Influence of circulating testosterone concentration on sperm cryoresistance: The ibex as an experimental model
Funding information
This study was funded by MICINN/AEI/FEDER and EU grant AGL2017-85753R.
Abstract
Background
Recent studies have noted that the circulating testosterone concentration may affect the ability of spermatozoa to survive cryopreservation. However, few attempts to confirm such a relationship have been made. Wild ruminant species have very marked seasonal changes in their reproductive function and strong annual changes in their plasma testosterone concentration.
Objectives
The present work examines the influence of induced changes in testosterone secretion on sperm variables following conventional slow freezing and ultra-rapid freezing, using the Iberian ibex as an experimental model.
Materials and Methods
In a first experiment, testosterone levels were reduced in the middle of the rutting season (December) using the antiandrogen cyproterone acetate (CA). In a second experiment, testosterone levels were increased at the end of the rutting season (January) via the use of the androgen testosterone propionate (TP).
Results
During December, the testosterone concentration was found to be higher in the blood and seminal plasma of untreated males than in those of CA-treated males (p < 0.001 and p < 0.05, respectively). Compared with controls, the TP-treated animals had higher blood plasma testosterone concentrations but lower seminal plasma testosterone concentrations during January (p < 0.01 and p < 0.001, respectively). The seminal vesicles of the TP-treated males were larger than those of untreated males (p < 0.05). When CA was administered, sperm viability improved compared with controls (p < 0.05), irrespective of the freezing protocol followed. For the ultra-rapid freezing procedure, the cryoresistance ratio for motility decreased when TP was administered (p < 0.05). The values for fresh sperm morphometric variables decreased during the 50 days after the end of CA treatment (p < 0.001) and increased over the same time after the end of TP treatment (p < 0.001).
Discussion and Conclusion
The circulating testosterone concentration appears to influence sperm cryoresistance. This may explain the seasonal changes seen in sperm freezability in some species, independent of fresh sperm quality.
1 INTRODUCTION
Sperm cryopreservation is routinely used to avoid subjecting azoospermic patients to repeated semen collection procedures,1 to preserve spermatozoa before radio- and chemotherapy,2 and as a back-up fertility measure for individuals who choose to undergo vasectomy.3 Spermatozoon also needs to be cryopreserved to provide animal models for the study of human disease,4 to maintain the genetic resources of rare domestic animals,5 for use in animal breeding programs,4 to improve traits that could help ensure global food security, and to establish germplasm banks for the conservation of threatened wild species.6 The damage caused to spermatozoa by freezing-thawing varies according to the protocol followed (ie, the sperm selection technique, the extender, cooling rate, packaging, and thawing procedure used),7, 8 the source of the spermatozoa (eg, ejaculated, epididymal),9 the semen collection method,10 and the quality of the freshly collected samples. Initial sperm quality may vary according to the presence of underlying disease,11 individual factors,12 the season of semen collection (especially in species with seasonal breeding13, 14), and the breed of certain domestic species.15 In addition, the concept of “good and bad freezers” postulated by Watson might extend beyond variations in the semen of different individuals16 to the endocrine status of the individual at the moment of semen collection, which may affect the ability of spermatozoa to survive cryopreservation.17
In most small ruminant species, the circulating testosterone concentration varies seasonally, affecting spermatogenesis and the functionality of sperm cells18 and accessory glands.19 High levels of testosterone during the rutting season appear to exert a harmful effect on the cryoresistance of ibex (Capra pyrenaica),20 mouflon (Ovis musimon), and ram (Ovis aries)17 spermatozoa. This suggests that the peak of the rutting season is not the best time to collect spermatozoa for cryopreservation.14
The influence of testosterone fluctuations on sperm freezability is difficult to evaluate, especially in species that are non-seasonal or moderately seasonal, for example, domesticated species that show a more continuous endocrine pattern as a result of human selection.21 Other changes in physiological status over the year make it even harder to definitively associate any differences in sperm cryoresistance with changes in testosterone, and few studies attempting to demonstrate such a cause-effect relationship have been undertaken. However, manipulating the testosterone concentration artificially in individuals from which semen is collected allows the influence of the hormone to be isolated.
The treatment with antiandrogens affects all androgen-dependent organs and functions, such as accessory sexual glands, spermatogenesis, libido, and male secondary sexual characteristics.22, 23 The effects of the antiandrogen cyproterone acetate (CA), a pharmaco-hormonal compound with antiandrogenic, antigonadotropic, and progestational properties, on reproductive functions have been widely reported in humans.24, 25 For instance, the antiandrogenic properties of CA have been used in urological clinic for treatment of advanced human prostate cancer26 or suppression of sexual disorders.24 CA induces a marked reduction in sperm concentration and motility, plus a moderate reduction in the volume of seminal fluid and testicular germ cell numbers.27, 28 On the other hand, the administration of androgens (eg, testosterone propionate, TP) raises testosterone plasma concentrations but decreases the intratesticular testosterone concentrations, which are required for the maintenance of spermatogenesis.29 In rams, the administration of TP improves many sperm kinetic variables in fresh samples.18 In any case, the overall effect of both CA and TP may vary according to the concentration administered.
Wild ruminant species could prove to be an excellent model for such experiments as, unlike domestic species, they have gone through no domestication process that might have affected the neuroendocrine control of reproductive function.30 In general, wild ruminants show marked reproductive seasonality and notable annual changes in their blood plasma testosterone concentration.31
The aim of the present work was to examine how changes in the testosterone concentration at key moments of the year influence sperm cryopreservation, using the ibex, a wild ruminant species, as a model. Testosterone levels were modified experimentally at the middle and end of the rutting season by administrating antiandrogens or androgens. As sensitivity to testosterone concentration-associated sperm damage could differ according to the cooling rate employed (sperm quality is usually more strongly affected by a very fast cooling rate),9 sperm samples were cryopreserved following a conventional slow freezing and an ultra-rapid freezing protocol.
2 MATERIAL AND METHODS
2.1 Animal handling
The animals used in this work were 12 male Iberian ibexes aged 3–9 years. All were handled according to procedures approved by the INIA Ethics Committee (Órgano Regulador de los Comités de Ética de Experimentación Animal), and in accordance with the Spanish Policy for Animal Protection (RD53/2013), which conforms to European Union Directive 86/609 regarding the protection of animals used in scientific experiments. The same ethics committee also approved the present work (ref. ORCEEA 2014/027; Madrid Regional Government ref. PROEX 271/14). All the above animals were born and raised in captivity at the INIA Department of Animal Reproduction (Madrid, Spain). They were housed in a 250 m2 enclosure with partial roof cover and fed Visan K-59 (Visan Ind. Zoot. S.A, Madrid, Spain), which contains 15% crude protein, 15.7% crude fiber, 4% crude fat, 10.6% crude ash, and 0.5% Na. This commercial feed was supplemented with barley grain, barley straw, and dry alfalfa. Water and vitamin/mineral blocks were available ad libitum. To minimize stress during experimental procedures, the animals were gradually accustomed to handling in the restraining stall (2 m2) where they would later be anesthetized. Anesthesia was induced with intravenous detomidine (50 µg/kg Domosedan, Pfizer Inc., Amboise Cedex, France), ketamine hydrochloride (0.5 mg/kg Imalgene-1000, Rhône Mérieux, Lyon, France), and tiletamine-zolazepam (0.5 mg/kg Zoletil-100, Virbac España S.A., Barcelona, Spain) and maintained with 1.5% isoflurane (Isobavet, Intervet/Schering-Plough Animal Health, Madrid, Spain) with oxygen supplementation via an endotracheal tube (flow rate 2.5 L/min). During anesthesia, all animals were monitored via pulse oximetry and capnography. Anesthesia was reversed after sperm collection using yohimbine hydrochloride (0.7 mg/kg: half intravenous and half intramuscular; Sigma Chemical Co. St. Louis, USA).
2.2 Experimental procedures
Marked monthly changes in testosterone secretion are observed over the year in ibexes, which are characterized by baseline levels from January to August, start to rise in September, reaching their highest values in October and November, and begin to decrease in December (coinciding with the highest number of matings in the wild), reaching again basal levels in January.14 Testosterone secretion was modified in the periods in which testosterone secretion is still high (Experiment 1) and when basal levels are attained (Experiment 2). Animals were grouped into three groups (two treated groups and one control group) allowing that the age variability among groups was similar. Control animals were the same in both Experiment 1 and Experiment 2.
2.2.1 Experiment 1: Influence of the antiandrogen cyproterone acetate at mid-rutting season on sperm cryoresistance
This experiment was performed with eight animals and lasted from the end of November until the end of February. The animals were divided into two groups: (1) cyproterone acetate (CA) group with four animals that received 200 mg of CA (Androcur, Schering A.G., Berlin, Germany) intramuscularly diluted in 2 mL of olive oil (vehicle) twice weekly (Tuesday and Thursday) throughout December (coinciding with the period in which testosterone plasma concentrations were expected to be high),32 and (2) control group composed of the other four ibexes. These received 2 mL olive oil intramuscularly without CA twice weekly on the same days as above. The CA dose was chosen according to Santiago-Moreno et al.33 This protocol has been successfully used at our laboratory to maintain plasma testosterone concentrations at basal levels.
A total of seven semen collections were performed from each animal, with a 15-day interval between collections. The first sample was collected just before the start of treatment (November; used only for morphometric analysis), two were collected during treatment (December), and four after treatment had ended (January and February). Coinciding with semen collection, the diameter of the testes, and the area of the seminal vesicles and bulbourethral glands were measured by ultrasonography. Blood samples were collected once weekly throughout the experimental period to measure testosterone concentrations.
2.2.2 Experiment 2: Influence of testosterone propionate at the end of the rutting season on sperm cryoresistance
This experiment was performed with eight animals and lasted from early January to the end of March. These animals were divided into two groups: (1) testosterone propionate (TP) group with four ibexes that received 25 mg of TP subcutaneously (Fluka, Sigma-Aldrich) diluted in 2 mL of olive oil (vehicle) twice weekly (Tuesday and Thursday) in January (coinciding with the seasonal fall in plasma testosterone), and (2) control group composed of the same control animals as in Experiment 1, which received 2 mL of olive oil without TP subcutaneously twice weekly (Tuesday and Thursday). The TP dose was chosen according to Santiago-Moreno et al.33 This protocol has been successfully used at our laboratory to induce high plasma testosterone concentrations.
Like Experiment 1, semen was collected seven times from each animal, with a 15-day interval between collections. The first sample was collected just before beginning TP treatment (used only for morphometric analysis), two during treatment (January), and four after treatment had ended (February and March). Coinciding with semen collection, the testes, seminal vesicles, and bulbourethral glands were again measured by ultrasonography. Blood samples were collected once weekly throughout the experimental period to measure the testosterone concentration.
Data were grouped into those covering the period coinciding with CA or TP treatment, and the 50 days after the end of treatment—the time needed for spermatogenesis to complete in caprines.34
2.3 Collection of blood samples and ultrasound analysis
Blood samples were collected by jugular venipuncture in heparinized tubes. Samples were centrifuged at 1500 g for 15 min, and the separated plasma was stored at −20°C until testosterone analysis.
Testicular diameter, the area of the bulbourethral glands and seminal vesicles, and the presence of spermatozoa in the ampulla of the vas deferens were examined by ultrasound using real-time transrectal ultrasonography employing a 7.5-MHz linear array probe (Prosound 2, Aloka Co., Ltd., Tokyo, Japan).19
2.4 Sperm evaluation
Sperm samples were collected by transrectal ultrasound-guided massage of the accessory glands (TUMASG).35, 36 The diluents and all other materials that came into contact with the semen were maintained at 37°C. The volume of the ejaculates was measured using a micropipette (Gilson, Villiers Le Bel, France). Sperm concentration was calculated before freezing using a Neubauer chamber (Marienfeld, Lauda-Königshofen, Germany). Sperm motility was evaluated using a computer-aided sperm analysis system (CASA) (SCA, Barcelona, Spain) coupled to a Nikon Eclipse model 50i phase-contrast microscope with negative contrast capability. The percentages of immotile spermatozoa, sperm showing progressive motility, and spermatozoa showing non-progressive motility were determined in a minimum of three fields and 500 sperm tracks (image acquisition rate 25 frames/s).35 Morphological abnormalities were assessed by phase-contrast microscopic examination of glutaraldehyde-fixed samples. Sperm viability and acrosome status were assessed by fluorescence microscopy using a fluorochrome combination of propidium iodide (PI) and fluorescein isothiocyanate-conjugated peanut (Arachis hypogaea) agglutinin (PNA-FITC), as previously described.37 These variables required the observation of 200 cells.
Finally, smears were prepared for morphometric analysis by placing 5 µl of the fresh semen on the clear end of a frosted slide and dragging the drop across it. The smears were allowed to air-dry before staining with Hemacolor and assessed by CASA using the morphometry module of Sperm-Class Analyzer v.5.3.0.1 software (Microptic S.L. Barcelona, Spain) as described by Esteso et al.38 The percentage of the head occupied by the acrosome was measured together with the length, width, perimeter, and area of the sperm head. For this analysis, a total of 100 sperm heads were measured on each slide. All sperm variables, except morphometry, were assessed before and after freezing with both cryopreservation methods. Morphometric variables were measured in fresh semen in the samples collected before the treatments and during the 50 days after the end of treatment.
2.5 Sperm cryopreservation
All semen samples were separated into two fractions and diluted 1:1 (vol:vol) with TCG (Tris 313.7 mm, citric acid 104.7 mm, glucose 30.3 mm) for centrifugation at 900 g for 20 min. After centrifugation, the supernatant was separated into another centrifuge tube (Sterilin, Stone, UK) and centrifuged a second time to separate out the seminal plasma. This was stored at −20°C until testosterone determination. In preparation for conventional slow freezing, sperm pellets were resuspended at room temperature (23°C) with TCG (Tris 313.7 mm, citric acid 104.7 mm, glucose 30.3 mm, 6% egg yolk [vol/vol], and 5% glycerol [vol/vol]) to a final concentration of 100 × 106 spermatozoa/mL. In preparation for ultra-rapid freezing, sperm pellets were similarly resuspended in the same extender but with glycerol substituted by 100 mM sucrose. All suspensions were adjusted to pH 6.8 with NaOH at room temperature. The osmolality (measured in the absence of cryoprotectants) of all suspensions was 345 mOsm/kg. All reagents used in the preparation of the extenders were purchased from Panreac Química S.A. (Barcelona, Spain) or the Sigma Chemical Co. (St. Louis, USA).
Sperm samples cryopreserved by conventional slow freezing were cooled at 5°C for 1 h, and then maintained at this temperature for 2 h. Aliquots were then loaded into 0.25-mL straws and frozen by placing them in nitrogen vapor 5 cm above the surface of a liquid nitrogen bath for 10 min.39 The sperm samples cryopreserved by ultra-rapid freezing were cooled for 30 min at 5°C and then plunged drop by drop (about 50 μL/drop) directly into liquid nitrogen as reported by Pradiee et al.40 All cryopreserved samples were kept in liquid nitrogen for 12 months until thawing. For the straws, this was performed using a water bath at 37°C for 30 s; for the pellets, it was achieved by placing them on a DPP70 thermo-regulated conical hotplate (INIA, Madrid, Spain) set at 60–65°C.
2.6 Testosterone analysis
Testosterone concentrations in blood plasma and seminal plasma aliquots (250 μL and 50 μL respectively) were measured (in duplicate) by radioimmunoassay as previously described.19 The sensitivity of the technique was 0.06 ng/mL; the intra-assay and inter-assay coefficients of variation were 7% and 11%, respectively.
2.7 Statistical analysis
Data were expressed as means ±SE. The plasma testosterone data showed a skewed distribution as determined by the Shapiro-Wilk test; values were therefore log-transformed before analysis. The same log transformation was made for the ultrasound measurements of testicular diameter, and for the area of the bulbourethral glands and the seminal vesicles. The values for sperm variables expressed in percentages that showed non-normal distributions, as determined by the Shapiro-Wilk test, were arcsine-transformed before analysis. The remaining sperm variables showing a non-normal distribution were log-transformed or arcsinh-transformed (in case of variables including zero values). The cryoresistance ratio (CR) for different variables was determined as the (value after thawing/value before thawing) x 100]).41
The effects of CA (Experiment 1) and TP treatment (Experiment 2) on the plasma testosterone concentration, the area of the sexual accessory glands, and the CR values for the different sperm variables returned by each freezing method were assessed by GLM ANOVA. The effects of the CA (Experiment 1) and TP treatment (Experiment 2), and of their interaction with the data period (ie, data collected during and after treatment), on fresh sperm variables and the CR values returned by each freezing method, were studied by GLM repeated-measures ANOVA. Morphometric variables were analyzed by factorial ANOVA, examining the interaction between the treatment and sample collection period. Significance was set at p < 0.05. All calculations were performed using Statistica for Windows v.12.0 software (StatSoft, Tulsa, OK, USA).
3 RESULTS
3.1 Experiment 1: Influence of CA treatment during the rutting season on sperm cryoresistance
During December, the testosterone concentration was higher in the blood and seminal plasma of the untreated than the CA-treated animals (p < 0.001 and p < 0.05, respectively) (Figure 1). In addition, the seminal vesicles of the CA-treated animals were smaller than those of the controls during December (p < 0.001) (Figure 2). No differences were seen between the CA and control animals in terms of testicular diameter or the area of the bulbourethral gland.
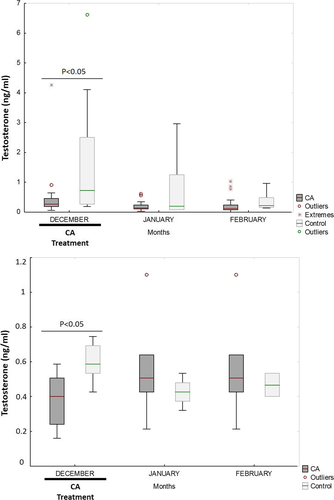
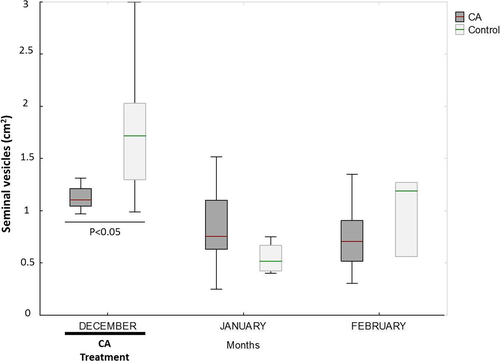
During the post-treatment period, the semen of the CA-treated males showed a greater percentage of spermatozoa with morphological abnormalities than did that of the untreated males (p < 0.05) (Table 1). During the period of treatment, the CR viability value was greater for the CA-treated than for the control animals irrespective of the freezing method used (p < 0.05; Table 2); no differences were seen in the post-treatment period. Moreover, during the treatment period, the CR motility value was greater for the CA-treated animals than for the controls (p = 0.09) when the spermatozoon was slow-frozen. During the post-treatment period, the CR-VCL value was lower for the CA-treated animals than the controls when the spermatozoon was slow-frozen (p < 0.01) (Table 2). The CR-VSL and CR-VAP values also tended to be lower than in the controls (p = 0.07 for both), while the CR acrosome integrity tended to be higher (p = 0.09) (Table 2).
| Sperm variables | Fresh during treatment | Fresh after treatment | ||
|---|---|---|---|---|
| Control | CA | Control | CA | |
| Motility (%) | 59.4 ± 9.8 | 46.7 ± 7.8 | 38.3 ± 6.5 | 43.7 ± 11.4 |
| VCL (µm/s) | 79.5 ± 14.5 | 86.3 ± 9.4 | 80 ± 11.9 | 66 ± 10.4 |
| VSL (µm/s) | 39.3 ± 7.7 | 33.4 ± 4.9 | 35.2 ± 6.9 | 27.8 ± 6.3 |
| VAP (µm/s) | 55.7 ± 10.3 | 52 ± 7.1 | 51.4 ± 8.2 | 42 ± 9 |
| ALH (µm/s) | 2.7 ± 0.7 | 3.1 ± 0.5 | 3.3 ± 0.6 | 2.5 ± 0.6 |
| Viability (%) | 71.6 ± 11 | 67.4 ± 6.6 | 76.2 ± 4.8 | 76.8 ± 4.1 |
| Acrosome integrity (%) | 95 ± 2.1 | 87.9 ± 5.4 | 91 ± 3.1 | 92.2 ± 0.9 |
| Morphological abnormalities (%) | 45 ± 12.5 | 37.1 ± 6.8 | 33.5 ± 7.6b | 59.2 ± 6.4a |
- Different letters indicate significant differences (p < 0.05) between the control group and the treatment group.
- Abbreviations: ALH, amplitude of lateral head displacement; CA, cyproterone acetate; VAP, average path velocity; VCL, curvilinear velocity; VSL, straight-line velocity.
| Sperm variables | CR slow freezing | CR ultra-rapid freezing | ||||||
|---|---|---|---|---|---|---|---|---|
| Frozen-thawed during treatment | Frozen-thawed after treatment | Frozen-thawed during treatment | Frozen-thawed after treatment | |||||
| Control | CA | Control | CA | Control | CA | Control | CA | |
| Motility | 28.5 ± 6.1 | 54.1 ± 10.5 | 61.4 ± 13.7 | 60 ± 10.4 | 7.2 ± 0.8 | 6.7 ± 1.6 | 18.6 ± 4.4 | 13.2 ± 3 |
| VCL | 87.8 ± 17.2 | 69.3 ± 7.6 | 95.9 ± 6.2a | 65.5 ± 3.5b | 45.2 ± 4.9 | 59.8 ± 11.4 | 67.1 ± 11.4 | 53.7 ± 9.3 |
| VSL | 117.1 ± 26.6 | 109.6 ± 24.4 | 145.3 ± 26 | 83.8 ± 13 | 43.9 ± 12.8 | 50.4 ± 7.6 | 97.5 ± 25.5 | 55.2 ± 14.1 |
| VAP | 101.4 ± 22.4 | 87.1 ± 16.1 | 125.3 ± 22.4 | 72.1 ± 9.2 | 41.9 ± 9.5 | 56.2 ± 10.7 | 77.4 ± 17.7 | 48.9 ± 10.8 |
| ALH | 87.7 ± 14.4 | 73.5 ± 5.8 | 62.2 ± 7.3 | 77.4 ± 4.4 | 50.3 ± 18.6 | 42.6 ± 13 | 61.3 ± 13.1 | 46.6 ± 14.9 |
| Viability | 48.2 ± 10.8b | 91.4 ± 15a | 46.4 ± 7.2 | 46.4 ± 12.3 | 22.4 ± 8.7B | 44.7 ± 5.4A | 23.8 ± 3.5 | 33.4 ± 7.6 |
| Acrosome integrity | 60.7 ± 13 | 75.3 ± 5.9 | 66.3 ± 6.8 | 82.8 ± 3.1 | 52.5 ± 11.2 | 65 ± 6.9 | 69 ± 7.2 | 75.1 ± 5.5 |
| Normal spermatozoa | 112.4 ± 39.5 | 105.6 ± 8 | 63.8 ± 11.8 | 111.2 ± 35 | 97.1 ± 30.4 | 105.4 ± 7.1 | 66 ± 13 | 128.9 ± 35.5 |
- Different letters indicate significant differences (p < 0.05) between the control group and the treatment group for slow freezing (lower case letters) and for ultra-rapid freezing (upper case letters).
- Abbreviations: ALH, amplitude of lateral head displacement; CA, cyproterone acetate; VAP, average path velocity; VCL, curvilinear velocity; VSL, straight-line velocity.
The fresh spermatozoa collected from the CA-treated animals had smaller heads than did those of the controls (p < 0.001) (Table 3).
| Control before | CA before | Control 50 days post-treatment | CA 50 days post-treatment | |
|---|---|---|---|---|
| Length (µm) | 8.25 ± 0.02 | 8.28 ± 0.02 | 8.07 ± 0.03 | 8.00 ± 0.02 |
| Width (µm) | 4.02 ± 0.01 | 4.02 ± 0.02 | 4.09 ± 0.02A | 4.02 ± 0.01B |
| Area (µm2) | 27.52 ± 0.13 | 27.67 ± 0.11* | 27.41 ± 0.18A | 26.68 ± 0.12B* |
| Perimeter (µm) | 21.23 ± 0.06 | 21.31 ± 0.04* | 21.12 ± 0.07A | 20.85 ± 0.05B* |
| Acrosome (%) | 55.26 ± 0.33 | 55.89 ± 0.14 | 56.32 ± 0.18 | 56.52 ± 0.14 |
- Different letters indicate significant differences (p < 0.05) between control and treatment groups post-treatment (upper case letters). Asterisks show significant differences (p < 0.05) between pre-treatment and 50 days post-treatment.
- Abbreviations: CA, cyproterone acetate. Acrosome (%): percentage of the head occupied by the acrosome.
3.2 Experiment 2: Influence of TP treatment at the end of the rutting season on sperm cryoresistance
The TP-treated animals had higher blood plasma testosterone concentrations, but lower seminal plasma testosterone concentrations, than the control animals during January (p < 0.01 and p < 0.001, respectively) (Figure 3). No differences were seen in the following months. While the seminal vesicles of the TP-treated males were larger than those of the untreated males during January (p < 0.05) (Figure 4), no differences in testicular diameter or the area of the bulbourethral gland were seen at any time.
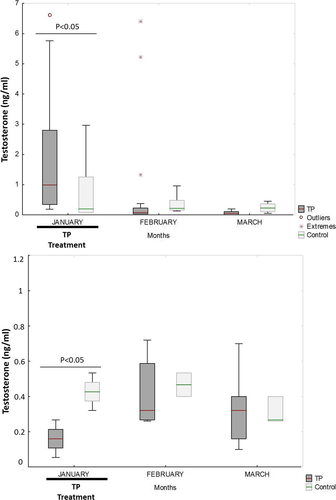
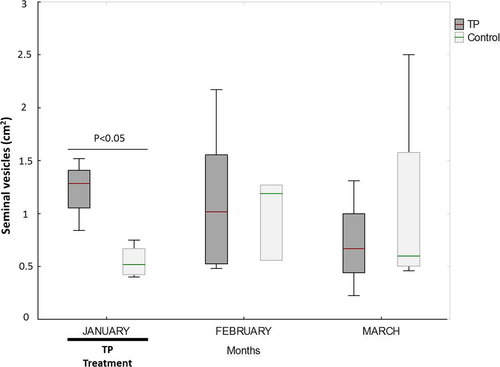
The fresh semen samples were not affected by the administration of TP during the treatment period. In the post-treatment period, the VSL and VAP were lower in the fresh spermatozoa of the treated males (p < 0.05) (Table 4). During the treatment period, the CR motility value was lower for the TP-treated than for the control males when the spermatozoon was ultra-rapidly frozen (p < 0.05) (Table 5).
| Sperm variables | Fresh during treatment | Fresh after treatment | ||
|---|---|---|---|---|
| Control | TP | Control | TP | |
| Motility (%) | 36.8 ± 4.1 | 50.4 ± 16.8 | 41.8 ± 13.1 | 31.8 ± 5.7 |
| VCL (µm/s) | 76.8 ± 42.8 | 71.3 ± 15 | 90 ± 12.6 | 66.3 ± 6.6 |
| VSL (µm/s) | 32 ± 21.5 | 32.6 ± 6 | 43.3 ± 8.3a | 24.8 ± 4.2b |
| VAP (µm/s) | 44.6 ± 23.9 | 50.4 ± 10.1 | 66.8 ± 11a | 40.8 ± 5.6b |
| ALH (µm/s) | 3.6 ± 1.9 | 2.8 ± 0.9 | 3 ± 0.3 | 2.4 ± 0.3 |
| Viability (%) | 78.8 ± 4 | 82.7 ± 11.8 | 68 ± 11.9 | 66.6 ± 6.4 |
| Acrosome integrity (%) | 90.5 ± 10.5 | 97.3 ± 2.1 | 93.3 ± 0.9 | 82.9 ± 3.2 |
| Morphological abnormalities (%) | 35.3 ± 24 | 42 ± 6.9 | 48.8 ± 12.6 | 51.5 ± 3.7 |
- Different letters indicate significant differences (p < 0.05) between the control group and the treatment group.
- Abbreviations: ALH, amplitude of lateral head displacement; TP, testosterone propionate; VAP, average path velocity; VCL, curvilinear velocity; VSL, straight-line velocity.
| Sperm variables | CR slow freezing | CR ultra-rapid freezing | ||||||
|---|---|---|---|---|---|---|---|---|
| Frozen-thawed during treatment | Frozen-thawed after treatment | Frozen-thawed during treatment | Frozen-thawed after treatment | |||||
| Control | TP | Control | TP | Control | TP | Control | TP | |
| Motility | 62.9 ± 21.6 | 40.1 ± 7.3 | 41.8 ± 11 | 42.9 ± 6.1 | 18.3 ± 4.4A | 1.9 ± 0.4B | 17.9 ± 6.7 | 11.6 ± 1.5 |
| VCL | 96.9 ± 9.1 | 85.1 ± 22.8 | 65.7 ± 16.8 | 90 ± 12.2 | 67.6 ± 16.3 | 36.4 ± 12 | 47.4 ± 14 | 63.5 ± 7.5 |
| VSL | 159.8 ± 38.5 | 112.5 ± 38.8 | 76.7 ± 27.3 | 163.7 ± 40.8 | 101.9 ± 31.3 | 34.7 ± 17.1 | 59.9 ± 31.5 | 75.2 ± 13.8 |
| VAP | 135.2 ± 33.1 | 92.6 ± 28.6 | 68.7 ± 22.6 | 116.6 ± 25.4 | 85.4 ± 23 | 27.7 ± 11.4 | 43.2 ± 18.7 | 64.2 ± 10.9 |
| ALH | 59.2 ± 11.1 | 62.27 ± 31.2 | 43.2 ± 14.6 | 53 ± 10.9 | 55.1 ± 19.6 | 19.1 ± 14.2 | 42.7 ± 18.1 | 21.2 ± 6 |
| Viability | 49.9 ± 10.8 | 64.3 ± 8.4 | 47.9 ± 9 | 67.6 ± 13.1 | 27.2 ± 4.3 | 45.8 ± 22.1 | 18.9 ± 3.3 | 29.9 ± 3.5 |
| Acrosome integrity | 70.1 ± 9.8 | 60 ± 16.7 | 66.1 ± 5.8 | 80.8 ± 3.5 | 73.8 ± 9.4 | 49.9 ± 18.3 | 65.1 ± 8.5 | 72.3 ± 4 |
| Normal spermatozoa | 54.7 ± 14.8 | 98.8 ± 40.4 | 126.7 ± 44.6 | 81.5 ± 6.9 | 56.1 ± 15.8 | 94.5 ± 45.1 | 125.6 ± 48.6 | 82.5 ± 9 |
- Different letters indicate significant differences (p < 0.05) between control and treatment groups for ultra-rapid freezing (upper case letters).
- Abbreviations: TP, testosterone propionate; VCL, curvilinear velocity; VSL, straight-line velocity; VAP, average path velocity; ALH, amplitude of lateral head displacement.
The fresh sperm cells of the TP-treated animals were longer and had a greater head area and perimeter than did those of the control animals, both before treatment and in the post-treatment period (p < 0.001) (Table 6).
| Control before | TP before | Control 50 days post-treatment | TP 50 days post-treatment | |
|---|---|---|---|---|
| Length (µm) | 8.07 ± 0.03b | 8.37 ± 0.02a* | 8.05 ± 0.02B | 8.24 ± 0.02A* |
| Width (µm) | 4.09 ± 0.02 | 4.04 ± 0.01* | 4.16 ± 0.01 | 4.20 ± 0.02* |
| Area (µm2) | 27.41 ± 0.18b | 27.99 ± 0.10a* | 27.83 ± 0.12B | 28.60 ± 0.15A* |
| Perimeter (µm) | 21.12 ± 0.07b | 21.60 ± 0.04a | 21.12 ± 0.05B | 21.50 ± 0.06A |
| Acrosome (%) | 56.32 ± 0.18 | 56.28 ± 0.14 | 56.10 ± 0.14 | 56.48 ± 0.13 |
- Different letters indicate significant differences (p < 0.05) between control and treatment groups before treatment (lower case letters) and post-treatment (upper case letters). Asterisks show significant differences (p < 0.05) between pre-treatment and 50 days post-treatment.
- Abbreviations: TP, testosterone propionate. Acrosome (%): percentage of the head occupied by the acrosome.
4 DISCUSSION
The two treatments modified the plasma testosterone concentration as expected33—modifications that were associated with changes in sperm cryoresistance. The rapid reduction in blood plasma testosterone levels after CA treatment was similar to that observed in white-tailed deer,42 fallow deer,23 and domestic goat.43 CA induced testosterone levels similar to those seen outside the reproductive season.14 TP administration induced physiological high plasma testosterone concentrations as previously described in ibexes.33 Plasma testosterone concentrations were highly variable with both CA and TP treatments. The reduced number of animals per group could have an influence on this variability. In addition, marked fluctuations, over relatively short periods, in peripheral plasma levels of testosterone secretion resulting from their intermittent secretion44 should also be taken into account. A regimen of sampling more frequent should be used to avoid this problem.19
A reduction in the plasma testosterone concentration improved cryoresistance, while an increase worsened certain cryoresistance variables. For example, the CR viability value improved when plasma testosterone was reduced by the CA treatment, whereas CR motility fell after TP treatment. It is interesting that most of the differences in the CR values were observed during the time that the treatments were being administered; they were not generally maintained after the end of treatment. Unlike that previously proposed,17 this suggests that they were not the consequence of modifying the early stages of spermatogenesis (which lasts about 48 days in goats).34 Rather, they would appear to be mainly related to changes occurring during the transit of spermatozoa in the epididymal cap and the corpus epididymis, during the final maturation in the cauda epididymis, and during the short period in which the sperm cells are exposed to seminal vesicle secretion during ejaculation. The changes might involve the remodeling of the lipid and protein components of the plasma membrane.45 The absorptive and secretory activity of the epididymal epithelium is regulated by androgens.46 Epididymosomes47 and extracellular vesicles48 contribute in the addition and replacement of sperm proteins during epididymal transit and ejaculation, respectively, inducing strong variations in seminal plasma composition. Certainly, testosterone can induce variations in the properties of sperm membranes,49 and the negative effects on CR values of high concentrations might be due to changes in membrane fluidity and susceptibility to glycerol toxicity.20 In rams, in vitro testosterone supplementation has been reported to reduce frozen-thawed acrosome integrity,50 but in the present work, neither treatment modified the CR value for this variable.
Androgens have an important role in differentiation, development, and maintenance of epithelial cells in seminal vesicles; techniques of transmission electron microscopy and laser Doppler flowmetry have shown that glands’ morphological features and blood flow, mainly in subepithelial capillaries, depend on androgens.51 This may explain fact that CA treatment reduced the area of the seminal vesicles, while the TP treatment increased it.52 Thus, besides their functional effects in the extra-testicular tract, the treatments appear to have had a direct impact on sperm CR values via their action at the level of the seminal vesicles. The reduction in the size of the seminal vesicles caused by CA treatment could have modified their activity52 and therefore the composition of the seminal plasma. The possible reduction in glucose and fructose production by the seminal vesicles53 during CA treatment might have influenced the metabolic status of the sperm cells, and perhaps their response to freezing-thawing. Further, in bulls the secretion of the seminal vesicles includes proteins, the synthesis of which is androgen-dependent. These bind to the sperm membrane53 and might influence CR values.
During the post-treatment period, the sperm heads were smaller in fresh samples collected from CA-treated individuals, suggesting that the spermatogenic cycle might have been affected. The sperm heads increased in size after the TP treatment, coinciding with that reported for domestic bucks.18 These findings suggest that higher concentrations of testosterone lead to a larger head size. Certainly, a close relationship exists between blood plasma testosterone and the dimensions of human sperm heads.54 During the non-breeding season of rams, that is, when the testosterone concentration is low, the size of the sperm head is also reduced,55 probably due to changes in the activity of the germinal epithelium and Sertoli cells.17 It is also possible that testosterone levels modify the organization of the manchette, a transient microtubular platform seen in elongating spermatids that plays an important role in head shaping.56 The influence of head dimensions in susceptibility to freezing-thawing is matter of debate,41 but small sperm cells have been reported less likely to be damaged by cryopreservation.39, 57 However, the present results show that, similar to that reported for some other wild species,41 a smaller head size does not appear to be associated with variations in CR values. It remains to be determined how different factors may interact to modify sperm head size and its relationship with sperm CR values.
During the post-treatment period, the CR-VCL value was reduced in the spermatozoa of CA-treated males that were subjected to slow freezing. This might be explained in that this is the period during which the percentage of sperms with morphological abnormalities increases.25 Certainly, the increase in morphological abnormalities observed in the present work during the post-treatment period would appear to reflect an effect of low testosterone levels on spermatogenesis. Surprisingly, and unlike that seen for blood plasma testosterone, the seminal plasma testosterone concentration was lower (compared to controls) in the TP-treated animals during the treatment period. This apparent lack of an association in the changes in blood and seminal plasma agrees with that reported for TP-treated domestic bucks.18 Indeed, it has been previously reported that the seminal plasma testosterone concentration need not correlate with the blood plasma concentration.58 It may be that testosterone is locally metabolized into other androgens, such as dihydrotestosterone and androstenedione,59, 60 with a more direct in situ action.
Although the model used in the present study (wild ruminants) does not allow the use of a large number of animals in the experiments, to increase the statistical power, it provided valuable information about the role of endocrine status on sperm response to freezing-thawing process. Future experiments should be designed to determine the mechanistic action of testosterone on sperm freezability. For instance, aquaporins (AQPs) adapt their membrane domain location to osmotic changes during freezing-thawing that can relate to variations in sperm cryosurvival.61 The expression of some AQPs is regulated by androgens in certain cells, for example, uterine cells62 or kidney cells,63 and thus, a possible influence of testosterone on AQP expression in the sperm cell should not be ruled out.
In conclusion, testosterone appears to negatively influence sperm cryoresistance independent of sperm head size. This may explain why sperm freezability varies seasonally in some species, independent of its fresh quality. The present results provide insight into the use of testosterone as a biological marker for predicting how spermatozoa will react to freezing-thawing, which could facilitate the optimization of cryopreservation techniques by endocrine manipulation of testosterone levels.
ACKNOWLEDGEMENTS
Paula Bóveda was the recipient of a grant for pre-doctoral researchers from MINECO (AEI/FSE, UE). Octavio Mejía was supported by a research fellowship from the PASPA-DGAPA-UNAM (Mexico).
CONFLICT OF INTEREST
The authors have no conflict of interest to disclose.
AUTHORS' CONTRIBUTIONS
PB collected data, performed statistical analyses, and wrote the first draft of the manuscript. MCE and ASLS designed the study. RV, CC, ATD, OM, and MGMB collected data. RU interpreted results and revised the manuscript critically. JSM designed the study, performed statistical analyses, and wrote the manuscript. All authors and co-authors approved the final version of the manuscript to be published.




