Bacterial secretion of histamine within the gut influences immune responses within the lung
Funding information
The authors are supported by Swiss National Science Foundation grants (project numbers CRSII3_154488, 310030_144219, 310030-127356 and 310030_144219) and funding from the Christine Kühne-Center for Allergy Research and Education (CK-CARE).
Abstract
Background
Histamine is an important immunomodulator influencing both the innate and adaptive immune system. Certain host cells express the histidine decarboxylase enzyme (HDC), which is responsible for catalysing the decarboxylation of histidine to histamine. We and others have shown that bacterial strains can also express HDC and secrete histamine; however, the influence of bacterial-derived histamine on the host immune responses distant to the gut is unclear.
Methods
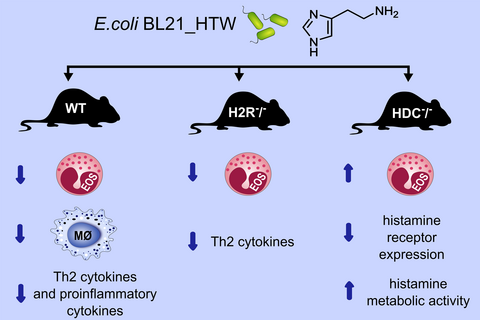
The Escherichia coli BL21 (E coli BL21) strain was genetically modified to express the Morganella morganii (M morganii)-derived HDC gene (E coli BL21_HTW). E coli BL21 and E coli BL21_HTW were gavaged to ovalbumin (OVA) sensitized and challenged mice to investigate the effect of bacterial-derived histamine on lung inflammatory responses.
Results
Oral administration of E coli BL21_HTW, which is able to secrete histamine, to wild-type mice reduced lung eosinophilia and suppressed ex vivo OVA-stimulated cytokine secretion from lung cells in the OVA respiratory inflammation mouse model. In histamine receptor 2 (H2R)-deficient mice, administration of histamine-secreting bacteria also reduced inflammatory cell numbers in bronchoalveolar lavage (BAL). However, the suppressive effect of bacterial-derived histamine on BAL inflammation was lost in HDC-deficient mice. This loss of activity was associated with increased expression of histamine degrading enzymes and reduced histamine receptor expression.
Conclusion
Histamine secretion from bacteria within the gut can have immunological consequences at distant mucosal sites, such as within the lung. These effects are influenced by host histamine receptor expression and the expression of histamine degrading enzymes.
Graphical Abstract
CONFLICT OF INTEREST
LOM is a consultant to Alimentary Health Ltd and has received research funding from GlaxoSmithKline. CA has received research support from Allergopharma, Actellion, SNF and CK-CARE. The other authors have no relevant conflicts of interest.