloride tablets (20 mg), 20 mg/10 mg/5 mg, PO, qod (dose decreased every 4 weeks) b. Qianlie Huichun capsule (0.3 g), 1.5 g, PO, bid c. tamsulosin hydrochloride (0.2 mg), 0.2 mg, PO, qd |
IIEF-5, Erection Quality Scale, potency recovery rate |
| Liu 2012 | 89/60 | 12 | ED and hyperprolactinemia | 21–69 | N/A | a. Shengjing capsule (0.4 g), 1.6 g, PO, tid | a. bromocryptine (2.5 mg), 2.5 mg, PO, bid | IIEF-5, potency recovery rate |
- Abbreviations: bid, twice a day; Biw, twice weekly; C, control group; CHD, coronary atherosclerotic heart disease; E, experimental group; ED, erectile dysfunction; N/A, not applicable; PO, per oral administration; PRN, pro re nata; qd, once a day; qn, once a day before sleep; qod, every other day; tid, three times a day.
3.2 Efficacy assessment of the IIEF-5 score
The IIEF-5 score was set as the primary outcome. Though there was a high degree of heterogeneity, significant improvement was found in the IIEF-5 score after TCM treatment as compared with controls (p < 0.001). Subgroup analysis revealed that different intervention combinations were the partial source of clinical heterogeneity (Figure 3). It turned out that TCM was more efficacious than placebo in improving the IIEF-5 score (p < 0.001), even compared with bromocriptine (p = 0.009). Furthermore, TCM combined with PDE5Is (p < 0.001) or other pharmaceuticals (p < 0.001) also significantly improved erectile function. However, we did not observe any difference between the comparison of TCM and PDE5Is (p = 0.27), but the superior efficacy of TCM came out when eliminating the study with high heterogeneity (Yang 2014b; p = 0.006).
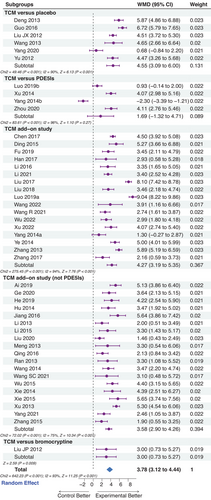
Sensitivity analysis by omitting one study in each turn found that Yang et al. in the first comparison (TCM vs. Placebo), Yang et al. in the second comparison (TCM vs. PDE5Is), Liu et al., and Luo et al. in the third comparison (TCM add-on study), and Liu et al. and Xu et al. in the fourth comparison (TCM add-on study [not PDE5Is]) dominated the clinical heterogeneity. In the first comparison, Yang et al.40 and Wang et al.39 enrolled patients with ED and type 2 diabetes mellitus and did not supply the basic diabetes treatment. However, the patients enrolled in the trial of Yang et al. had a higher fasted plasma glucose level (Yang et al., 8.05 ± 1.32 mmol/L of the experimental group, 8.18 ± 1.09 mmol/L of the control group; Wang et al., 6.80 ± 0.92 mmol/L of the experimental group, 6.71 ± 0.98 mmol/L of the control group), which might influence the efficacy.7 In the second comparison, Yang et al. reported superior efficacy of low-dose daily administration of tadalafil; meanwhile, significant improvement was also achieved by the Shugan Yiyang capsule (IIEF-5, before treatment, 10.13 ± 1.55; after treatment, 15.77 ± 2.05; p < 0.05).81 In the third comparison, Zhang et al.,49 Liu et al.,55 and Luo et al.80 all applied the invigorating blood method, which might be beneficial for vascular ED.25 Since Zhang et al. employed de-escalation therapy in the experimental group, the gap was narrowed. In the fourth comparison, Liu et al.64 and Xu et al.78 both reported impressive efficacy, and we could not find a convincing argument currently.
As age is a recognized factor of ED,1-3 it could account for the heterogeneity. However, we performed a meta-regression analysis based on age, and the results indicated that age might not be the critical source of heterogeneity (p = 0.230); neither the duration (p = 0.272). In addition, we conducted the subgroup analysis based on the kind of comparators (especially the kind of PDE5Is). The results also validated the efficacy of TCM and indicated that the drug used for control or essential treatment might be the source of heterogeneity (test for subgroup differences: χ2 = 112.18, I2 = 95.5%, p < 0.001; Figure S1).
3.3 Efficacy assessment of the clinical recovery rates
The clinical recovery rate was set as the percentage of participants with IIEF-5 scores over 21 after treatment. No significant heterogeneity was observed in the assessment. Substantial improvement in clinical recovery rate by TCM was shown in Figure 4. Interestingly, the clinical recovery rates of Ding et al.45 (50.0%), Wang et al.56 (67.7%), Xu et al.48 (52.2%), Ye et al.58 (63.8%), Zhang et al.49 (82.1%), and Zhou et al.44 (57.1%) were relatively higher. Zhou et al. conducted a head-to-head trial based on the treatment of chronic prostatitis, while the other five trials applied add-on therapy with PDE5Is.
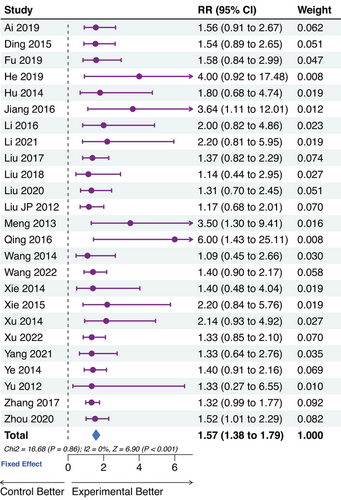
3.4 Efficacy assessment of the testosterone levels
There were 12 trials that reported changes in testosterone levels. High heterogeneity was observed and did not decrease significantly by omitting each trial or applying subgroup analysis based on whether supplementing testosterone was undecanoate. From the data in Figure 5, it was apparent that the overall effect of TCM was beneficial for the improvement of testosterone levels, though three trials40, 63, 77 did not show meaningful changes (p < 0.001). Li et al. and Wu et al. supplemented testosterone undecanoate soft capsules as basic treatment, however, the dosages of the experimental group were lower than the control.
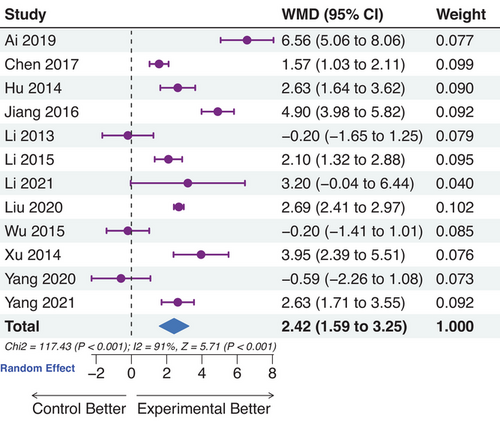
As for the participants in the study of Yang et al., the experimental group achieved a superior effect in improving testosterone levels (p = 0.042), but the lower baseline value of testosterone in the experimental group covered the efficacy (13.50 ± 4.65 nmol/L of the experimental group, 14.40 ± 4.55 nmol/L of the control group). Unexpectedly, the testosterone levels in the trial of Liu et al.65 were lower than the lower limit of the normal level, which could be due to the longer course or serious condition of diabetes.85
3.5 Safety assessment
Safety assessment was operated by calculating the percentage of participants reporting adverse events. There were 21 trials involving adverse events, 13 not reporting adverse events, and 13 not mentioning adverse events (Table S2). Only one adverse event reportedly resulted in the dropout due to the control intervention.49 Among the trials reporting adverse events, no significant difference was observed between the experimental and control groups (p = 0.12; Figure 6). Particularly in the trial of Zhou et al.,44 nearly half of the participants reported slight adverse events which did not need special treatment.
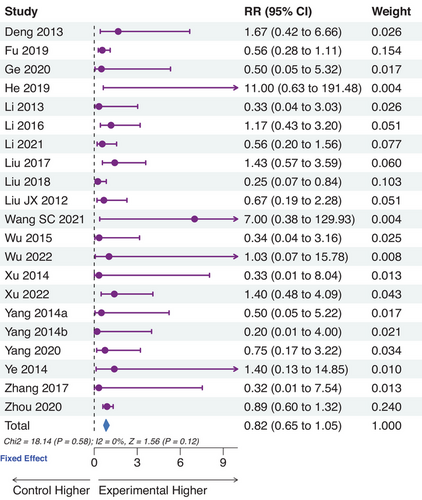
3.6 Publication bias
Publication bias was preliminarily assessed using funnel plots (Figure S2), and no significant publication bias was observed based on Begg's test (p = 0.252). The result of Egger's test indicated the existence of publication bias (P < 0.01), thus, we used the trim and fill method to correct the bias. After correction, we got the effect of 5.10 (95% CI: 4.43, 5.78; P < 0.001) to support the efficacy (Figure S3).
3.7 Trial sequential analysis
We performed TSA to determine the primary outcome's robustness and calculate the RIS. The results of IIEF-5 showed that the optimal sample size required for a credible conclusion of the beneficial effect of TCM treatment on ED was 634 participants, which was exceeded by the participants enrolled in the current study. Thus, the cumulative Z-curve crossed both the conventional and TSA boundaries (Figure 7A). Subgroup analyses were also conducted, and all the results indicated that the cumulative evidence is reliable and authentic (Figure 7B–D).
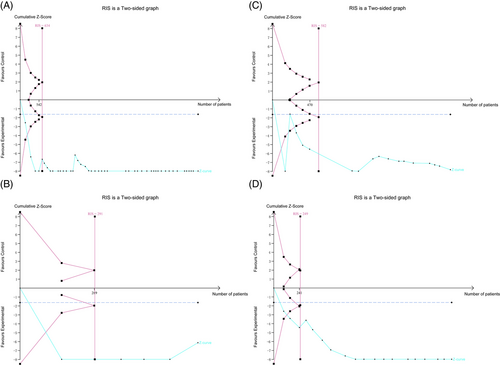
4 DISCUSSION
As mentioned in the literature review, several reports have indicated that TCM can ameliorate ED. The present study was designed to determine the efficacy and safety of TCM (or a combination of TCM and Western medicine) in treating ED. The most important clinically relevant finding was TCM can increase the IIEF-5 scores, clinical recovery rates, and testosterone levels of patients with ED. Surprisingly, TCM was found to improve erectile function under all the conditions of single-use, combined with PDE5Is, and combined with pharmaceuticals for complications or comorbidities.
The trials of the current study were assigned into five subgroups. The comparison between TCM and placebo indicated the absolute effect of TCM, though the heterogeneity was high. Further head-to-head trials provided solid evidence of certain efficacy and safety. The integrative therapy of on-demand PDE5Is and regular TCM administration is one of the most common therapies for ED in China currently.22 In accordance with the present results, a previous meta-analysis has validated the efficacy and safety of TCM combined with tadalafil in treating ED.29 Furthermore, the efficacy of oral Chinese patent medicine combined with western medicine for chronic prostatitis/chronic pelvic pain syndrome with sexual dysfunction was also accepted.83 Particularly, Zhang et al.49 applied the low-dose de-escalation therapy to reduce the dose of tadalafil for better efficacy and lower financial burden. Whether the efficacy of add-on therapy origins from the improvement of the tolerability profile under prolonged use of PDE5Is or the additive or even synergistic effect remains unknown. As for the combination with pharmaceuticals for complications or comorbidities, type 2 diabetes mellitus and diabetic nephropathy, hyperuricemia, late-onset hypogonadism, hypertension, and benign prostatic hyperplasia are involved. Several lines of evidence suggest that diabetes,7, 84, 85 hypogonadism,86, 87 hypertension,88 and benign prostatic hyperplasia89, 90 are all closely associated with ED, and the corresponding treatment or combination therapy could alleviate ED.90-92 As for hyperuricemia, a strong association with ED is only observed in patients with type 2 diabetes mellitus.93 Whether hyperuricemia is an independent predictor of ED remains controversial.94, 95
We have summarized involving TCM prescriptions in Table 2. The TCM prescriptions could be classified as TCM patent drugs, classic formulas, and empirical formulas. As can be seen from Table 1, several trials set the criteria for TCM syndrome. Correspondingly, we have presented the therapeutic functions in Table 2, which mainly converged on warming and supplementing kidney yang, supplementing the kidney essence, soothing the liver, boosting qi, and invigorating blood. However, some of the trials did not mention or set criteria for the TCM syndrome in which the prescriptions were created based on the understanding of the TCM disease mechanism of ED. As the TCM syndrome differentiations differ, the prescriptions diverge, not to say the intervention mechanism. The PDE5 pathway,96 Protein Kinase C and Protein Kinase C β pathways,25, 97 oxidative stress and endoplasmic reticulum stress-induced apoptosis pathways,26 endothelial nitric oxide synthase dysfunction and fibrosis,98 and testosterone secretion99 all reportedly associated with the amelioration of erectile function by TCM intervention.
| Study ID | TCM prescriptions | Components | Therapeutic functions |
|---|---|---|---|
| Ai 2019 | Yishen Huoxue Decoction | Dihuang (Rehmanniae Radix), Shanzhuyu (Corni Fructus), Shanyao (Dioscoreae Rhizoma), Yimucao (Leonuri Herba), Niuxi (Achyranthis Bidentatae Radix), Tusizi (Cuscutae Semen), Roucongrong (Cistanches Herba), Nvzhenzi (Ligustri lucidi Fructus), Gouqizi (Lycii Fructus), Ziheche (Placenta Hominis), Chuanxiong (Chuanxiong Rhizoma), Danggui (Angelicae Sinensis Radix). | supplement kidney and essence, invigorate blood, and dissolve stasis |
| Chen 2017 | Bushen Huanyang Decoction | Roucongrong (Cistanches Herba), Gouji (Cibotii Rhizoma), Sangjisheng (Taxilli Herba), Tusizi (Cuscutae Semen), Dangshen (Codonopsis Radix), Xuanshen (Scrophulariae Radix), Yangqishi (Actinolite), Yinyanghuo (Epimedii Folium), Niuxi (Achyranthis Bidentatae Radix), Gouqizi (Lycii Fructus), Nüzhenzi (Ligustri lucidi Fructus), Xuduan (Dipsaci Radix), Gancao (Glycyrrhizae Radix et Rhizoma), Dihuang (Rehmanniae Radix), Shanzhuyu (Corni Fructus). | supplement kidney and essence |
| Deng 2013 | Wuzi Yanzong capsule | Gouqizi (Lycii Fructus), Tusizi (Cuscutae Semen), Fupenzi (Rubi Fructus), Cheqianzi (Plantaginis Semen), Wuweizi (Schisandrae Chinensis Fructus), Lujiaojiao (Cervi Cornus Colla), Niuxi (Achyranthis Bidentatae Radix), Shuizhi (Hirudo), Shechuangzi (Cnidii Fructus), Shanyao (Dioscoreae Rhizoma), Fuling (Poria), Gancao (Glycyrrhizae Radix et Rhizoma). | supplement kidney and essence |
| Ding 2015 | Congrong Yishen granule | Roucongrong (Cistanches Herba), Bajitian (Morindae Officinalis Radix), Tusizi (Cuscutae Semen), Wuweizi (Schisandrae Chinensis Fructus), Fuling (Poria), Cheqianzi (Plantaginis Semen). | supplement kidney and essence |
| Fu 2019 | Jiajian Xiaoyaosan | Danggui (Angelicae Sinensis Radix), Baishao (Paeoniae Radix Alba), Chaihu (Bupleuri Radix), Zhishi (Aurantii Fructus Immaturus), Bohe (Menthae Haplocalycis Herba), Xiangfu (Cyperi Rhizoma), Zhizi (Gardeniae Fructus), Yujin (Curcumae Radix), Wangbuliuxing (Vaccariae Semen), Chuanxiong (Chuanxiong Rhizoma), Gancao (Glycyrrhizae Radix et Rhizoma). | soothe the liver and resolve constraint |
| Ge 2020 | Quyu Huatan Erxian Decoction | Yiyiren (Coicis Semen), Cheqianzi (Plantaginis Semen), Zhebeimu (Fritillariae Thunbergii Bulbus), Dilong (Pheretima), Yinyanghuo (Epimedii Folium), Xianmao (Curculiginis Rhizoma ) | dissolve phlegm, drain dampness and dispel stasis, fortify the spleen, warm the kidney, and assist yang |
| Guo 2016 | Zhenyang Decoction | Yinyanghuo (Epimedii Folium), Wugong (Scolopendra), Dihuang (Rehmanniae Radix), Gouqizi (Lycii Fructus), Shanzhuyu (Corni Fructus), Guijiajiao (Testudinis Carapacis Et Plastri Colla), Tusizi (Cuscutae Semen), Danggui (Angelicae Sinensis Radix), Honghua (Carthami Flos), Danshen (Salviae Miltiorrhizae Radix et Rhizoma), Niuxi (Achyranthis Bidentatae Radix), Chenpi (Citri reticulatae Pericarpium), Xiangfu (Cyperi Rhizoma), Chaihu (Bupleuri Radix), Gancao (Glycyrrhizae Radix et Rhizoma) | supplement kidney, invigorate blood, and rectify qi |
| Han 2017 | Yougui Pill | Dihuang (Rehmanniae Radix), Fuzi (Aconiti Lateralis Radix Praeparata), Rougui (Cinnamomi Cortex), Shanyao (Dioscoreae Rhizoma), Shanzhuyu (Corni Fructus), Tusizi (Cuscutae Semen), Lujiaojiao (Cervi Cornus Colla), Gouqizi (Lycii Fructus), Danggui (Angelicae Sinensis Radix), Duzhong (Eucommiae Cortex) | warm the kidney and supplement yang |
| He 2019 | Compound Xuanju capsule | Heimayi (Polyrhachis vicina Roger), Yinyanghuo (Epimedii Folium), Gouqizi (Lycii Fructus), and Shechuangzi (Cnidii Fructus). | warm the kidney, assist the yang, and supplement the essence |
| Hu 2014 | Jiawei Buyang Huanwu Decoction | Huangqi (Astragali Radix), Chuanxiong (Chuanxiong Rhizoma), Taoren (Persicae Semen), Honghua (Carthami Flos), Danggui (Angelicae Sinensis Radix), Chishao (Paeoniae Radix Rubra), Dilong (Pheretima), Chaihu (Bupleuri Radix), Dangshen (Codonopsis Radix), Dihuang (Rehmanniae Radix), Shanyao (Dioscoreae Rhizoma), Shanzhuyu (Corni Fructus), Gegen (Puerariae Lobatae Radix), Sanqi (Notoginseng Radix Et Rhizoma) | boost qi, invigorate blood, and unblock the collaterals |
| Jiang 2016 | Xuefu Zhuyu tablets | Taoren (Persicae Semen), Honghua (Carthami Flos), Danggui (Angelicae Sinensis Radix), Chishao (Paeoniae Radix Rubra), Dihuang (Rehmanniae Radix), Chuanxiong (Chuanxiong Rhizoma), Zhiqiao (Aurantii Fructus), Jiegeng (Platycodonis Radix), Chaihu (Bupleuri Radix), Niuxi (Achyranthis Bidentatae Radix), Gancao (Glycyrrhizae Radix et Rhizoma) | invigorate blood and dissolve stasis |
| Li 2013 | Compound Xuanju capsule | Heimayi (Polyrhachis vicina Roger), Yinyanghuo (Epimedii Folium), Gouqizi (Lycii Fructus), and Shechuangzi (Cnidii Fructus). | warm the kidney, assist the yang, and supplement the essence |
| Li 2015 | Qiyang Zhuyu Decoction | Renshen (Ginseng Radix et Rhizoma), Roucongrong (Cistanches Herba), Yinyanghuo (Epimedii Folium), Cheqianzi (Plantaginis Semen), Zhimu (Anemarrhenae Rhizoma), Huangbo (Phellodendri Chinensis Cortex), Zexie (Alismatis Rhizoma), Chishao (Paeoniae Radix Rubra), Danggui (Angelicae Sinensis Radix), Chuanxiong (Chuanxiong Rhizoma), Puhuang (Typhae Pollen), Ruxiang (Olibanum), Moyao (Myrrha), Wulingzhi (Faeces Togopteri), Yanhusuo (Corydalis Rhizoma) | unblock the collaterals and assist yang, clear heat, and drain dampness |
| Li 2016 | Zhuangyang Decoction | Baishao (Paeoniae Radix Alba), Huangqi (Astragali Radix), Shanzhuyu (Corni Fructus), Baizhu (Atractylodis Macrocephalae Rhizoma), Yinyanghuo (Epimedii Folium), Chaihu (Bupleuri Radix), Hehuanpi (Albiziae Cortex), Suanzaoren (Ziziphi Spinosae Semen), Danggui (Angelicae Sinensis Radix), Dangshen (Codonopsis Radix). | supplement the kidney, soothe the liver, and invigorate blood |
| Li 2021 | Xianlu Oral Liquid | Tusizi (Cuscutae Semen), Maidong (Ophiopogonis Radix), Yinyanghuo (Epimedii Folium), Lujiaojiao (Cervi Cornus Colla), Dihuang (Rehmanniae Radix), Gouqizi (Lycii Fructus), Guijiajiao (Testudinis Carapacis Et Plastri Colla), Huangjing (Rhizoma polygonati), Nüzhenzi (Ligustri lucidi Fructus), Zexie (Alismatis Rhizoma), Renshen (Ginseng Radix et Rhizoma), Shanyao (Dioscoreae Rhizoma) | enrich yin and supplement the kidney, supplement essence and boost marrow |
| Liu 2012 | Shengjing capsule | Lurong (Cervi Cornu Pantotrichum), Gouqizi (Lycii Fructus), Renshen (Ginseng Radix et Rhizoma), Dongchongxiacao (Cordyceps), Tusizi (Cuscutae Semen), Shayuanzi (Astragali Complanati Semen), Yinyanghuo (Epimedii Folium), Huangjing (Rhizoma polygonati), Heshouwu (Polygoni Multiflori Radix), Sangshen (Mori Fructus), Buguzhi (Psoraleae Fructus), Gusuibu (Drynariae Rhizoma), Xianmao (Curculiginis Rhizoma ), Jinyingzi (Rosae Laevigatae Fructus), Fupenzi (Rubi Fructus), Duzhong (Eucommiae Cortex), Daxueteng (Sargentodoxae Caulis), Mabiancao (Verbenae Herba), Yinxingye (Ginkgo Folium) | warm and supplement kidney yang, enrich kidney yin and essence, invigorate blood and dissolve stasis |
| Liu 2012 | Wenshen Huoxue Decoction | Yinyanghuo (Epimedii Folium), Tusizi (Cuscutae Semen), Rougui (Cinnamomi Cortex), Duzhong (Eucommiae Cortex), Danggui (Angelicae Sinensis Radix), Wulingzhi (Faeces Togopteri), Chuanxiong (Chuanxiong Rhizoma), Dilong (Pheretima), Yinyanghuo (Epimedii Folium). | warm the kidney and invigorate blood |
| Liu 2017 | Wenshen Huoxue Tongluo Decoction | Huangqi (Astragali Radix), Yinyanghuo (Epimedii Folium), Tusizi (Cuscutae Semen), Chuanniuxi (Cyathulae Radix), Rougui (Cinnamomi Cortex), Duzhong (Eucommiae Cortex), Danggui (Angelicae Sinensis Radix), Chuanxiong (Chuanxiong Rhizoma), Dilong (Pheretima), Chishao (Paeoniae Radix Rubra), Shanyao (Dioscoreae Rhizoma), Taoren (Persicae Semen), Wulingzhi (Faeces Togopteri), Honghua (Carthami Flos), Wugong (Scolopendra) | supplement the kidney and boost qi, invigorate blood, and unblock the collaterals |
| Liu 2018 | Canrong Zhutian capsules | Can'e (Bombycidae), Yinyanghuo (Epimedii Folium), Bajitian (Morindae Officinalis Radix), Dihuang (Rehmanniae Radix), Shanyao (Dioscoreae Rhizoma), Shanzhuyu (Corni Fructus), Gouqizi (Lycii Fructus), Tusizi (Cuscutae Semen), Lurong (Cervi Cornu Pantotrichum), Duzhong (Eucommiae Cortex), Danggui (Angelicae Sinensis Radix), Rougui (Cinnamomi Cortex), Fuzi (Aconiti Lateralis Radix Praeparata), Wugong (Scolopendra), Tianma (Gastrodiae Rhizoma), Renshen (Ginseng Radix et Rhizoma), Lushen (Penis et Testis Cervi) | warm and supplement kidney yang, supplement essence, and blood |
| Liu 2020 | Jinshuibao capsule | Dongchongxiacao (Cordyceps). | supplement the lung, kidney, and essential qi |
| Luo 2019 | Xuefu Zhuyu tablets | Chaihu (Bupleuri Radix), Danggui (Angelicae Sinensis Radix), Dihuang (Rehmanniae Radix), Chishao (Paeoniae Radix Rubra), Honghua (Carthami Flos), Taoren (Persicae Semen), Zhiqiao (Aurantii Fructus), Gancao (Glycyrrhizae Radix et Rhizoma), Chuanxiong (Chuanxiong Rhizoma), Niuxi (Achyranthis Bidentatae Radix), Jiegeng (Platycodonis Radix) | invigorate blood and dissolve stasis |
| Meng 2013 | Bushen Yixin Decoction | Gouteng (Uncariae Ramulus Cum Uncis), Yinyanghuo (Epimedii Folium), Cheqianzi (Plantaginis Semen), Shijueming (Haliotidis Concha), Shengtieluo (Frusta Ferri), Guijia (Testudinis Carapax), Niuxi (Achyranthis Bidentatae Radix), Yuanzhi (Polygalae Radix), Shihu (Dendrobii Caulis), Shouwuteng (Polygoni Multiflori Caulis), Huashi (Talcum), Rendongteng (Lonice Raejaponicae Caulis), Zhuru (Bambusae Caulis In Taenias), Gancao (Glycyrrhizae Radix et Rhizoma) | supplement essence and assist yang, drain dampness and dissolve stasis |
| Qing 2016 | Bushen Yixin tablet | Yinyanghuo (Epimedii Folium), Cheqianzi (Plantaginis Semen) | warm and supplement kidney yang, clear liver heat, and promote urination |
| Ran 2013 | Bushen Jiangya Formula | Sangjisheng (Taxilli Herba), Nvzhenzi (Ligustri lucidi Fructus), Mohanlian (Ecliptae Herba), Xianmao (Curculiginis Rhizoma ), Yinyanghuo (Epimedii Folium), Yimucao (Leonuri Herba), Zexie (Alismatis Rhizoma), Xixiancao (Siegesbeckiae Herba), Digupi (Lycii Cortex), Duzhong (Eucommiae Cortex) | supplement kidney |
| Wang 2013 | Tongxinluo capsule | Renshen (Ginseng Radix et Rhizoma), Shuizhi (Hirudo), Quanxie (Scorpio), Chishao (Paeoniae Radix Rubra), Chantui (Cicadae Periostracum), Tubiechong (Eupolyphaga Steleophaga), Wugong (Scolopendra), Tanxiang (Santali Albi Lignum), Jiangxiang (Dalbergiae odoriferae lignum), Ruxiang (Olibanum), Suanzaoren (Ziziphi Spinosae Semen), Tianranbingpian (Borneolum) | boost qi and invigorate blood, unblock the collaterals, and relieve pain |
| Wang 2014 | Jinshuibao capsule | Dongchongxiacao (Cordyceps) | supplement the lung, kidney, and essential qi |
| Wang 2022 | Shugan Qiwei Decoction | Chaihu (Bupleuri Radix), Baishao (Paeoniae Radix Alba), Danggui (Angelicae Sinensis Radix), Chuanxiong (Chuanxiong Rhizoma), Zhiqiao (Aurantii Fructus), Chenpi (Citri reticulatae Pericarpium), Xiangfu (Cyperi Rhizoma), Gancao (Glycyrrhizae Radix et Rhizoma), Shichangpu (Acori Tatarinowii Rhizoma), Yuanzhi (Polygalae Radix), Yujin (Curcumae Radix), Jili (Tribuli Fructus), Yinyanghuo (Epimedii Folium), Xianmao (Curculiginis Rhizoma) | soothe the liver and resolve constraints, assist yang |
| Wang 2021 | Shanhaidan granule | Sanqi (Notoginseng Radix Et Rhizoma), Renshen (Ginseng Radix et Rhizoma), Huangqi ( Astragali Radix), Honghua (Carthami Flos), Shanyangxue(the blood of goats), Juemingzi (Cassiae Semen), Gegen (Puerariae Lobatae Radix), Foshou (Citri sarcodactylis Fructus), Haizao (Sargassum), Heshouwu (Polygoni Multiflori Radix), Danshen (Salviae Miltiorrhizae Radix et Rhizoma), Chuanxiong (Chuanxiong Rhizoma), Maidong (Ophiopogonis Radix), Lingzhi (Ganoderma), Xiangfu (Cyperi Rhizoma), Puhuang (Typhae Pollen) | invigorate blood and dissolve stasis |
| Wang 2021 | Bushen Yixin tablet | Yinyanghuo (Epimedii Folium), Cheqianzi (Plantaginis Semen). | warm and supplement kidney yang, clear liver heat, and promote urination |
| Wu 2015 | Shenrong Qiangshen tablets | Renshen (Ginseng Radix et Rhizoma), Lurong (Cervi Cornu Pantotrichum), Lushen (Penis et Testis Cervi), Niushen (Yak whip), Haigoushen (Ursine Seal's Penis and Teste), Huangqi (Astragali Radix), Danggui (Angelicae Sinensis Radix), Roucongrong (Cistanches Herba), Yangqishi (Actinolite), Gouqizi (Lycii Fructus), Duzhong (Eucommiae Cortex), Fuzi (Aconiti Lateralis Radix Praeparata), Tusizi (Cuscutae Semen), Dihuang (Rehmanniae Radix), Yinyanghuo (Epimedii Folium), Jiucaizi (Allii Tuberosi Semen) | warm the kidney, assist the yang, and supplement the essence |
| Wu 2022 | Bushen Shugan Xingyang Decoction | Chaihu (Bupleuri Radix), Baishao (Paeoniae Radix Alba), Jinjuye (Oval kumquat leaf), Jili (Tribuli Fructus), Jiuxiangchong (Aspongopus), Suanzaoren (Ziziphi Spinosae Semen), Dihuang (Rehmanniae Radix), Dihuang (Rehmanniae Radix), Shanzhuyu (Corni Fructus), Shanyao (Dioscoreae Rhizoma), Yinyanghuo (Epimedii Folium), Tusizi (Cuscutae Semen), Yujin (Curcumae Radix), Wugong (Scolopendra), Chuanniuxi (Cyathulae Radix) | supplement the kidney, soothe the liver, and assist the yang |
| Xie 2014 | Bushen Huoxue Decoction | Fuzi (Aconiti Lateralis Radix Praeparata), Duzhong (Eucommiae Cortex), Shanzhuyu (Corni Fructus), Dihuang (Rehmanniae Radix), Chishao (Paeoniae Radix Rubra), Danshen (Salviae Miltiorrhizae Radix et Rhizoma), Shuizhi (Hirudo), Dangshen (Codonopsis Radix), Huangqi (Astragali Radix), Fuling (Poria) | supplement the kidney and fortify the spleen, invigorate blood, and dissolve stasis |
| Xie 2015 | Bushen Huoxue Decoction | Fuzi (Aconiti Lateralis Radix Praeparata), Duzhong (Eucommiae Cortex), Shanzhuyu (Corni Fructus), Dihuang (Rehmanniae Radix), Chishao (Paeoniae Radix Rubra), Danshen (Salviae Miltiorrhizae Radix et Rhizoma), Shuizhi (Hirudo), Dangshen (Codonopsis Radix), Huangqi (Astragali Radix), Fuling (Poria) | supplement the kidney and fortify the spleen, invigorate blood, and dissolve stasis |
| Xu 2013 | Shugan Yiyang capsule | Jili (Tribuli Fructus), Chaihu (Bupleuri Radix), Fengfang (Vespae Nidus), Dilong (Pheretima), Shuizhi (Hirudo), Jiuxiangchong (Aspongopus), Zishaohua (Spongilla fragills), Shechuangzi (Cnidii Fructus), Yuanzhi (Polygalae Radix), Roucongrong (Cistanches Herba), Tusizi (Cuscutae Semen), Wuweizi (Schisandrae Chinensis Fructus), Bajitian (Morindae Officinalis Radix), Wugong (Scolopendra), Shichangpu (Acori Tatarinowii Rhizoma) | soothe the liver and resolve constraint, invigorate blood and unblock the collaterals, raise yang and improve impotence |
| Xu 2014 | Bailing capsule | Dongchongxiacao (Cordyceps). | supplement the lung, kidney, and essential qi |
| Xu 2022 | Jinjie tablet | Jinyingzi (Rosae Laevigatae Fructus), Gejie (Gecko), Yinyanghuo (Epimedii Folium), Jiucaizi (Allii Tuberosi Semen), Shanzhuyu (Corni Fructus). | warm the kidney, assist the yang, and consolidate the essence |
| Yang 2014 | Shugan Yiyang capsule | Jili (Tribuli Fructus), Chaihu (Bupleuri Radix), Fengfang (Vespae Nidus), Dilong (Pheretima), Shuizhi (Hirudo), Jiuxiangchong (Aspongopus), Zishaohua (Spongilla fragills), Shechuangzi (Cnidii Fructus), Yuanzhi (Polygalae Radix), Roucongrong (Cistanches Herba), Tusizi (Cuscutae Semen), Wuweizi (Schisandrae Chinensis Fructus), Bajitian (Morindae Officinalis Radix), Wugong (Scolopendra), Shichangpu (Acori Tatarinowii Rhizoma) | soothe the liver and resolve constraint, invigorate blood and unblock the collaterals, raise yang, and improve impotence |
| Yang 2020 | Qiyao Xiaoke oral liquid | Gouqizi (Lycii Fructus), Tianhuafen (Trichosanthis Radix), Dihuang (Rehmanniae Radix), Shanyao (Dioscoreae Rhizoma), Shanzhuyu (Corni Fructus), Renshen (Ginseng Radix et Rhizoma), Huanglian (Coptidis Rhizoma), Shigao (Gypsum Fibrosum), Gancao (Glycyrrhizae Radix et Rhizoma). | boost qi and nourish yin |
| Yang 2021 | Buyang Huanwu Decoction | Shanzhuyu (Corni Fructus), Huangqi (Astragali Radix), Taoren (Persicae Semen), Danggui (Angelicae Sinensis Radix), Shanyao (Dioscoreae Rhizoma), Honghua (Carthami Flos), Chishao (Paeoniae Radix Rubra), Dilong (Pheretima), Chaihu (Bupleuri Radix), Dangshen (Codonopsis Radix), Chuanxiong (Chuanxiong Rhizoma), Dihuang (Rehmanniae Radix), Gegen (Puerariae Lobatae Radix), Sanqi (Notoginseng Radix Et Rhizoma). | boost qi, invigorate blood, and unblock the collaterals |
| Ye 2014 | Liuwei Dihuang Pill | Dihuang (Rehmanniae Radix), Shanzhuyu (Corni Fructus), Mudanpi (Moutan Cortex), Shanyao (Dioscoreae Rhizoma), Fuling (Poria), Zexie (Alismatis Rhizoma) | enrich yin and supplement kidney |
| Yu 2012 | Yikan capsule | Wugong (Scolopendra), Danggui (Angelicae Sinensis Radix), Baishao (Paeoniae Radix Alba), Chuanxiong (Chuanxiong Rhizoma), Chaihu (Bupleuri Radix), Niuxi (Achyranthis Bidentatae Radix). | soothe the liver and resolve constraint |
| Zhang 2013 | Jiawei Sinisan | Zishiying (Fluoritum), Jili (Tribuli Fructus), Huangqi (Astragali Radix), Zhiqiao (Aurantii Fructus), Baishao (Paeoniae Radix Alba), Danggui (Angelicae Sinensis Radix), Chaihu (Bupleuri Radix), Niuxi (Achyranthis Bidentatae Radix), Tusizi (Cuscutae Semen), Yinyanghuo (Epimedii Folium), Fengfang (Vespae Nidus), Wugong (Scolopendra) | soothe the liver and rectify qi, invigorate blood, and unblock the collaterals |
| Zhang 2015 | Jinkui Shenqi Pill | Dihuang (Rehmanniae Radix), Shanyao (Dioscoreae Rhizoma), Shanzhuyu (Corni Fructus), Fuling (Poria), Mudanpi (Moutan Cortex), Zexie (Alismatis Rhizoma), Guizhi (Cinnamomi Ramulus), Fuzi (Aconiti Lateralis Radix Praeparata). | warm and supplement kidney yang |
| Zhang 2017 | Hongjing Yihao Formula | Hongjingtian (Rhodiolae Crenulatae Radix et Rhizoma), Huangqi (Astragali Radix), Dangshen (Codonopsis Radix), Gouqizi (Lycii Fructus), Niuxi (Achyranthis Bidentatae Radix), Danshen (Salviae Miltiorrhizae Radix et Rhizoma). | boost qi and invigorate blood |
| Zhou 2020 | Bushen Tianjing Decoction | Dihuang (Rehmanniae Radix), Gouqizi (Lycii Fructus), Shanyao (Dioscoreae Rhizoma), Fuling (Poria), Bajitian (Morindae Officinalis Radix), Dangshen (Codonopsis Radix), Buguzhi (Psoraleae Fructus), Xianmao (Curculiginis Rhizoma), Yinyanghuo (Epimedii Folium), Shanzhuyu (Corni Fructus), Fengfang (Vespae Nidus), Shechuangzi (Cnidii Fructus). | supplement kidney and essence |
These findings may be somewhat limited by the intervention duration, the subtypes of ED both in modern medicine and TCM, and the therapy combinations. We have set 12 weeks as the minimum treatment course based on previous clinical trials that applied PDE5Is to treat ED in China.103, 104 Whether longer treatment could achieve clinical benefits without increasing adverse events remains unknown. Besides, there is not an international or widely accepted standardized TCM syndrome differentiation of ED, and several complications or comorbidities were involved in the current study, which has caused clinical heterogeneity. Nevertheless, we validated the potential roles of TCM in treating ED to address the objective of the current study. The clinical application of TCM needs more trials to choose suitable prescriptions for different TCM syndromes, different stages of disease progression, and so forth. Furthermore, the enrolled trials in some subgroups have limited sample sizes; therefore, these results need to be interpreted cautiously. However, we have applied the TSA to determine the robustness of the outcome, and the cumulative evidence is reliable and authentic.
5 CONCLUSION
In general, these findings suggest that TCM can gain better responses in improving IIEF-5 scores, clinical recovery rates, and testosterone levels as an alternative and complementary treatment, with no increase in side effects. To enrich the therapeutic applications for ED with higher creditability, long-term efficacy, and safety assessment including within treatment and after drug discontinuation, standardized TCM, as well as integrative medicine diagnosis and treatment guidelines of ED, will be needed.
AUTHOR CONTRIBUTIONS
Haoran Wu drafted the manuscript. Xuemei Chen and Qi Wang reviewed the research and manuscript. Zezheng Gao, Xing Liu, and Yini Fang searched the literature and extracted data. Haoran Wu and Zezheng Gao made statistical analyses of all the data. Haoran Wu and Dan Dai conducted data visualization and revised the manuscript. All of the authors participated in the design and approved the final manuscript, approving the published version and agreeing to be accountable for the accuracy and integrity.
ACKNOWLEDGMENTS
The authors thank all the researchers of every trial involved above for their outstanding work.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING INFORMATION
This work was partially supported by the National Social Science Fund of China (No. 17ZDA331), Beijing Tong Ren Tang (Bozhou) Master of TCM Inheritance Studio Construction Program, and the Commission Project of Shenzhen Municipal Health Commission (No. 2020A00113). The design, management, analysis, and reporting of the study are independent of the funding programs.
Open Research
DATA AVAILABILITY STATEMENT
The data supporting this study's findings are included in the article.