Antioxidant effect of Elamipretide on bull's sperm cells during freezing/thawing process
Funding information
This research received no external funding.
Abstract
Background
Spermatozoa are subjected to drastic changes in temperature, ice crystal formation, and diverse types of stresses (chemical, physical, osmotic, and oxidative) during the cryopreservation process, which severely compromise sperm quality and fertility. In this study, we aimed to investigate the protective role of Elamipretide in the cryopreservation of bull's spermatozoa.
Materials and methods
The study included 36 healthy Simmental bulls with an average age of 2 ± 0.5 years housed individually in pens. Two ejaculates were collected from each bull using an artificial vagina at 7 a.m. Subsequently, the semen was extended with animal protein-free commercial BIOXcell® extender (IMV Technologies) to a final concentration of 160 × 106 spermatozoa/ml, and rated in terms of motile sperm percentage, progressive motility, viability, and morphological abnormality of spermatozoa. Semen samples that showed more than 60% motility and 60% viability, were selected for the experiment. The fresh semen was then divided into five equal fractions. The first fraction was left for the control group (without Elamipretide), to the next were added in succession 0.1; 1; 5; and 10 μM of Elamipretide TFA (Trifluoroacetic) (MedChemExpress). After that semen was subjected to freezing and thawing. Next semen was assessed for motility, viability, mitochondrial membrane potential and acrosome integrity, and antioxidant activity (SOD, CAT, MDA).
Results
It has been shown that a concentration of 5 and 10 μM proved to be the most effective in terms of tested parameters of the quality of sperm cells subjected to cryopreservation.
Conclusion
In conclusion, addition of the Elamipretide to the cryopreservation extender significantly improved frozen-thawed sperm cells quality and their function. The results of this study indicate that Elamipretide can be used as a cryoprotective agent to protect cells against the devastating effects of oxidative stress and increasing sperm survival after cryopreservation.
1 INTRODUCTION
Spermatozoa are subjected to drastic changes in temperature, ice crystal formation, and diverse types of stresses (chemical, physical, osmotic, and oxidative) during the cryopreservation process, which severely compromise sperm quality and fertility.1-3 Although the continuous optimization of cryopreservation methods has improved the quality of spermatozoa after freezing, changes in sperm structure, epigenetic modification, and the long-term effects caused by freezing injury including enzyme inactivation, ion changes, and oxidative stress cannot be ignored, and the optimization of the freezing system is still an ongoing task that needs further research.4, 5 Despite the development of many agents such as different proteins, antioxidants, and cryoprotective agents (incorporated into the freezing medium) for increasing sperm cryosurvival have not yet reached the desired level because many spermatozoa still lose their viability after cryopreservation.6
Elamipretide (formerly referred to as Bendavia, MTP-131, and SS-31) is an aromatic-cationic tetrapeptide that readily penetrating cell membranes and transiently localizing to the inner mitochondrial membrane where it interacts with cardiolipin. Through this, elamipretide is able to restore energy production, to reduce the production of reactive oxygen species, and ultimately to increase the energy (adenosine triphosphate [ATP]) supplied into affected cells.7, 8 As recent studies show, it can increase the synthesis of ATP and reduced reactive oxygen species (ROS) production (in humans) independently of the specific mitochondrial abnormality causing the impaired mitochondrial respiration (in rats).9, 10
However, there are no reports on the application of Elamipretide in bull's spermatozoa preservation and it is unclear whether it can effectively protect spermatozoa from cryodamage during freezing. In this study, we aimed to investigate the protective role of Elamipretide in the cryopreservation of bull's spermatozoa.
2 MATERIALS AND METHODS
2.1 Semen processing
The experiment has been performed as part of routine activities during the current semen production in the reproductive station and did not require the approval of the ethics committee.
These experiments were performed on the Breeding and Insemination Centre “MCB” (Krasne, Poland), from February to June. The study included 36 healthy Simmental bulls with an average age of 2 ± 0.5 years housed individually in pens, animals were fed the same (a mixture of farm fodder: haylage, maize silage, apple pomace, basic mineral, and vitamin premix), all bulls had free access to freshwater. Two ejaculates were collected from each bull using an artificial vagina at 7 a.m. The semen was held in a water bath at 37°C, where the sperm concentration and initial percentage of motile spermatozoa were estimated. Sperm concentration was assessed using a digital photometer (Dr Lange, LP 300 SDM; Minitube, Tiefenbach b. Landshut, Germany) at 560 nm.
Semen samples were transferred immediately after collection into graduated test tubes, placed in a water bath at 37°C. The fresh undiluted semen was then evaluated microscopically (Nikon E 200, China) for mass motility. Subsequently, the semen was extended with animal protein-free commercial BIOXcell® extender (IMV Technologies, L’aigle, France) to a final concentration of 160 × 106 spermatozoa/ml, and rated in terms of motile sperm percentage, progressive motility, viability, and morphological abnormality of spermatozoa (morphology were examined in nigrosine-eosin smears (300 cells/slide) and assessed at 1250× magnification, under light microscope (Nikon Eclipse E100). Semen samples that showed more than 60% motility and 60% viability, were selected for the experiment. After a positive evaluation, semen samples were pooled to eliminate individual differences.
The fresh semen was then divided into five equal fractions. The first fraction was left for the control group (without Elamipretide), to the next were added in succession 0.1; 1; 5; and 10 μM of Elamipretide TFA (Trifluoroacetic) (MedChemExpress). Semen was automatically packed (Bloc Machine FIN, IS 4, France) into polyvinyl chloride (PVC) straws (0.25 ml) (IMV, France) which were filled and equilibrated for 1.5 h at 4°C. After equilibration, the straws were frozen in liquid nitrogen vapor using a computer-controlled automatic freezer from 4°C to −15°C at the rate of −3°C/min and from −15 to −80°C at the rate of −10°C/min (IMV Technologies). After reaching −80°C, semen straws were plunged into liquid nitrogen and packaged in plastic goblets for 24 hours of storage in the liquid nitrogen container. After one day, the straws were thawed in a water bath at 38°C for 20 s and then were examined.
2.2 Computerized assessment of sperm motility
Sperm motility was examined using a Sperm Class Analyzer (SCA, version 5.1, Microptic, Barcelona, Spain), a light microscope (Nikon Eclipse E200). Just prior to analysis, semen was diluted 1:10 in a warm (25°C) physiological solution (sodium chlorate 0.9%). Then, 2 µL of the prepared sample was placed in a Leja 4 analysis chamber (Leja Products B.V., Holland) of a thickness of 20.0 µm. The slide was placed on a stage warmer (38°C). The following motility parameters were included in this study: percentage of motile spermatozoa, curvilinear velocity (VCL), straight-line velocity (VSL), path velocity (VAP), linearity (LIN), and amplitude of lateral head displacement (ALH). Minimum 500 cells were evaluated, and depending on sperm concentration, five analyses were performed per sample.
2.3 Viability
The double stain SYBR-14 with propidium iodide (L-7011 LIVE/DEAD Sperm Viability Kit; Invitrogen, Molecular Probes) using a flow cytometer was applied (CytoFlex Beckman Coulter, B3-R1-V0, China). For this purpose, 50 ml of thawed semen was measured (37°C for 20 s) and 940 ml NaCl (0.9%) and 5 ml SYBR14 were added. The whole was thoroughly mixed and then incubated (36°C for 10 min) without light access. Subsequently, 5 ml of PI was remixed and incubated 3 min without light, followed by a test.
2.4 Mitochondrial membrane potential
Mitochondrial membrane potential was evaluated by JC-1 probe (5,5’,6,6'-tetrachloro-1,1′,3,3′-tetraethyl- benzimidazolylcarbocyanine chloride, (Invitrogen). This probe emits green fluorescent at low (LMP) and medium (MMP) mitochondrial potential or red-orange fluorescent at high potential (HMP). The procedure was performed with 200,000 cells diluted in SP-Talp and stained with JC-1 (76.5 μmol/L in DMSO). Samples were analyzed by flow cytometry after 10 min. Minor modification of the methodology was applied to the previously published procedure.11
2.5 Acrosome integrity
Acrosome integrity was evaluated by propidium iodide (PI) and fluorescein isothiocyanate-conjugated Pisum sativum agglutinin (FITC-PSA), respectively. This association divides sperm populations into two groups: intact acrosome (IA), damaged acrosome (DA). The procedure was performed with 200,000 cells diluted in SP-Talp, stained with PI (0.5 mg/ml NaCl 0.9%) and FITC-PSA (FITC-PSA L-0770, Sigma, 100 μg/ml in sodium azide NaN 3 solution at 10% in DPBS). Samples were analyzed by flow cytometry after 10 min. Minor modification of the methodology was applied to the previously published procedure.11
2.6 Biochemical assays
Semen samples were centrifuged at 750 rpm for 10 min to remove the supernatants, obtained cell pellets were washed three times with PBS and then incubated with 0.2% Triton X-100 on ice for 20 min. The supernatants were pipetted for subsequent biochemical analyses. Superoxide dismutase (SOD) and Catalase (CAT) activities were examined using the spectrophotometric method by commercially available enzyme activity test kits (Jiancheng Bioengineering Institute), Malondialdehyde (MDA) content was determined using the thiobarbituric acid (TBA) method by chemical reaction kits (Jiancheng Bioengineering Institute), which performed following manufacturer's instructions as described previously.12
2.7 Statistical analysis
Analysis of variance (ANOVA) was used to assess differences between concentrations of Elamipretide supplementation on all semen characteristics. When the F ratio was significant (p < 0.05), Duncan's multiple range test was used to compare treatment means. The statistical analysis of the results was performed with Statistica 12.0 (StatSoft).
3 RESULTS
Figure 1a and b present the results of the bull's semen quality analysis before and after the cryopreservation process.
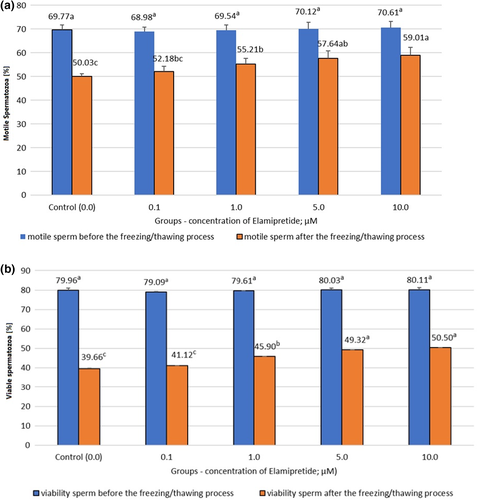
Figure 1a and b show the effects of different concentrations of Elamipretide on sperm cell motility and the stability of their plasmic membrane. The analyzed samples before the cryopreservation process showed no significant differences in terms of sperm motility and viability in any of the studied groups. The highest percentage of motile spermatozoa was observed in the group with the addition of 10 μM of Elamipretide (increase by 8.98%). Significantly higher (p < 0.05) cell motility was observed in samples containing 1; 5; and 10 μM of Elamipretide relative to the additive-free group. Plasmic membrane stability increased in studied groups, the best sperm viability result was obtained in the group with the addition of 10 μM of Elamipretide (50.50%), which was higher by 10.84% than the result obtained in the control group (39.66%). In both parameters tested, the lowest concentration of Elamipretide (0.01 μM) did not significantly affect the obtained results.
Table 1 shows the results of parameters for the motility of frozen/thawed spermatozoa in the presence (or not) of the Elamipretide supplement. In terms of PM, a significant (p < 0.05) improvement of this parameter was observed in the group with the addition of 5.0 and 10.0 μM. Moreover, VCL and VAP also significantly (p < 0.05) improved in the samples containing the two highest concentrations of the additive. Within VSL and LIN, only samples in the presence of 10.0 μM of Elamipretide showed significantly better parameters. There were no significant differences in terms of ALH in the tested samples.
| Parameters | Control group (without Elamipretide) | Elamipretide treatments (concentration, µM) | |||
|---|---|---|---|---|---|
| 0 | 0.1 | 1.0 | 5.0 | 10.0 | |
| Progressive motility (%) (PM) | 48.91a ± 1.9 | 49.98a ± 0.9 | 50.00a ± 1.4 | 54.33b ± 1.7 | 56.51b ± 2.1 |
| Curvilinear velocity (μm/s) (VCL) | 62.44a ± 2.2 | 63.97a ± 1.2 | 65.14a ± 1.2 | 68.89b ± 1.6 | 70.01b ± 1.7 |
| Average path velocity (μm/s) (VAP) | 45.99a ± 1.8 | 46.65a ± 2.6 | 47.10a ± 1.3 | 51.74b ± 1.2 | 52.05b ± 2.2 |
| Straight-line velocity (μm/s) (VSL) | 42.13a ± 1.6 | 42.20a ± 2.3 | 42.69a ± 1.2 | 46.12a ± 1.9 | 47.81b ± 1.8 |
| Linearity (%) (LIN) | 60.02a ± 0.6 | 60.10a ± 0.7 | 61.00a ± 0.7 | 62.94a ± 1.6 | 64.01b ± 2.1 |
| Amplitude of lateral head displacement (μm) (ALH) | 2.70a ± 0.1 | 2.54a ± 0.1 | 2.57a ± 0.1 | 2.55a ± 0.1 | 2. 59a ± 0.1 |
- Explanations: a, b, c, d – means with different superscript letters in the same row differ significantly at p < 0.05.
- The values are expressed as mean ± SD.
Table 2 shows the results of the acrosome integrity and mitochondrial membrane potential after the freezing/thawing process. Significant (p < 0.05) improvement in acrosome integrity was observed in the 1.0, 5.0, and 10.0 µM of Elamipretide supplementation groups. The percentage of sperm cells with MMP was the highest in the group with the highest concentration of the supplement and it was 51.91%, at the same time in the same group the lowest of the percentage of cells with LMP and the highest with HMP was obtained. In the groups with the addition of 1.0 and 5.0 0 μM of Elamipretide, a significantly lower percentage of sperm cells with LMP was obtained compared with the control group.
| Groups | Acrosome Integrity (%) | Mitochondrial Membrane Potential (%) | ||||
|---|---|---|---|---|---|---|
| IA | DA | LMP | MMP | HMP | ||
| Control group (without Elamipretide) | 39.10a ± 2.3 | 60.90a ± 2.3 | 36.66a ± 1.3 | 41.24a ± 1.2 | 22.10b ± 1.3 | |
| Elamipretide treatments (concentration, µM) | 0.1 | 42.22a ± 2.1 | 57.78a ± 2.5 | 38.01a ± 1.2 | 40.71a ± 1.3 | 21.28b ± 1.3 |
| 1.0 | 46.50b ± 2.4 | 53.50b ± 2.1 | 29.30b ± 1.2 | 46.77b ± 1.2 | 23.93ab ± 1.5 | |
| 5.0 | 52.41bc ± 2.3 | 47.59bc ± 2.3 | 23.16b ± 1.1 | 49.89bc ± 1.3 | 26.95a ± 1.4 | |
| 10.0 | 53.27c ± 2.5 | 46.73c ± 2.0 | 22.32b ± 1.4 | 51.91c ± 1.7 | 25.77a ± 1.1 | |
- Explanations: a, b, c, d – means with different superscript letters in the same row differ significantly at p < 0.05.
- The values are expressed as mean ± SD.
- Description:
- LMP – low mitochondrial membrane potential.
- MMP – medium mitochondrial membrane potential.
- HMP – high mitochondrial membrane potential.
It was also observed that with increasing Elamipretide concentration (0.1; 1; 5; 10 µM) DA decreased by 3.12, 7.4, 13.31, and 14.17%, respectively. Significant (p < 0.05) improvement in acrosome damage was observed in the 1.0, 5.0, and 10 µM of Elamipretide groups.
Table 3 shows the influence of Elamipretide addition on SOD, CAT activity, and intracellular MDA in frozen/thawed sperm cells. After cryopreservation, the antioxidant enzyme SOD and CAT activities were also reduced, in the studied groups compared with the control group, while the concentration of MDA was significantly lowered (p < 0.05). Significant differences between the studied groups with Elamipretide supplement and the control group within SOD activity were observed in samples containing 1; 5; and 10 μM of Elamipretide. In terms of CAT enzymatic activity, significant differences were observed in samples containing 0.1; 1; 5; and 10 μM of Elamipretide compared to the control group. MDA concentration in samples containing 1; 5; and 10 μM of Elamipretide significantly (p < 0.05) lowered relative to the control group by 0.63, 0.64, and 0.72 nmol/ml, respectively.
| Groups (concentration; μM) | SOD (U/ml) | CAT (mU/ml) | MDA (nmol/ml) |
|---|---|---|---|
| Control (0) | 3.50c ± 0.31 | 89.11c ± 8.80 | 2.12a ± 0.19 |
| 0.1 | 3.73bc ± 0.44 | 98.83b ± 9.63 | 1.70a ± 0.22 |
| 1.0 | 3.84ab ± 0.37 | 103.56a ± 10.10 | 1.49b ± 0.24 |
| 5.0 | 3.89a ± 0.32 | 103.79a ± 9.98 | 1.48b ± 0.21 |
| 10.0 | 3.93a ± 0.41 | 104.99a ± 13.14 | 1.40b ± 0.23 |
- Explanations: a, b, c, d – means with different superscript letters in the same row differ significantly at p < 0.05.
- The values are expressed as mean ±SD.
4 DISCUSSION
In this study, dose-related improvement in bulls’ semen quality parameters was observed after Elamipretide addition. Analyzes in the field of motility and viability of sperm cells and antioxidant activity of frozen/thawed semen showed that the addition of Elamipretide positively affects the percentage of live spermatozoa obtained after cryopreservation by an average of about 10%, which was also associated with an increased percentage of motile cells by an average of 9% compared with the control group. We also showed that in the tested groups containing 5 and 10 µM, an improvement in sperm movement parameters was achieved. Moreover, we did not observe any changes (including deterioration) in sperm quality immediately after adding Elamipretide to the semen extender. The results of our own research showed that Elamipretide has a protective effect on spermatozoa during freezing, thus reducing the degree of damage of their acrosome. Similar results were obtained by Bai13 who reported that in humans, Elamipretide may reduce acrosome damage induced by cryopreservation.
Mitochondria comprise the main center for energy transfer between intracellular and extracellular compartments and play important roles in the maintenance of cell function and in apoptosis.14 The results of this experiment showed that Elamipretid plays an important role in maintaining the high mitochondrial potential of sperm cells, the use of 5 and 10 µM of the supplement allowed to protect the mitochondria from the negative effects of oxidative stress during freezing/thawing. The results are in accordance with previous reports that Elamipretide protects the integrity of mitochondria by maintaining the MMP level, and protects against open the mitochondrial permeability transition pore (mPTP) in mouse.15
The stress on the plasma membrane during sperm cooling may occur because changes in the asymmetry of the phospholipid bilayer and the altered functional state of the membrane. Lipids and proteins in a fluid state, solidifies into the gel, producing a rigid and fragile structure, more sensitive to injuries.16 Furthermore, sperm cells are exposed to a hyperosmotic environment that induces an influx of water and ions across the membrane, leading to cell dehydration.17 Our study shows that a progressive loss of integrity occurred in both acrosome and plasma membranes during cryopreservation. Nevertheless, the addition of Elamipretid to semen extender used in our experiment was effective in preventing damage to the acrosome and cytoplasmic membrane.
As a result of the fact that sperm cells have less cytoplasm and the plasma membrane is rich in unsaturated fatty acids, it is susceptible to oxidative stress damage during cryopreservation.18, 19 Moreover, the reduced antioxidant activity of SOD observed in bull and ram spermatozoa after cryopreservation could explain in part the increased susceptibility of frozen/thawed spermatozoa to oxidative damage.20, 21
So far it has been shown Elamipretide effectively resists stress and fights diseases in the myocardium, nervous, and endocrine systems.22-24 In research conducted by Bai et al.13 it has been shown that Elamipretide supplement significantly improved post-thaw sperm parameters including motility and viability, the stability of the plasma membrane, and mitochondria and chromosomes. In our own research, it was observed a decrease in SOD and CAT activity and the accumulation of MDA that has also been confirmed by other researchers.25, 26 Production of ROS triggers the oxidative attack of polyunsaturated phospholipids, resulting in the formation of malondialdehyde (MDA).27 Our own research has shown that the concentration of MDA decreased as the concentration of the Elamipretide added increased.
The present study showed that samples containing Elamipretide at a concentration of 5 and 10 μM proved to be the most effective in terms of the tested parameters of the quality of sperm cells subjected to cryopreservation. No toxic or limited effect on the spermatozoa of the above additive at a concentration of 10 μM has been demonstrated.
5 CONCLUSION
In conclusion, the addition of the Elamipretide to the cryopreservation extender significantly improved frozen-thawed sperm cells’ quality and their function. The results of this study indicate that Elamipretide can be used as a cryoprotective agent to protect cells against the devastating effects of oxidative stress and increasing sperm survival after cryopreservation.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
We declare that all authors made substantial contributions to this manuscript. A.K. in conducting the experiment, in the conception and design of the study, and in establishing the methodology. E.C-P., in the collection, assembly of data and in the analysis and interpretation of results. A.K., E.C-P., in the preparation of the manuscript.
ETHICAL APPROVAL
The experiment has been performed as part of routine activities during the current semen production in the reproductive station and did not require the approval of the ethics committee.
Open Research
DATA AVAILABILITY STATEMENT
The datasets used during the current study are available from the corresponding author on reasonable request.




