The future direction of imaging in prostate cancer: MRI with or without contrast injection
Funding information
This research did not receive any specific grant from funding agencies in the Public, Commercial, or not-for-profit sectors.
Abstract
Background
Multiparametric MRI (mpMRI) is the “state of the art” management tool for patients with suspicion of prostate cancer (PCa). The role of non-contrast MRI is investigated to move toward a more personalized, less invasive, and highly cost-effective PCa diagnostic workup.
Objective
To perform a non-systematic review of the existing literature to highlight strength and flaws of performing non-contrast MRI, and to provide a critical overview of the international scientific production on the topic.
Materials and Methods
Online databases (Medline, PubMed, and Web of Science) were searched for original articles, systematic review and meta-analysis, and expert opinion papers.
Results
Several investigations have shown comparable diagnostic accuracy of biparametric (bpMRI) and mpMRI for the detection of PCa. The advantage of abandoning contrast-enhanced sequences improves operational logistics, lowering costs, acquisition time, and side effects. The main limitations of bpMRI are that most studies comparing non-contrast with contrast MRI come from centers with high expertise that might not be reproducible in the general community setting; besides, reduced protocols might be insufficient for estimation of the intra- and extra-prostatic extension and regional disease. The mentioned observations suggest that low-quality mpMRI for the general population might represent the main shortage to overcome.
Discussion
Non-contrast MRI future trends are likely represented by PCa screening and the application of artificial intelligence (AI) tools. PCa screening is still a controversial topic; bpMRI has become one of the most promising diagnostic applications, as it is a more sensitive test for PCa early detection, compared to serum PSA level test. Also, AI applications and radiomic have been the object of several studies investigating PCa detection using bpMRI, showing encouraging results.
Conclusion
Today, the accessibility to MRI for early detection of PCa is a priority. Results from prospective, multicenter, multireader, and paired validation studies are needed to provide evidence supporting its role in the clinical practice.
1 INTRODUCTION
Prostate cancer (PCa) is becoming a global health major issue: it is the second most common cancer in men1 and its prevalence surges with aging.2 PCa incidence differs considerably in distinct geographical areas. Australia/New Zealand and Northern America show the highest incidence (age-standardized rates [ASR] per 100,000 of 111.6 and 97.2, respectively), followed by Western and Northern Europe (ASRs of 94.9 and 85, respectively), this is probably due to the aging population and the spread of prostate-specific antigen (PSA) testing. Oppositely, Eastern and South Central Asia present the lowest incidence of PCa (ASRs of 10.5 and 4.5, respectively). Nonetheless, other regions, especially Eastern and Southern Europe, are facing a steady incidence increase.3, 4 PCa is an insidious pathology, mostly due to its multifactorial etiology and as such should be correctly diagnosed at an early stage, since if diagnosed at an advanced stage it is usually aggressive requiring multimodal therapy.5 Indeed, there is a significant variability in the behavior of prostate cancer. Clinically significant prostate cancer (csPCa) is often aggressive and potentially metastatic and requires early detection; instead, clinically insignificant prostate cancer (ciPCa) is a low-grade indolent neoplasm, that rarely progresses and that can be safely treated with active surveillance (AS) strategies. At the present time the most accepted criteria for the definition of ciPCa are the ones provided by the European Association of Urology (EAU) guidelines, which include: (1) organ-confined Gleason 3+3 tumors, with no grade 4 or 5; (2) when specified <2–3 positive cores with <50% cancer involvement in every positive core; (3) a clinical-stage T1c or T2a; (4) a PSA <10 ng/mL and a PSA density <0.15 ng/mL/cc (albeit the latter criterion remains controversial).6
Multiparametric MRI (mpMRI), plays a fundamental role in this scenario, as in other fields of oncological imaging.7-11 MRI provides clinicians a non-invasive tool to detect, localize, and stage PCa, allowing prostate biopsy planning. Indeed, mpMRI scored using the PI-RADS v2.1 is recommended as first-line study in biopsy naïve patients by the EAU guidelines.6, 7 The aim of this non-systematic review was to highlight the strength and flaws of performing non-contrast MRI and to provide a critical overview of the international scientific production on the topic.
2 EVIDENCE ACQUISITION
This non-systematic review was performed using existing literature on the role of non-contrast or biparametric MRI for prostate cancer detection. Online databases (Medline, PubMed, and Web of Science) were searched for original articles, systematic review and meta-analysis, and expert opinion papers.
3 PROSTATE CANCER AND CURRENT UROLOGICAL GUIDELINES ON IMAGING
It is worldwide recognized that MRI is the most appropriate imaging tool for the detection of PCa. In this paragraph, the most important guidelines on MRI recommendations for PCa detection are described.
3.1 European Association of Urology (EAU)
Albeit EAU guidelines6 do not recommend mpMRI as first screening step for PCa diagnosis, it recommends mpMRI as first-line study in naïve patients with suspicion of PCa, before considering prostate biopsy, following the PI-RADS recommendations for mpMRI acquisition and interpretation. Naïve patients should undergo targeted and systematic prostate biopsy, when mpMRI is positive, with a PI-RADS score ≥3. When negative (PI-RADS score ≤2) without any clinical suspicion of PCa exist, prostate biopsy is not recommended. Patients who have already undergone prostate biopsies, with negative results, must undergo MRI-targeted biopsy whenever mpMRI is positive.
3.2 National Comprehensive cancer Network (NCCN)
According to NCCN recommendations, mpMRI should be considered before performing transrectal ultrasound-guided biopsies (TRUS-GB), to accurately characterize prostatic tissue and possible neoplastic foci, to guide the procedural planning. However, NCCN panel affirms that the available data are not consistent enough to recommend a generalized use of pre-biopsy mpMRI, mostly due to the lack of an appropriate inter-reader variability. Furthermore, they state that MRI-guided targeted biopsies may be recommended in centers where MRI is available and where there is a high expertise among radiologists in prostate mpMRI reading. NCCN panel highlights the importance of mpMRI to approach high-risk patients with a prior negative prostate biopsy, to efficiently rule-out csPCa. Furthermore, MRI and MRI-targeted biopsies are proving extremely promising performances; however, the NCCN guidelines state that a combination of MRI-targeted biopsy with the systematic technique can better detect csPCa, with higher sensitivity. Nonetheless, the topic is a very controversial point in literature and the use of MRI-targeted techniques, if available, should always be pursued.12, 13
3.3 National Institute for Health and Care Excellence (NICE)
NICE guidelines state that MRI should be routinely proposed to patients when a radical treatment is being considered. On the other hand, MRI must be proposed as first-line examination to all men with clinical suspect of localized PCa. According to NICE, MRI should be scored using the Likert assessment scale, and patients with findings scored as >3 should be counseled for MRI-directed biopsy. MRI-directed biopsies have proved to be more cost-effective than systematic sextant biopsy since csPCa is more likely to be detected. Patients with ciPCa enrolled in active surveillance protocol, should be investigated with mpMRI, especially if they have never undergone it. If MRI reports do not correspond to biopsy results, a new MRI-directed biopsy is recommended.14
3.4 American Urological Association (AUA)
American Urological guidelines have been also endorsed by the American Society of clinical Oncology (ASCO). No clear recommendations exist for naïve patients. According to AUA, mpMRI should be considered for all patients in active surveillance protocols, in men with localized PCa, and MRI-directed biopsy should be offered to these patients for a correct management.15-19
4 THE PROSTATE IMAGING-REPORTING AND DATA SYSTEM (PI-RADS) SCORE
The most used and popular MRI scoring system is the PI-RADS score. The first version of the PI-RADS was released in 2012 and promoted clinical guidelines to correctly perform and report mpMRI.20 In 2015, the second version of the system was published and included a renovated and more detailed assessment category algorithm.21 Finally, in 2019, the latest version (v. 2.1) of PI-RADS was released, which applied further changes concerning the scoring system and imaging acquisition parameters.22, 23 PI-RADS v2.1 guideline proposes a structured report template with clear definitions of the terms used in the scoring system's lexicon, allowing an efficient diffusion of its application and a wider comprehension. Analyzing image acquisition parameters improvements, PI-RADS v2.1 suggests acquiring T2-weighted imaging (T2WI) on the axial plane and on at least an orthogonal plane. Diffusion-weighted imaging (DWI) should be obtained with one low b-value set (preferentially 50–100 s/mm2), one intermediate (800–1000 s/mm2) and one high (≥ 1400 s/mm2). Several research groups have investigated the role of the Intravoxel Incoherent Motion Diffusion-Weighted Imaging (IVIM)-DWI, a technique able to separate the incoherent motion of water molecules within the capillaries from the extravascular molecular diffusion, for PCa detection. In a recently published systematic review and meta-analysis, He et al. showed that diffusion coefficients allowed to stratify the tumor Gleason grades in PCa and that IVIM parameters were adequate to differentiate PCa from non-cancerous tissues, however, did not prove to be superior to the ADC value.24, 25 The application of IVIM is not currently recommended. Concerning the dynamic contrast enhancement (DCE) sequence, PI-RADS v2.1 recommends using a temporal resolution ≤15 s (Table 1; Figures 1-4). Regarding the scoring system, PI-RADS v2.1 changed mostly the evaluation of the transition zone (TZ), defining the chance to up-graded PI-RADS 2 findings to PI-RADS 3 if showing significant restricted diffusion.
| Sequence | ||
|---|---|---|
| T2WI (FSE or TSE) | Slice thickness | 3mm |
| FOV | 12–20 cm | |
| In plane dimension | ≤0.7 mm (phase) × ≤0.4 mm (frequency) | |
| DWI | TE | ≤90 msec |
| TR | ≥3000 msec | |
| Slice thickness | ≤4 mm | |
| FOV | 16–22 cm | |
| In plane dimension | ≤2.5 mm phase and frequency | |
| low b-value | 0–100 s/mm2 (preferably 50–100 s/mm2) | |
| Intermediate b-value | 800–1000 s/mm2 | |
| high b-value | ≥1400 s/mm2 | |
| DCE (T1W gradient echo) | temporal resolution | <15 s |
| TE | <5 msec | |
| TR | <100 msec | |
| Slice thickness | 3 mm | |
| In plane dimension | ≤2 mm × ≤2 mm | |
| Temporal resolution | ≤15 s | |
| Total observation rate | >2 min | |
| Dose | 0.1 mmol/kg standard GBCA or equivalent high relativity GBCA | |
| Injection rate | 2–3 cc/s |
- T2WI, T2-Weghited Images; FSE, fast- spin-echo; TSE, turbo-spin-echo; FOV, Field of View; DWI, Diffusion-Weighted Imaging; TE, Time of Echo; TR, Time of Repetition; DCE, Dynamic Contrast-Enhanced.
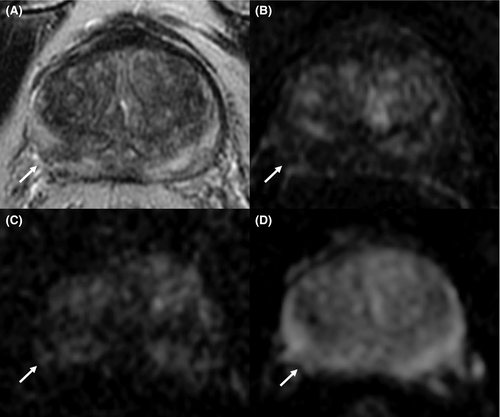
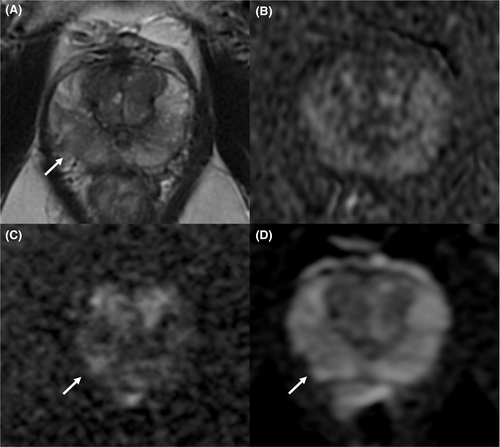
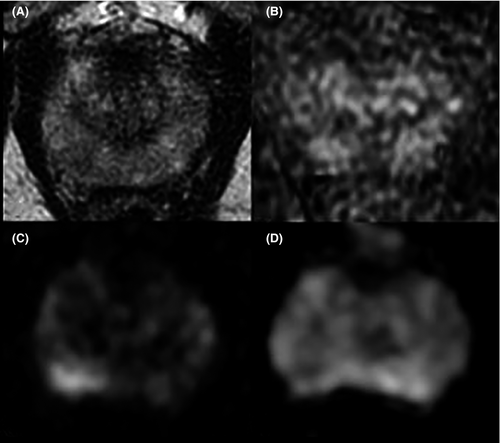
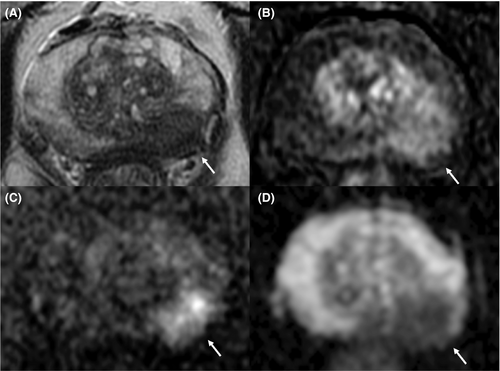
Considering the peripheral zone (PZ), PI-RADS v2.1 defined an improvement of DWI with a clearer definition of DWI score 2. Furthermore, PI-RADS v2.1 proposed a new definition of DCE positive findings, describing them as focal enhancement arising earlier or simultaneously than the adjacent normal prostatic tissue. However, despite the DCE is still endorsed in the clinical practice, in the PI-RADS v2.1 document the issue of non-contrast MRI has been faced for the first time. Several studies have investigated the power of these modifications, especially in terms of inter-reader agreement and diagnostic performance. Bhayana et al.26 demonstrated that version 2.1 improved the inter-reader agreement between expert readers for findings at the PZ, while it was found comparable to v2 for TZ lesions. Rudolph et al.27 found that the use of v2.1 did not significantly improve the diagnostic performance of per score csPCa; this point suits the aim of v2.1, as indicated in the original document. In fact, v2.1 has been defined to improve inter-reader agreement, maintaining the same fundamental structure as v2. Regarding the study of TZ, they found that lesions with a score of 2 decreased significantly, while score 1 increased, in line with the presence of typical BPH nodule in TZ and a low frequency of PCa. Byun et al.28 demonstrated that PI-RADS v2.1 shows higher sensitivity and specificity than v2, even though the difference was not statistically significant. Recently, Park et al.29 published a systematic review and meta-analysis on the evaluation of the diagnostic performance of PI-RADS v2.1 in comparison with v2, on a cohort of 1248 patients with 1406 lesions from 10 eligible selected articles. Authors showed a pooled sensitivity and specificity of PI-RADS v2.1 of 87% (95% CI, 82–91%) and 74% (95% CI, 63–82%), respectively. Although PI-RADS v2.1 provided technical updates and revision of the imaging interpretation criteria, some issues remain unresolved and will be likely addressed in future.23
5 THE MRI PATHWAY
MpMRI and its “pathway” has been recognized as “state of the art” management tool and the most cost-effective exam for PCa investigation.30, 31 Indeed, mpMRI has considerably improved the detection of PCa and patients’ management, this is mostly linked to its high performance in terms of negative predictive value (NPV) and positive predictive value (PPV). Indeed, mpMRI shows a pooled NPV of 91% (86%–94%),32, 33 the variation is mostly related to non-comparable PCa prevalence among countries and to different definitions of csPCa. Also, it is thought to be due to different centers’ expertise and lack of general quality standards.34, 35 The clinical benefit of prostate MRI comes from its NPV to rule-out csPCa that allows biopsy avoidance and reduces the detection of ciPCa. MRI PPV is generally lower than NPV. Westphalen et al.36 reported an estimated PPV was 35% (95% CI: 27–43%) for a PI-RADS score ≥3 and 49% (95% CI: 40–58%) for a PI-RADS score ≥4, across 26 centers. The reasons for such heterogeneity are multifactorial and likely related to inaccurate targeting, to mischaracterization of cancer grade, and again to differences in the prevalence of disease across centers. In 2019, Barkovich et al.37 showed how the low PPV is due to the very low value for PI-RADS 3 lesions, for which agreement is still very low (pooled PPV were 6%, 12%, 48%, and 72% for PI-RADS scores of 2, 3, 4, and 5, respectively). Nonetheless, MRI can still underestimate specific types of lesions: ciPCa, non-index csPCa <1 cm in size, anterior tumors <0.5 ml, and tumors presenting particular histopathological patterns.38-41
Four level 1a studies allowed to include MRI as first-line study, for PCa detection, in international guidelines: the PRECISION trial,42 the PROMIS trial,43 the MRI First, and the 4 M validating pairing studies.30, 44 The 4 M trial compared the MR-directed biopsy and the TRUS-guided biopsy pathway, performed in naïve men with serum PSA levels ≥3 ng/ml. Authors demonstrated a relative sensitivity of the MRI pathway versus to TRUS-GB pathway of 1.09 for csPCa (p = 0.17) and 0.57 for ciPCa (p < 0.0001), with a reduction biopsy cores of about 89%. The PROMIS study is a paired validating confirmatory study, investigating the diagnostic accuracy of mpMRI and TRUS-GB against a template prostate mapping biopsy in naïve men. MpMRI showed a higher sensitivity for csPCa detection (93%, 95% CI 88–96%) than TRUS-GB (48%, 42–55%; p < 0.0001), but a lower specificity (41%, 36–46% for mpMRI vs. 96%, 94–98% for TRUS-GB; p < 0.0001). The use of mpMRI in naïve patients allowed 27% of men to avoid a primary biopsy and to detect 5% fewer ciPCa.43 The PRECISION multicenter randomized trial compared MRI-targeted biopsy to TRUS-GB. CsPCa was diagnosed in 95 men (38%) of the group approached with MRI-targeted biopsy and in 64 patients (26%) in the one with TRUS-GB (adjusted difference, 12% points; 95% CI, 4–20; p = 0.005). 28% of men presented negative MRI and avoided prostate biopsy.42 The MRI-FIRST study analyzed the performance of MRI in the diagnosis of csPCa in naïve men. A cohort of 275 men was enrolled, of whom 14% were diagnosed with systematic biopsy, 20% with targeted biopsy, and 66% using both techniques. No statistically significant difference in csPCa detection between the two techniques was found.44 Drost et al. published the latest Cochrane systematic review and meta-analysis. Authors’ first aim was to investigate the diagnostic accuracy of the MRI pathway (MRI with or without MRI-targeted biopsy) and systematic biopsy compared to template-guided biopsy, considered as the reference standard in the diagnosis of csPCa. Authors found MRI pathway pooled sensitivity and specificity for the identification of csPCa of 0.72 (95% CI: 0.60–0.82) and 0.96 (95% CI: 0.94–0.98), respectively.45 In addition, a recently published study compared the Gleason grade group concordance between pre-operative targeted biopsy and radical prostatectomy histopathologic analysis and showed that MRI-guided in-bore biopsies offered superior sampling accuracy with a lower incidence of GG upgrades compared with MRI–transrectal US fusion biopsies upon radical prostatectomy.46
5.1 MRI performance predictive factors
The correct protocol and image acquisition are essential for proper interpretation and reporting of prostate MRI. The appropriate protocol acquisition parameters are fully described in the PI-RADS document and more explicitly enlisted in the paper published by Engels et al. in 2019.47 The lack of adequate equipment, protocol optimization, and patient preparation often determines suboptimal signal-to-noise ration that does not allow to safely rule in and out all significant lesions. Recently, Giganti et al.48 published the first attempt to develop an MRI quality scoring system: The Prostate Imaging-Quality (PI-QUAL) from the PRECISION trial. PI-QUAL might offer clinicians a scoring system for evaluating and reporting the quality of their prostate MRI scans. De Rooij et al.34, 35 released a list of consensus statements on MRI images quality. Recommendations included checking and reporting on image quality, to visually assess images adequacy for determining diagnostic acceptability, to control image quality at 6 months intervals or in 5% of studies, and to standardize ADC measurements on phantom.
As poor image quality, also reader expertise is a crucial point of a highly diagnostic prostate MRI report. Beginners can often misinterpret a suspicious lesion on MRI, performing both under- and overdiagnosis and treatment, lowering the MRI potential to detected csPCa. Both reading errors, MRI learning curve, and training needs to be considered.
Pitfalls in prostate MRI exist and paraphysiologic prostate appearances can often mimic cancer.49, 50 Most common signs mimicking PCa are the bilateral basal hypointense zones (mustache sign) due to the compression of the central zone by benign prostatic hyperplasia (BPH); the median posterior hypointense area at the middle third of the gland, in which the central zone appears compressed between the transition and peripheral zone (reversed teardrop sign); BPH foci of stromal hyperplasia; ectopic BPH nodules at the periphery of the gland; granulomatous prostatitis after intravesical BCG instillation; hypertrophic anterior fibromuscular stroma; hypertrophic periprostatic venous plexus and neurovascular bundle.
As of young trainees learning curve for prostate cancer diagnosis with MRI and MRI-Directed Biopsy (MRDB), there is little evidence in literature. In 2019, Gatti et al.51 investigated the detection of csPCa by readers with different experience, comparing performance of bpMRI, with mpMRI as reference. Authors showed that readers with no experience performed significantly worse in bpMRI and indicated 700–800 cases as threshold for reliable interpretation with bpMRI. In 2017, Calio et al.,52 showed that after an early learning period, fusion biopsy detected higher rates of csPCa and lower rates of ciPCa compared with systematic biopsy. Later, X. Meng et al.53 showed a significant improvement in csPCa detection with fusion biopsy in 4 years. In 2019, panelists from the European Society of Urogenital Radiology (ESUR) and EAU Section of Urologic Imaging (ESUI) released a consensus statement on prostate MRI quality requirements for image acquisition, interpretation, and radiologists’ training. It represents a starting point for certification of individual radiologists and for accreditation of centers for their prostate MRI diagnostic pathway.34 Twenty-four out of 31 of agreement items and 11/16 of other questions reached consensus using the Delphy method. Considering radiologists’ training, authors concluded that theoretical and hands-on courses, followed by supervised education, regular self-, and external performance assessments are essential. The highest agreement was reached on radiologists’ training, specifically on criteria and minimum requirements for basic and expert prostate MRI knowledge. Among others they set as minimum number of cases per year at 150 and 200, for basic and expert, respectively. Also, suggested agreement in double reads with expert centers at 80% and ≥90%, for basic and expert, respectively.35 Recently, Park et al.54 published a systematic review and meta-analysis on PI-RADS v. 2 inter-reader agreement. Authors showed a substantial inter-reader agreement with a PI-RADS cutoff of 4 or higher (k = 0.61) and a moderate agreement with a cutoff of 3 or higher (k = 0.57). They found that the difference in reader experience was the single significant factor affecting study heterogeneity (p = 0.01).
In addition, PCa is well known for its heterogeneity, complexity, and variable outcomes. Such features make its management perfectly suited to a multidisciplinary (MTD) approach. In 2016, Pillay et al.55 investigated the impact of multidisciplinary team meetings on patient assessment, management, and outcomes on oncologic patients in a systematic review. Twenty-seven studies were included, and they found that 4%–45% of patients discussed at MTD meetings experienced changes in diagnostic reports. Also, that MTD discussed patients are more likely to receive more accurate and complete pre-operative staging and neoadjuvant/adjuvant treatment planning. However, no improved survival outcomes were demonstrated. Similarly, Heidenreich56 stated that the benefit of MTD meetings has been shown to lead to major individual treatment changes in 40–60% of the patients.
6 THE ROLE OF CONTRAST AND NON-CONTRAST-ENHANCED MRI
Today, as previously mentioned, contrast-enhanced MRI is the imaging gold standard for prostate cancer diagnosis, in the clinical practice. Indeed, multiparametric MRI (mpMRI), consisting of morphologic and functional sequences, sees dynamic contrast-enhanced (DCE) images, as part of the standard acquisition protocol.7 However, in PI-RADS recommendations the DCE sequence has a marginal role for PCa detection; it is part of the assessment score for the evaluation of equivocal foci in the peripheral zone of the gland, but with no clear indication for lesions of the transitional zone. Nonetheless, different investigations reported that DCE may assist in the detection of csPCa (eg, less experienced readers, and degraded DWI). As a matter of fact, the basic tenant of acquiring contrast-enhanced images is related to the role as “safety-net” to increase the sensitivity of the technique. The PI-RADS recommendations suggest the use of contrast-enhanced MRI to be reserved for select patients subgroup: with prior negative biopsies and persistently elevated PSA levels, in active surveillance protocols, with a prior negative non-contrast MRI and persistent suspicion of csPCa, with previous interventions and/or medical therapy and at high risk of developing csPCa, such as patients with prostate cancer family history, and elevated urinary genomic scores and risk-calculator scores.7 However, in research settings, the role of MRI without contrast medium (commonly referred to as “biparametric MRI” or “bpMRI”) is investigated to push toward a more personalized, less invasive, and highly cost-effective diagnostic workup. Indeed, in the diagnostics of prostate cancer, due to the steady increase in MRI demand, there are growing concerns about the lack of availability of qualified radiologist and radiographers that would allow to face the upcoming large demand for prostate MRI. In addition, Porter et al.57 modeled the potential cost of bpMRI to determine cost savings and predicted that performing short protocol non-contrast MRI would lead to an increase in gross profit of $10,710.98 in a 9 h business day. Recently, the PI-RADS steering committee released a position statement on the role of non-contrast MRI in biopsy naïve patients with clinical suspicion of PCa.58 The authors detailed possible approaches to the growing demand for prostate MRI, proposing solutions that should increase operational benefits without compromising diagnostic performance, quality, and service delivery. Also, they recommended the use of risk grouping for the risk assessment of biopsy naïve men for whom contrast medium injection is advisable and to guide biopsy and/or focal treatment. The proposed risk stratification consisted of grouping men into low-, intermediate-, and high-risk categories, using proven clinical risk assessment calculators and nomograms. In detail low-risk patients should be “screened” to rule-out csPCa using bpMRI and lower the diagnosis of ciPCa; intermediate- and high-risk patients should undergo contrast-enhanced MRI in order to rule-out/in and rule in csPCa, respectively (discriminating PI-RADS 3 foci and confirming the disease); for patients at very high-risk of PCa, non-contrast MRI should be performed to rule in csPCa, to confirm the diagnosis, for biopsy/therapy planning and for local staging. When non-contrast MRI is used as the default approach, patients with foci scored as PI-RADS 1 and 2 should be followed-up and monitored with clinical data and repeated imaging as safety-net, as PI-RADS 3 and 4 should complement the examination with DCE sequences either during on-table monitoring or after hospital recalls, to improve stratification and for biopsy planning and as score PI-RADS 5 could undergo contrast injection for local staging and management planning.
6.1 Evidence in literature on non-contrast MRI performance
The diagnostic performance of non-contrast MRI has been investigated by many researchers in the last decade. As mentioned above, eliminating contrast-enhanced sequences offers many advantages in terms of operational logistics, costs, time, capacity, and side effects. When looking at the diagnostic accuracy of non-contrast MRI, one of the largest and earliest cohort was presented in 2017 by Kuhl et al.59 in which the bpMRI diagnostic accuracy for detection of csPCa (89.1%, 483 of 542), comparable to that of mpMRI. Obmann et al.60 in a prospective single-institution study showed that bpMRI detected csPCa with a sensitivity of 95.1% with an NPV of 95.1% and positive predictive value of 53.2%, taking as standard of reference biopsy results. Also, in 2017 Jambor et al.61 showed the results from the IMPROD trial where bpMRI and targeted biopsy improved risk stratification of patients with prostate cancer suspicion. Recently, Knaapila et al.62 externally validated the results of the IMPROD, showing an NPV for csPCa in bpMRI Likert 3–5 and 4–5 score groups of 93% and 92%, respectively, with PPV of 57% and 72%, respectively. Authors defined as optimal the combination of IMPROD bpMRI Likert score 4–5 or Likert 3 with PSAd ≥0.20 ng/ml/cm3, with 35% of the biopsy avoided and 13 csPCa missed.
In 2019, Boesen et al.63 presented the results from the BIDOC study, in which patients with suspicion of PCa underwent non-contrast MRI. All patients underwent systematic biopsy, patients with positive MRI underwent systematic plus targeted biopsy. Authors showed how performing combined biopsies for men with suspicious bpMRI findings, 30% of men could avoid biopsies. Diagnosis of csPCa improved by 11% (396 vs. 351 men; p < 0.001), and diagnosis of ciPCa were reduced by 40% (173 vs. 288 men; p < 0.001). The NPV of bpMRI in ruling out csPCa was 97% (95% CI, 95%–99%). Van der Leest et al.30 in a prospective, multireader, head-to-head study compared the performance of monoplanar "fast" bpMRI and triplanar bpMRI. Sensitivity for high-grade PCa for all protocols was 95% (180/190; 95% confidence interval [CI]: 91–97%). Specificity was 65% (285/436; 95% CI: 61–70%) for "fast" bpMRI and 69% (299/436; 95% CI: 64–73%) for bpMRI. With fast bpMRI, 0.96% (6/626) more low-grade PCa was detected. In a multicenter study, Choi et al.64 on a total of 113 patients with prostate cancer who underwent radical prostatectomy showed that the diagnostic performance of pre-biopsy bpMRI is similar to that of mpMRI, with a moderate inter-reader agreement on PI-RADS scoring for both bpMRI (k = 0.540) and mpMRI (k = 0.478). Results from one of the largest head-to-head study investigating the role of bpMRI, Van der Leest et al. 65 prospectively proved that sensitivity for high-grade PCa for all protocols was 95% (180/190; 95% CI: 91–97%), specificity was 65% (285/436; 95% CI: 61–70%) for "fast" bpMRI and 69% (299/436; 95% CI: 64–73%) for bpMRI and mpMRI. However, authors acknowledge the poor generalizability of these results in less experienced centers. Recently, Christophe et al.66 investigated on the applicability of non-contrast MRI as staging tool for prostate cancer and did not show any reduction in local staging accuracy. Several systematic reviews and meta-analysis have been published on the topic.67-74 In 2018, Woo et al.71 selected 20 studies with 2142 patients. The pooled sensitivity and specificity were 0.74 (95% CI, 0.66–0.81) and 0.90 (95% CI, 0.86–0.93) for bpMRI and 0.76 (95% CI, 0.69–0.82) and 0.89 (95% CI, 0.85–0.93) for mpMRI. The MRI protocol (bpMRI vs mpMRI) was not a significant factor in heterogeneity in any subgroup of patients, stratified according to clinic-pathological data and MRI characteristics (p = 0.25–0.97). In 2020, Bass et al.69 included 44 studies and with a pooled sensitivity for clinically significant prostate cancer was 0.87 (95% CI, 0.78–0.93), specificity 0.72 (95% CI, 0.56–0.84) and the AUC value was 0.87. No difference was revealed in the pooled diagnostic estimates between bpMRI and mpMRI. Finally, Knaapila et al.67 published a pooled data analysis on the NPV of bpMRI based on clinical data from four prospective studies and showed NPVs of bpMRI Likert scores 3–5 and 4–5 for csPCa were 0.932 and 0.909, respectively, and the corresponding PPV were 0.589 and 0.720.
Several investigations have shown comparable diagnostic accuracy of bpMRI with that of mpMRI in the detection of PCa and the identification of csPCa 75, 76 (Table 2). The possibility of applying non-contrast MRI for csPCa diagnosis as default approach is the consequence of many investigations evaluating the diagnostic performance of MRI for prostate cancer detection with and without contrast media. However, the detection of more ciPCa implies the need to define patient subgroups where the benefits and harms of contrast enhancement are aligned to their clinical priorities.58
| First author | Research type, study design | Y. of Publ. | N. of Patients |
bpMRI Sn (%) (csPCa) |
bpMRI Sp (%) (csPCa) |
mpMRI Sn (%) (csPCa) |
mpMRI Sp (%) (csPCa) |
Reference standard |
|---|---|---|---|---|---|---|---|---|
| Van der Leest (Netherlands)65 | Original Research, P, h-to-h comparison | 2019 | 699 | 95 (91–97) * | 69 (64–73) * | 95 (91– 97) * | 69 (64–73) * | TB + SB |
| Kuhl (Germany)59 | Original Research, R, h-to-h comparison | 2018 | 236 | 93.9 (88.7–97.2) | 87.6 (83.9–90.7) | 94.6 (88.8–97.2) | 84.8 (80.1–88.2) | TB or SB or RP |
| Choi (South Korea) | Original Research, R, h-to-h comparison | 2018 | 113 | 63.1 (53/84)§ | 72.4 (21/29)§ | 79.8 (67/84)§ | 44.8 (13/29)§ | RP |
| Liang (China)74 | Meta-analysis, no h-to-h comparison | 2020 | 5217 | 0.78 (0.66–0.87) | 0.77 (0.66–0.85) | 0.81 (0.66–0.90) | 0.70 (0.50–0.84) | TB or SB or RP |
| Alabousi (Canada)70 | Meta-analysis, h-to-h comparison | 2019 | 9480 | 0.91 (0.82–0.96) | 0.73 (0.37–0.92) | 0.92 (0.91–0.94) | 0.65 (0.33–0.87) | TB or SB or RP |
| Niu (China)68 | Meta-analysis, no h-to-h comparison | 2018 | 2383 | 0.80 (0.71–0.90) | 0.80 (0.64–0.96) | 0.85 (0.78–0.93) | 0.77 (0.58–0.95) | TB or SB or RP |
| Woo (South Korea)71 | Meta-analysis, h-to-h comparison | 2018 | 2142 | 0.86 (0.76–0.96) | 0.82 (0.75–0.89) | 0.87 (0.78–0.97) | 0.83 (0.76–0.89) | TB or SB or RP |
| Bass (UK)69 | Meta-analysis, h-to-h comparison | 2020 | 3672 | 0.84 (0.73–0.91) | 0.79 (0.70–0.85) | 0.89 (0.80–0.94) | 0.74 (0.56–0.87) | TB or SB or RP |
| Tamada76 (Japan) | Original Research, R, h-to-h comparison | 2021 | 117 | 62.5 (50/80) | 92.9 (79/85) | 72.5 (58/80) | 78.8 (67/85) | TB or SB or RP |
| Cuocolo (Italy)73 | Meta-analysis, no h-to-h comparison | 2021 | 3964 | 0.83 (0.76–0.88) | 0.71 (0.63–0.79) | – | – | TB or SB or RP |
- P, prospective; R, retrospective; h-to-h, head-to-head; bpMRI, biparametric MRI; mpMRI, multiparametric MRI; Sn, sensitivity; Sp, specificity; csPCa, clinically significant prostate cancer; TB, Targeted biopsy; SB, systematic biopsy; RP, radical prostatectomy.
- * High grade PCa.
- § Values in parentheses are raw data.
7 CURRENT TRENDS AND FUTURE APPLICATIONS
7.1 Prostate Cancer Screening using non-contrast MRI
PCa screening is still a controversial topic in the urology community, this is mostly related to the low specificity of PSA value that has been the cause of overdiagnosis of ciPCa for many years, with lack of survival improvement.77, 78 Recently, Van Poppel et al.79 proposed a novel early detection algorithm for PCa that sees risk stratification for prostate biopsy with multivariable risk prediction models and/or contrast MRI mandatory for an individualized assessment of the potential risk of having a csPCa detection, defining annual PSA testing as redundant. Non-contrast MRI, on the other hand, has become one of the most promising MRI applications as it is a more sensitive test able to perform PCa early detection. Indeed, the Prostate Cancer Prevention Trial (PCPT) showed that the sensitivity for GGG ≥2 was 58%, at a PSA threshold of 3 ng/ml.80 In 2016 by R. Nam et al.81 performed a pilot study to assess the feasibility of MRI to detect PCa during a screening protocol. In this pilot study, PCa was detected with a significantly higher rate with MRI than with PSA level (2.7, 95% CI 1.4–5.4, p = 0.004 vs. 1.1, 95% CI 0.9–1.4, p = 0.21). One of the most significantly results derived from the ERSPC screening study; in particular, the Gothenburg arm82, 83 investigated and reported MRI performance at different PSA thresholds. Authors demonstrated that MRI combined with a PSA threshold >1.8 ng/mL reached a sensitivity of 73% and an NPV of 92% for ruling out and ruling in csPCa. The abovementioned studies were pilot study investigating the feasibility of MRI as screening tool for PCa. Today, the first large ongoing trial has been presented by Eldred-Evans et al.,84 the IP1-PROSTAGRAM Study. Authors compared the performance of PSA testing, MRI, and ultrasonography as screening tests for PCa and demonstrated that a positive MRI (PI-RADS 4–5) compared with PSA alone >3 ng/mL was associated with more men diagnosed with csPCa, without an increase in the number of men advised to undergo biopsy or overdiagnosis of ciPCa. Also, Panebianco et al.85 proposed an emerging innovative method to meet prostate cancer screening needs, using the Network Medicine (NM) approach. Large, randomized controlled trials are needed to further support the role of non-contrast MRI for the early detection of PCa in the screening setting.
7.2 Predictive factors & nomograms
Currently, the use of nomograms and predictive factors is strongly encouraged by EAU guidelines to further improve the complex risk stratification of patients with suspected PCa. The use of such additional tools has been also investigated in the setting of non-contrast MRI. One of the most accepted and validated clinical parameter for PCa detection in adjunct to MRI is the PSA density (PSAd). In EAU guidelines, the suggested threshold for PCa suspicion is 0.2 ng/ml.2 Nonetheless, many research groups are still trying to identify the best risk stratification strategy for csPCa early detection that often include clinical parameters, such as PSAd, and bpMRI. Back in 2015, Rais-Bahrami et al.86 were among the first groups to demonstrate that using a specific formula [14 × (PSAD) + (the number of SPL) >4.25], the AUC for PCa diagnosis was 0.87 with an overall accuracy of prostate cancer detection of 79%. Falagario et al.87 used the Prostate Magnetic Resonance Imaging Outcome Database (PROMOD) to assess the combined use of PSAd and bpMRI for biopsy decision planning. The authors applied ten strategies based on PSAd values and MRI reporting scores (PI-RADS]/Likert/IMPROD biparametric prostate MRI Likert) to assess the best way of avoiding ciPCa diagnosis. They found that the best strategy to avoid biopsy in about 40% of the cases was by using PI-RADS/Likert 4–5 or PI-RADS/Likert 3 if PSAd >0.2, the missed GGG1 PCa were 44% and the csPCa were 10.9%. Recently, several groups are investigating the role of nomograms and risk calculators.88-91 Reesink et al.89 evaluated men with suspicion of PCa (suspicious PSA and/or DRE) using non-contrast MRI and the European Randomized Study of Screening for Prostate Cancer (ERSPC) risk calculator. Authors interestingly found that using bpMRI as a risk stratification tool outperforms risk-calculator in detecting csPCa. Instead, applying the risk-calculator first to decide on whether to perform MRI, avoids 1 out of 2 MRIs. However, applying this strategy would lead to missing up to 1 out of 5 csPCa. In 2019, Boesen et al.,90 achieved impressive results developing a predictive model on a cohort of 876 patients, defined by bpMRI scores, age, tumor stage, and PSAd, that showed a high discriminatory power, a good calibration on internal validation, and resulted in a reduction in ciPCa detection of about 50% with only 7% of significant cancers missed. At the same time, Perez et al.91 aimed at developing and validating bpMRI-based nomograms using qualitative and quantitative derived variables csPCa prediction. They retrospectively analyzed data from the IMPROD and MULTI-IMPROD trial and showed that on a multivariate analysis the model with the highest performance was the MRI model (PSA, age, use of 5-alpha-reductase inhibitors, and IMPROD bpMRI Likert score). Notably, Li et al.92 developed a radiomic-clinicopathologic nomogram (RadClip) for post-surgical biochemical recurrence-free survival (bRFS) and adverse pathology (AP) prediction in men with prostate cancer (PCa), from pre-operative bpMRI. Authors demonstrated that the RadClip performed better than existing nomograms in terms of bRFS and AP.
7.3 Radiomics & Artificial intelligence
The rapid technological innovation in radiology has led to continuous advances causing a strong transformation in the specialty. Soon, the highest impact on medical imaging will likely derive from artificial intelligence (AI) technology developments. Indeed, in the age of the Big Data, AI, and its application, and radiomics have been the object of many investigations, including the diagnosis of PCa with non-contrast MRI (Table 3). In 2018, Niu et al.93 investigated the performance of non-contrast MRI texture analysis (TA) in detecting high-grade prostate cancer in zone-specific region. The study included 184 biopsy-naïve patients who underwent contrast MRI. Two readers scored the images with contrast using the PI-RADS system and biparametric MRI TA. Authors showed that the diagnostic performance of the TA-based model was improved (transition zone AUC, 0.87; peripheral zone AUC, 0.89) in comparison with PI-RADSv2. Similarly, Xu et al.94 applied radiomics signature to similar non-contrast MRI findings for discriminating benign from malignant lesions. The MRI signature, based on six selected radiomics features, showed better discrimination (AUC, 0.92) than on each subcategory images (AUC, 0.81 on T2WI; AUC, 0.77 on DWI; AUC, 0.89 on ADC), which improved when applying it to clinical factors (AUC, 0.93). Recently, Baek et al.95 evaluated the association of texture features extracted from non-contrast MRI with Gleason score for differentiating ciPCa from csPCa. The gray-level co-occurrence matrix (GLCM) entropy on ADC map allowed to discriminate csPCa with an accuracy of 82%. Also, Algohary et al.96 evaluated the role of peri-tumoral radiomic features on non-contrast MRI to distinguish PCa risk categories according to the D'Amico Risk Classification System. Authors suggested that peri-tumoral radiomic features were independent predictive value to intra-tumoral radiomic features for PCa risk assessment, improving it by 3–6%. Finally, Gong et al.97 assessed the role of radiomic features derived from non-contrast MRI to retrospectively evaluate the use of different sequences for noninvasively distinguishing high-grade PCa. Authors found that all radiomic models showed significant (p < 0.001) predictive performances and that the DWI model achieved the most encouraging results. Bonekamp et al.98 prospectively compared non-contrast MRI free radiomic machine learning (RML), mean apparent diffusion coefficient (ADC), and radiologist assessment on a cohort of 316 patients. The quantitative measurement of the mean ADC values improved detection of malignant lesions, compared with clinical assessment, but no significant differences were detected compared to the radiomic machine learning. Also, Jensen et al.99 evaluated the role of a k-nearest neighbor classifier to assess the grade grouping of PCa using features extracted from T2WI and DWI. Authors showed that classifying PCa lesions into grade groups (GG) resulted in AUC of 0.87, 0.88, 0.96, 0.98, and 0.91 for GG1, GG2, GG1 + 2, GG3, and GG4 + 5 for the peripheral zone, respectively. Recently, Vente et al.100 presented a neural network for the diagnosis and grading of PCa, using ProstateX-2 challenge dataset. The proposed model outperformed standard multiclass classification and multi-label ordinal regression. Winkel et al.101 evaluated the performance of bpMRI on a small cohort of patients (49) using a deep learning method in a screening setting. The DL method yielded a sensitivity of 87% and a specificity of 50%, suggesting that the software solution was able to identify highly suspicious lesions and has the potential to effectively guide biopsy.
| First Author | Research Type, Study Design | Y. of Publ. | Type | N. of Patients (Training cohort) | N. of Patients (Test cohort) | bpMRI Sn (%) (csPCa) | bpMRI Sp (%) (csPCa) |
|---|---|---|---|---|---|---|---|
| Jensen (Denmark)99 | Original Research, R | 2018 | Radiomic | 112 | 70 | 0.78–1.00 | 0.80–1.00 |
| Bonekamp (Germany)98 | Original Research, R | 2018 | Radiomic | 183 | 133 | 0.79–0.97 | 0.58–0.63 |
| Niu (China)93 | Original Research, R | 2018 | Radiomic | - | 184 | 66.3–87.6 * | 61.2–81.3 * |
| Winkel (Switzerland)101 | Original Research, P | 2020 | Deep Learning | - | 49 | 0.87 | 0.50 |
| Xu (China)94 | Original Research, R | 2019 | Radiomic | 331 | 99 | 0.83–0.87 | 0.84–0.89 |
| Baek (South Korea)95 | Original Research, R | 2020 | Radiomic | 111 | 51 | 0.86 | 0.71 |
| Gong (China)97 | Original Research, R | 2020 | Radiomic | 326 | 163 | 0.54–0.79 | 0.64–0.78 |
- P, prospective; R, retrospective; bpMRI, biparametric MRI; Sn, sensitivity; Sp, specificity; csPCa, clinically significant prostate cancer.
- * High grade PCa.
The application of radiomics features and/or AI systems would likely aid in PCa diagnostics. Indeed, AI may meet multiple PCa diagnostic open issues. First, the image quality and inter-reader reproducibility issue. Second, MRI reading optimization. AI might work as second reader for less experienced radiologists, evaluate and assess quantitative MRI metrics and reduce variability of predictive values. Third, AI might have a role in determining whether to perform contrast MRI and support the MRI role for opportunistic screening as triage test.63 Fourth, AI might adapt MRI performance to meet patient clinical priorities and to manage multiple imaging time point. Fifth, AI might support radiologists in follow-up MR imaging for disease monitoring.
8 CONCLUSION
Although systematic reviews and individual studies support the bpMRI approach, the impact of using it in men with suspected prostate cancer are not yet documented for clinical practice. It is recognized, however, that high-quality examinations, expertise, and multidisciplinary teams are required to group patients according to clinical risk and to correctly manage patients. Major disadvantages of applying non-contrast MRI as a default approach are linked to centers expertise and reproducibility in the general community, lack of widespread imaging quality standards and impossibility of performing loco-regional staging. However, the accessibility to MRI for early detection of PCa is a priority. The answer to these issues might be represented by results from prospective, multicenter, multireader, and paired validation studies, in which all the possible situations that can be encountered in “real life” clinical practice are fulfilled (different scans and different readers’ experience). This becomes even truer when looking at the health and economic benefits of scaling up imaging modalities for prostate cancer, especially for low-income countries, to enable the development of affordable imaging technologies and solutions.
DISCLOSURE
V. Panebianco certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (e.g., employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are none.
AUTHORS’ CONTRIBUTIONS
MP: Project development, data analysis/collection, manuscript writing; EM: Data analysis/collection, manuscript writing; MB: Data analysis; GC: Data collection; MDM: Data collection; BI: Manuscript editing; GLT: Manuscript editing; CC: Project development; VP: Project development, Manuscript writing/editing.