Contribution of airway eosinophils in airway wall remodeling in asthma: Role of MMP-10 and MET
Funding information
The U-BIOPRED project is supported through an Innovative Medicines Initiative Joint Undertaking under grant agreement 115010, resources of which are composed of financial contributions from the European Union's Seventh Framework Programme (FP7/2007-2013) and European Federation of Pharmaceutical Industries and Associations companies’ in-kind contributions (www.imi.europa.eu).
Abstract
Background
Eosinophils play an important role in the pathophysiology of asthma being implicated in airway epithelial damage and airway wall remodeling. We determined the genes associated with airway remodeling and eosinophilic inflammation in patients with asthma.
Methods
We analyzed the transcriptomic data from bronchial biopsies of 81 patients with moderate-to-severe asthma of the U-BIOPRED cohort. Expression profiling was performed using Affymetrix arrays on total RNA. Transcription binding site analysis used the PRIMA algorithm. Localization of proteins was by immunohistochemistry.
Results
Using stringent false discovery rate analysis, MMP-10 and MET were significantly overexpressed in biopsies with high mucosal eosinophils (HE) compared to low mucosal eosinophil (LE) numbers. Immunohistochemical analysis confirmed increased expression of MMP-10 and MET in bronchial epithelial cells and in subepithelial inflammatory and resident cells in asthmatic biopsies. Using less-stringent conditions (raw P-value < 0.05, log2 fold change > 0.5), we defined a 73-gene set characteristic of the HE compared to the LE group. Thirty-three of 73 genes drove the pathway annotation that included extracellular matrix (ECM) organization, mast cell activation, CC-chemokine receptor binding, circulating immunoglobulin complex, serine protease inhibitors, and microtubule bundle formation pathways. Genes including MET and MMP10 involved in ECM organization correlated positively with submucosal thickness. Transcription factor binding site analysis identified two transcription factors, ETS-1 and SOX family proteins, that showed positive correlation with MMP10 and MET expression.
Conclusion
Pathways of airway remodeling and cellular inflammation are associated with submucosal eosinophilia. MET and MMP-10 likely play an important role in these processes.
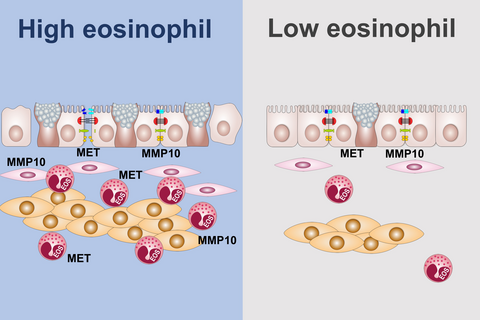
Graphical Abstract
Analysis of differentially expressed genes in eosinophil-high biopsies of asthma yielded MMP10 and MET as potential drivers of airway wall remodeling. Important pathways include extracellular matrix organization, mast cell activation, C-C receptor binding, and regulation of leukocyte activation. Bronchial eosinophils are important drivers of airway wall remodeling in asthma. MMP10: Matrix metalloprotease 10 gene. MET: Hepatocyte growth factor receptor gene.
CONFLICTS OF INTEREST
Dr. Djukanovic reports personal fees from TEVA, grants and personal fees from Novartis, and personal fees and other support from Synairgen, outside the submitted work. Dr. Howarth reports part-time employment by GSK as Global Medical Expert. Dr. Krug, Dr. Kuo, Dr. Wilson, Dr Guo, Dr Pandis, Dr Pavlidis, Dr Gibeon, Dr Hoda, Dr Fowler, Dr. Zhu, Dr Corfield, and Dr. Sousa have nothing to disclose. Dr. Chung reports personal fees from advisory board membership, grants for research, and personal fees from payments for lectures, outside the submitted work. Dr F. Baribaud is a current employee and shareholder of Janssen R&D. Dr. Dahlén reports personal fees from advisory board membership and personal fees from payments for lectures, outside the submitted work. Dr. Auffray reports grants from Innovative Medicine Initiative and grants from Innovative Medicine Initiative, during the conduct of the study. Dr. Loza reports other from Janssen R&D, outside the submitted work. Dr Shaw reports personal fees from advisory board meetings, outside the submitted work. Dr. Sterk reports grants from Innovative Medicines Initiative (IMI), during the conduct of the study. Dr Chanez had consultancy services for Boehringer Ingelheim, Johnson & Johnson, GlaxoSmithKline, Merck Sharp & Dohme, AstraZeneca, Novartis, Teva, Chiesi, Sanofi, and SNCF; served on advisory boards for Almirall, Boehringer Ingelheim, Johnson & Johnson, GlaxoSmithKline, AstraZeneca, Novartis, Teva, Chiesi, and Sanofi; received lecture fees from Boehringer Ingelheim, Centocor, GlaxoSmithKline, AstraZeneca, Novartis, Teva, Chiesi, Boston Scientific, and ALK; and received industry-sponsored grants from Roche, Boston Scientific, Boehringer Ingelheim, Centocor, GlaxoSmithKline, AstraZeneca, ALK, Novartis, Teva, and Chiesi. Dr A. Rowe is a current employee and shareholder of Janssen R&D. Dr. Adcock reports personal fees from advisory board membership, grants from grants, and personal fees from payments for lectures, outside the submitted work. Dr. Sandström reports personal fees from advisory board membership and personal fees from payments for lectures, outside the submitted work. Dr. Fleming reports personal fees from advisory board membership, grants for research, and personal fees from payments for lectures, outside the submitted work.