Renal inflamm-aging provokes intra-graft inflammation following experimental kidney transplantation
An He and Attia Sarwar equally contributed as first authors.
Abstract
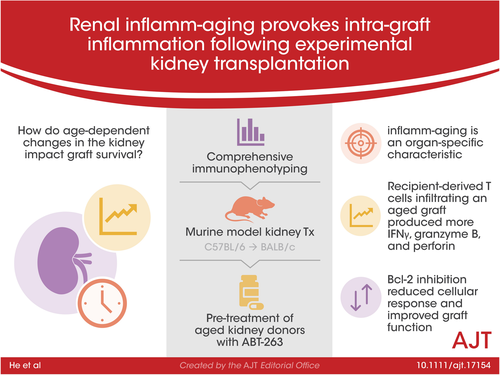
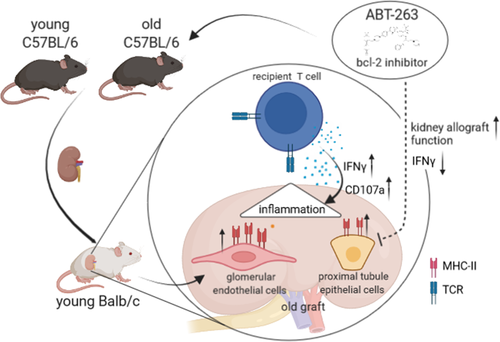
Donor age is a major risk factor for allograft outcome in kidney transplantation. The underlying cellular mechanisms and the recipient's immune response within an aged allograft have yet not been analyzed. A comprehensive immunophenotyping of naïve and transplanted young versus aged kidneys revealed that naïve aged murine kidneys harbor significantly higher frequencies of effector/memory T cells, whereas regulatory T cells were reduced. Aged kidney-derived CD8+ T cells produced more IFNγ than their young counterparts. Senescent renal CD8+ T and NK cells upregulated the cytotoxicity receptor NKG2D and the enrichment of memory-like CD49a+CXCR6+ NK cells was documented in aged naïve kidneys. In the C57BL/6 to BALB/c kidney transplantation model, recipient-derived T cells infiltrating an aged graft produced significantly more IFNγ, granzyme B and perforin on day 7 post-transplantation, indicating an enhanced inflammatory, cytotoxic response towards the graft. Pre-treatment of aged kidney donors with the senolytic drug ABT-263 changed the recipient-derived effector molecule profile to significantly reduced levels of IFNγ and IL-10 compared to controls. Graft function after ABT-263 pre-treatment was significantly improved 28 days post kidney transplantation. In conclusion, renal senescence also occurs at the immunological level (inflamm-aging) and aged organs provoke an altered recipient-dominated immune response in the graft.
Abbreviations
-
- CKD
-
- chronic kidney disease
-
- DC
-
- dendritic cell
-
- DGF
-
- delayed graft function
-
- GBM
-
- glomerular basement membrane
-
- PTECs
-
- proximal tubule epithelial cells
-
- SASP
-
- senescence-associated secretory phenotype
-
- Trm
-
- tissue-resident memory T cells
1 INTRODUCTION
Worldwide, the number of individuals with an advanced age >65 years is steadily increasing. This results in a higher number of patients diagnosed with progressive chronic kidney disease (CKD) constituting a potential kidney transplant recipient group.1, 2 Chronological donor age is also a major risk factor for allograft dysfunction, as grafts from older donors are more susceptible to ischemic injury and prone to develop delayed graft function (DGF) post kidney transplantation.3-5 Aged donor kidneys are characterized by structural changes, including glomerular basement membrane (GBM) permeability, changes in podocyte morphology and nephron loss, resulting in reduced glomerular filtration rate and altered homeostasis.6-12
Renal aging also entails chronic, sterile low-grade inflammation paralleled with the development of fibrosis occurring at different molecular and cellular levels including DNA damage, dysfunctional telomeres, and protein aggregation.13 One major mechanism is mediated via cyclin-dependent kinase inhibitor p16INK4a resulting in growth arrest of viable cells and changes in the cell's secretory phenotype, called senescence-associated secretory phenotype (SASP).14-16 In addition, the reduced regenerative capacity of stem cells and their progeny accelerates cellular senescence.17
Despite these findings, the exact mechanisms of age-dependent alterations in the kidney impacting long-term overall graft survival still remain unclear. Although it has been speculated that the SASP present in older allografts creates a pro-inflammatory milieu, literature on this topic is scarce. Some experimental data suggested that parenchymal changes seen in older allografts are associated with enhanced immunogenicity, therefore accelerating rejection, whereas other studies failed to detect such differences.18, 19 The process of progressive, multidimensional, physiological degeneration of the immune system resulting in a low-grade chronic inflammation has been described as inflamm-aging.20, 21 However, it has not been addressed as to how kidney-resident lymphocytes may undergo senescence. In this context we recently demonstrated that frequencies of tissue-resident memory CD8+ T cells (Trm) residing in human kidneys correlate with chronological age and that CD4+ Trm correlate with kidney function.22 Here, using experimental mouse models, we show how age impacts immunity in the naïve kidney, but also assess how aged organs shape the recipients´ allo-response, with possible implications for therapeutic targeting.
2 MATERIALS AND METHODS
2.1 Animals
Young BALB/c and C57BL/6 mice (8–12 weeks) and C57BL/6 mice (20 months) were purchased from Charles River Laboratories (Charles River). Male mice weighing 24–30 g were used. Animals were housed under standard conditions and received human care in compliance with the ‘Principles of Laboratory Animal Care’ prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH Publication No. 86–23, revised 1985). All animal experiments were approved by the Landesamt für Gesundheit und Soziales Berlin, Germany.
2.2 Kidney and heart transplantation
Renal transplantations were performed as previously described.23 Briefly, after procurement of the left donor kidney, end-to-side anastomoses between the donor renal vessels and the recipient's abdominal aorta and inferior vena cava were performed following a knotless technique. Animals were either sacrificed on day 7 or on day 28 without receiving immunosuppression. For long-term survival experiments, the contralateral kidney was removed 1 day before animals were sacrificed, allowing determination of graft function after 24 h of observation. For sensitization of recipients, fully allogeneic BALB/c- (donor) derived hearts were transplanted into C57BL/6 recipients using a heterotopic cardiac transplantation model as described elsewhere.24
2.3 In vivo treatment
ABT-263 (50 mg/kg/day Navitoclax, Hycultech GmbH) was administered in two cycles by oral gavage with a 1-week interval in-between. Control animals received corn oil. Animals were sacrificed either on day 7 (short-term survival) or on day 28 (long-term survival). In the long term group, the contralateral kidney was removed 1 day before animals were sacrificed in order to analyze serum creatinine and urea being indicative for graft function.
2.4 Serum analysis of kidney function parameters
Serum samples were stored in aliquots at −20°C until serum creatinine and urea were measured using the CREP2 Creatinine Plus version 2 and Urea/BUN assays, respectively, on a Roche/Hitachi Cobas C 701/702 system (Roche Diagnostics).
2.5 Isolation of cells
For isolating renal MNCs, tissues were mechanically dissociated and digested in 10 ml of RPMI medium supplemented with collagenases II and IV (Gibco/Invitrogen, Worthington) and DNase I (Roche Diagnostics) for 45 min at 37°C. Following digestion, recovered leukocytes were enriched using CD45 Microbeads over MACS LS columns (Miltenyi Biotec, Inc.). Leukocytes from spleen and lymph nodes were isolated by density gradient centrifugation. For analysis and culture of PTECs, kidneys were minced into 2 mm3 pieces, processed through a 180 μm stainless steel sieve and collected in DMEM/F12 medium. Flow-through was applied to a 100 μm cell strainer from which renal tubular segments and glomeruli were recovered by reverse flushing with medium. After centrifugation, tubular segments were digested using collagenase II for 20 min at 37°C in a shaking waterbath. Thereafter, cells were either immediately analyzed by FACS or transferred to 6 well plates for outgrow cultures; 80% confluence of PTECs was reached after 5–6 days. Glomerular endothelial cells (gECs) were isolated as already published.25 Briefly, glomeruli were digested using Trypsin–EDTA 0.25% (Life Technologies) for 23 min at 37°C in a shaking waterbath, with pellet resuspension every 5 min. gECs were harvested by filtering the suspension through a 40 μm strainer for immediate FACS analysis.
2.6 Flow cytometry
Typically 1 × 106 cells were stained with antibodies listed in Table S1. Cells were measured using a FACS Fortessa X20 (BD Bioscience). FACS data analysis was conducted using FlowJo software 10.0 (Tree Star Inc.). A gating strategy for identification of the various lymphocyte subsets is illustrated in Figures S1 and S2. Polyfunctionality was assessed via Boolean gating. Generation of t-Distributed Stochastic Neighbor Embedding (t-SNE) plots, FlowSOM analysis and heatmaps was conducted using Cytobank (Beckman Coulter).
2.7 In vitro assays
Functional analysis of T and NK was performed as previously described.23 Cells were rested in 200 U/ml murine IL-2 (Miltenyi Biotec) over night, followed by stimulation with 50 ng phorbol 12-myristate 13-acetate (PMA) and 1 μg ionomycin (Sigma-Aldrich) for 4 h in the presence of 10 μg/ml brefeldin A and 2 μM monensin (Biolegend). CD107a expression was used as a correlate for degranulation. Following activation, cells were surface stained, fixed and permeabilized (Transcription Factor Staining Buffer Set; Thermofisher), followed by intracellular staining (representative raw data shown in Figure S3). To assess alloreactivity, DCs were isolated from young and old BALB/c kidneys by magnetic purification using the MojoSort panDC kit (Biolegend) in combination with MACS LD-type columns for depletion of unwanted cells (Miltenyi Biotec). Splenic CD3+ T cells were equally enriched using the MojoSort T cell kit (Biolegend) from C57BL/6 mice sensitized with a BALB/c heart on day 7 post transplantation, followed by staining with the proliferation dye cell trace violet (CTV, 1 μM, Thermofisher). Allo-induced T cell proliferation was monitored by FACS based on loss of CTV after 4 days of co-culture of 104 DCs with 105 T cells.
2.8 Real-Time RT-PCR
Real-Time PCR was performed as previously described.26 Total RNA was extracted from snap-frozen samples using RNeasy Mini Kit (Qiagen). RT-PCR was performed for gene expression analysis on ABI 7500 Fast Real-Time PCR System (Applied Biosystems, Life Technologies) using Taqman® gene expression assays (Table S2). Gene expression was normalized (2-ΔCt formula) to hypoxanthine-guanine phosphoribosyltransferase (HPRT).
2.9 Histology
Kidney tissues were formalin-fixed and paraffin-embedded; 1 μm thick sections were prepared, processed and stained with periodic acid schiff (PAS) and counterstained with hematoxilin as previously described.27 Samples were assessed for necrosis, acute tubular damage, and glomerulitis. Semiquantitative scores in alignment to the Banff criteria were adapted and designed for each lesion. Following scores were used: acute tubular damage: 0 = non, 1 = mild, 2 = moderate, 3 = severe; necrosis: 0 = non, 1 = 1–10% of tissue, 2 = 11–20% of tissue, 3 = more than 20% of tissue; interstitial inflammation in non-fibrotic cortex: 0 = < 10%, 1 = 11–25%, 2 = 26–50%, 2 = more than 50%; glomerulitis: 0 = non, 1 = present in at least one glomerulus. Scoring was performed in a blinded fashion based on the whole slide of each sample. Staining of ß-galactosidase was performed according to the manufacturer's instructions (Abcam).
2.10 Immunofluorescence microscopy
Immunofluorescence microscopy (IF) was done as previously published.28 Briefly, transplanted kidneys were excised and a ¼ of each kidney was fixed overnight at 4°C with PLP fixative. Following extensive wash with PBS, kidneys were dehydrated in 30% sucrose in PBS overnight at 4°C, snap frozen in OCT and stored at −80°C. 16 μm kidney cryosections were mounted on Superfrost Plus microscope slides (Fisher) and dried overnight at room temperature. Sections were blocked and stained in a humidified box in the dark at room temperature overnight with purified or directly conjugated antibodies (Table S3). Next morning, following washes with PBS sections were stained with the respective secondary antibody for 1 h at room temperature, counterstained with Hoechst and mounted with Fluoromount-G (eBiosciences). Samples were scanned on a Zeiss LSM780 confocal microscope using a dry 20× 0.8 N.A. objective. Tiled, z-stacks were acquired at 1024 × 1024 pixels, with line averaging of 4 and pinhole size 1, and were stitched together using Zen Blue software (Zeiss). Fuji (version 1.53r) was used to process the acquired images and generate maximal intensity projection snapshots.
2.11 Statistics
Statistical analysis was performed using GraphPad Prism 8 (GraphPad Software). Significant differences between groups were calculated applying either T- or Mann–Whitney U test (two groups) or Kruskal–Wallis test (multiple groups) after testing for normality distribution using the D'Agostino & Pearson test. Statistical significance was considered for the following p values: ns = p > .05, *p ≤ .05, **p ≤ .01, ***p < .001, ****p ≤ .0001.
3 RESULTS
3.1 Solid and lymphoid organs are differentially affected by inflamm-aging
Applying a viSNE algorithm approach to assess FACS derived data for T cell diversity, we observed no clear differences between young and aged kidneys (Figure 1A, top). Next, we applied FlowSOM, a technique for unsupervised clustering of FACS data. Generated viSNE maps were overlayed with metaclusters identified in FlowSOM, allowing the identification of multiple T cell subpopulations based on their memory markers present in both young and aged kidneys (Figure 1A, bottom). Based on the annotated clusters, manual gating of FACS data for T cells revealed that frequencies of CD8+ T cells were significantly increased in aged kidneys, spleen and lymph nodes whereas frequencies of CD4+ T cells were decreased. Histology of young and old naïve kidneys did not reveal histopathological changes regarding necrosis, edema or interstitial inflammation (Figure S5A), however aged kidneys showed slightly more acute tubular injury (Figure 1B).
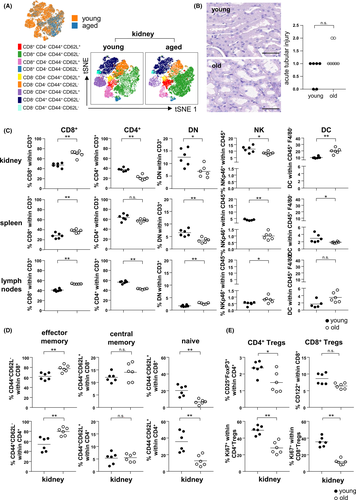
We further observed significantly reduced frequencies of double-negative (DN) T cells as well as NKp46+ NK cells in aged kidneys and spleens compared to young organs, whereas both subsets were significantly increased in lymph nodes. Aged kidneys and lymph nodes were also characterized by significantly higher frequencies of CD11c+MHC-II+ dendritic cells (DCs) than their young counterparts (Figure 1C).
Both, FlowSOM and manual gating demonstrate that kidney, spleen and lymph nodes harbor significantly higher frequencies of effector memory CD4+ and CD8+ T cells (Figure 1A,D, Figure S5). In contrast, frequencies of natural CD4+CD25+FoxP3+ regulatory T (Treg) cells were significantly reduced in the aged kidney, being characterized by diminished portions of proliferating Ki67+ cells; both features also applied to CD122+ CD8+ regulatory T cells (Figure 1E). As opposed to the kidney, CD8+ Treg frequencies were significantly higher in aged spleens and lymph nodes, but showing less proliferative capacity compared to their young counterparts (Figure S6). Thus, aging results in a change of the intra-renal lymphocyte compartment towards an effector memory phenotype paralleled with a loss of regulatory T cells.
3.2 A distinct subset of NKp46+ NK cells expressing CD49a and CXCR6 resides in senescent kidneys
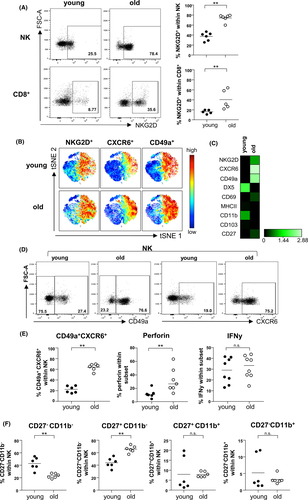
We previously described increased mRNA expression of the activating cytotoxicity receptor NKG2D in human renal zero-hour biopsies as being indicative for biological donor age.29 Here, we identified higher frequencies of NKG2D+ NK and CD8+ T cells in relation to age, confirming that NKG2D is upregulated on senescent renal lymphocytes (Figure 2A). This observation was also corroborated by viSNE analysis for NKp46+ NK cells and equally applied to the chemokine receptor C-X-C chemokine receptor type 6 (CXCR6) and the alpha 1 subunit of α1β1 integrin (CD49a) (Figure 2B). Heatmap analysis confirmed this induction, whereas other NK cell markers including DX5 and CD11b were downregulated (Figure 2C). Interestingly, CXCR6+CD49a+ NK cells have already been identified as tissue-resident NK cells in the human liver,30 a feature that we confirm for murine kidneys and that quantitatively increases with age (Figure 2D,E). Aged CXCR6+CD49a+ NK cells contain significantly higher portions of perforin+, but not IFNγ+ cells (Figure 2E). Finally, despite lower overall frequencies of NK cells in aged kidneys (Figure 1B), we detected increased frequencies of differentiated CD27+CD11b− NK cells (Figure 2F), being associated with greater effector function and responsiveness to chemokines.31
3.3 Intra-renal lymphocytes display a distinct inflammatory effector function profile according to age
Both aged renal CD8+ and CD4+ populations contained significantly higher frequencies of degranulating CD107a+ as well as IFNγ+ cells, whereas portions of granzyme B producers were reduced (Figure 3A). Senescent NK cells less frequently expressed the cytotoxic mediators granzyme B and perforin, along with reduced proportions of IFNγ+ cells, indicating an impairment of classical NK effector functions. Increased frequencies of IL-17+ cells were confined to the senescent CD4−CD8− DN T and NK cell compartment (Figure 3A). Aged kidney-derived CD8+ and CD4+ T cells further demonstrated an increase in polyfunctionality reflected by higher portions of cells secreting two or three effector molecules at a time, whereas senescent NK cells lost that ability (Figure 3B,C). In summary, our phenotypic analysis revealed a re-composition and altered effector profile of intra-graft lymphocytes in aged kidneys, which needs to be considered for their potential role as passenger leukocytes.
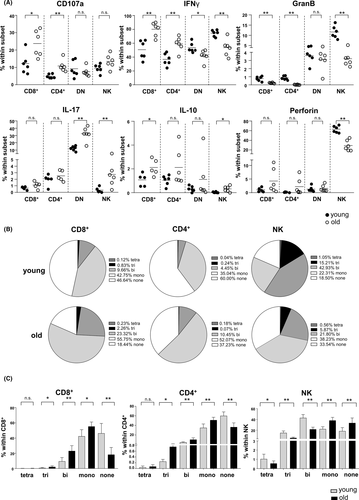
3.4 The senescent kidney graft provokes an inflammatory immune response
In order to gain knowledge into the kinetics of graft infiltration, using congenic strains, we transplanted CD45.2 BALB/c kidneys into CD45.1 C57BL/6 recipients. Analysis of the intra-renal leukocyte composition revealed that whereas on day 3 post-transplantation donor-derived leukocytes were still detectable, the kidney was completely re-populated on day 7 by recipient-derived cells (Figure 4A). Thus, the majority of cells isolated from the graft in subsequent experiments are derived from the kidney recipient. Already on day 3, both recipient-derived, graft-infiltrating CD45.1+ CD4+ and CD8+ T cells express the integrin αE (CD103) as well as the activation/retention marker CD69 being indicative for tissue-residency (Trm). This expression increased for CD8+ T cells until day 7, demonstrating that graft-infiltrating CD4+ and CD8+ T cells acquire a Trm phenotype after entering the graft (Figure 4B). We hypothesized that aged grafts provoke the formation of higher intra-renal Trm frequencies. However, whereas no differences between young and aged grafts were observed for CD8+ Trm, frequencies of CD4+ Trm were significantly decreased in aged kidney grafts on day 7 post-transplantation (Figure S7A).
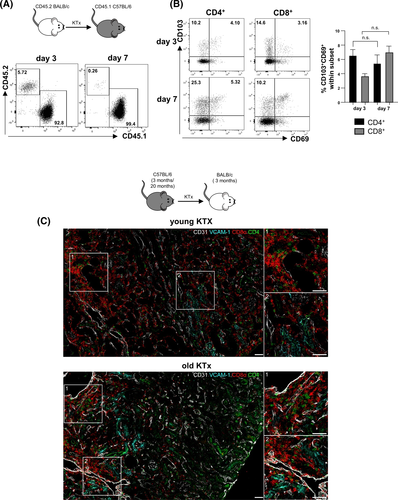
Finally, we transplanted kidneys derived from either young (3 months) or aged (20 months) C57BL/6 mice into young (3 months) BALB/c recipients. Although we used in previous experiments BALB/c mice as donors,23 this was not possible in this experimental set-up, as only aged C57BL/6 mice were available. On day 7 post-transplantation, CD4+ and CD8+ T cells were identified in young and aged organs. Both cell types were located in close proximity to CD31+ (endothelial) cells and, although not exclusively, in close proximity to VCAM-1+ tubuli in the cortex of kidney grafts (Figure 4C). Higher graft-infiltrating CD4+ T cell frequencies were observed in aged organs, which was not observed for the spleen or lymph nodes (Figure 5A, Figure S7B). On the contrary, frequencies of total NK cells and CD49a+CXCR6+NKp46+ NK cells were significantly decreased in aged kidneys compared with young grafts, but contained higher portions of IFNγ+ cells (Figure 5B). Furthermore, we observed higher infiltration of effector memory CD4+ T (TEM) cells in aged kidneys (Figure S7C). Recipient-derived CD8+ T cells infiltrating an aged kidney graft showed enhanced degranulation capacity reflected by CD107a, granzyme B, perforin and IFNγ expression with the latter feature also accounting for CD4+ and DN T cells. Surprisingly, recipient-derived CD4+ and CD8+ T cell subsets isolated from a senescent kidney engrafted into a young recipient produced significantly more IL-10 than cells from a young graft (Figure 5C). Taken together, although frequencies of graft infiltrating cells are not dramatically different between young and aged kidneys, old grafts provoke a significantly higher inflammatory immune response mediated by graft-infiltrating recipient-derived T cells.
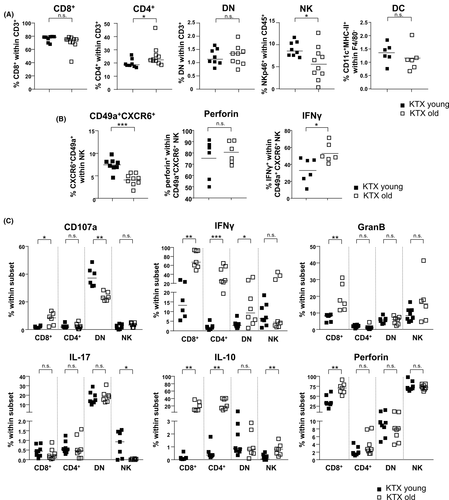
3.5 Senescent proximal tubular epithelial cells upregulate MHC class II and co-stimulatory molecules
Aged allografts exhibited a higher incidence of glomerulitis, but not necrosis or tubular damage compared with young grafts (Figure 6A,B). Both young and aged grafts showed the presence of CD3+ T cells in glomeruli (Figure 6C).
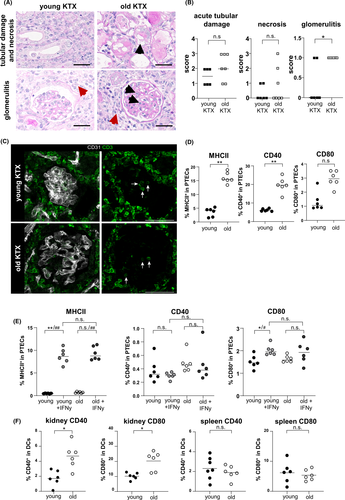
We further addressed whether proximal tubule epithelial cells (PTECs) might also contribute to age-related local alloinflammation. Freshly isolated PTECs from naive aged kidneys contained significantly higher portions of MHC class II+, CD40+ and CD80+ cells than their young counterparts (Figure 6D). To gain sufficient cells for allo-stimulatory assays with T cells, we expanded PTECs from young versus old naïve mice for 5 days in vitro. As shown in Figure 6E, however, PTECs lose MHC class II and CD40 during culture as compared to ex vivo expression (Figure 6D). To re-induce costimulatory molecules, the expansion culture medium was supplemented with IFNγ, resulting in significant MHC class II upregulation; however, no differences between young and aged PTECs could be observed under these conditions (Figure 6E). CD80 expression was upregulated following IFNγ treatment solely on young kidney-derived PTECs but did not demonstrate higher expression levels than aged kidney-derived PTECs (Figure 6E). As an alternative celltype for stimulation of allo-specific T cells, DCs were isolated. In line with data from PTECs, aged renal DCs showed significantly higher expression of CD40 and CD80 (Figure 6F), a feature not accounting for splenic DCs. However, the allo-stimulatory capacity of aged versus young kidney-derived DCs by adding allo-sensitized T cells was comparable (Figure S8).
3.6 ABT-263 pre-treatment reduces inflammation in the aged graft
ABT-263 (Navitoclax) is a Bcl-2/w/xL inhibitor targeting the Bcl-2 pathway in senescent cells, resulting in cellular apoptosis.32 Currently, ABT-263 is used in phase 1/2 clinical trials for both hematologic and solid organ malignancies, suggesting additional potential in other conditions requiring depletion of senescent cells, such as kidney transplantation.33 We therefore treated naïve aged C57BL/6 mice with ABT-263 dosages. To examine potential effects of ABT-263 on renal senescent cells, we analyzed the contralateral kidney for classical markers indicative for renal senescence, tubular injury and inflammation.18, 34, 35 Staining with ß-galactosidase, a classical senescence marker, demonstrated a reduction of senescent PTECs in ABT-263 pre-treated animals confirming recent data (Figure 7A).34 Both aged mice treated with ABT-263 and untreated controls demonstrated a significantly higher mRNA expression of CDKN2a (p16INK4a), HAVCR1 (kidney injury molecule 1, KIM 1) and CCL2 (Monocyte Chemoattractant Protein-1, MCP-1) compared to untreated young kidneys (Figure 7B). Intriguingly, p16INK4a was induced, whereas KIM-1 and MCP-1 showed a reduced mRNA expression in ABT-263 kidneys. In order to evaluate the impact of ABT-236 treatment of renal endothelial cells, we isolated glomerular endothelial cells (gECs) from naïve young, old untreated and old ABT-263 treated mice. Comparable with PTECs, aged renal gECs demonstrated a significantly higher expression of MHCII compared with young gECs, which was partially observed for CD40 and CD80. This expression was downregulated in kidneys from ABT-263 pretreated mice, although not statistically significant (Figure 7C). Interestingly, CD4+ and CD8+ T cells isolated from ABT-263 pre-treated untransplanted kidneys showed significantly less degranulation capacity and IFNγ production (Figure 7D).
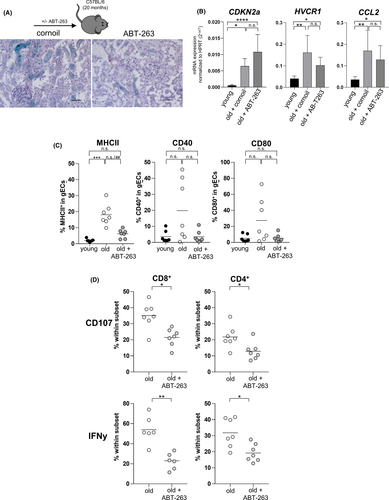
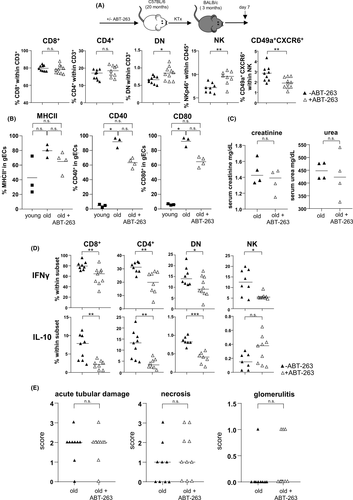
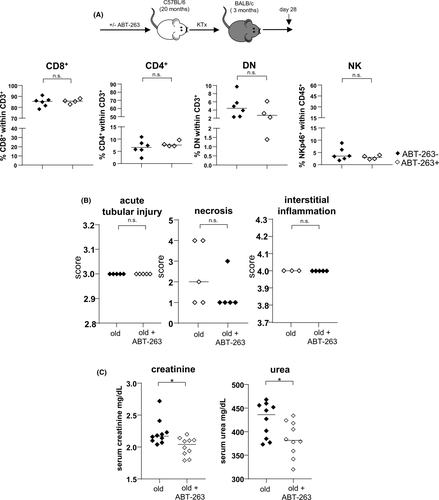
On day 7 post kidney transplantation, higher frequencies of DN T cells and NK cells infiltrated ABT-263 pre-treated allografts. Despite the overall increase of NK cells, frequencies of CD49a+CXCR6+ NKp46+ NK cells were significantly lower in grafts from ABT-263 treated mice (Figure 8A). Moreover, gECs isolated on day 7 post kidney transplantation from ABT-263 pre-treated mice showed still reduced levels of MHCII, CD40 and CD80 compared with aged untreated controls although not statistically significant (Figure 8B). Although no difference for graft function was detected on day 7 post transplantation (Figure 8C), recipient-derived, graft-infiltrating cells demonstrated a significant reduction of IFNγ and IL-10 for all T cell subsets (Figure 8D). We did not detect any histological changes between the ABT-263 treated and non-treated groups (Figure 8E). To prove a long-term effect of ABT-263 treatment, we repeated the aforementioned experiments, sacrificing the animals on day 28. Again, no dramatic changes for the various intra-renal lymphocyte populations analyzed were detected (Figure 9A), but creatinine and urea levels were significantly lower in the ABT-263 treated versus non- treated group, indicating an improvement of graft function after ABT-263 pre-treatment despite of unaltered histology (Figure 9A–C). Figure 10 summarizes the main findings of the study.
4 DISCUSSION
Strategies to address the current organ shortage request a critical re-examination of donor eligibility, especially including the elderly.36, 37 Immune activation within a renal allograft may be mediated via the vasculature and/or passenger immune cells, yet no experimental studies have determined the impact of aging on any of these compartments. As molecular mechanisms of senescence have already been documented,38-40 we aimed to understand the immunological component first, by assessing the intra-renal cellular composition according to kidney senescence. A shift of the CD4:CD8 ratio has already been described in association with aging and obesity in humans,41 however, our analysis confirms higher frequencies of CD8+ T cells, paralleled by a reduction of CD4+ T cells, in the aged kidney. According to studies performed in human peripheral blood and murine studies, we detected a significantly higher portion of CD4+ and CD8+ TEM, whereas naïve T cells were decreased.42, 43 Our findings further demonstrate that renal NK cells were significantly reduced in naïve, older kidneys displaying reduced cytotoxicity and impaired polyfunctionality. Although an age-dependent decline of peripheral blood NK cells in humans has been described,44, 45 alternative studies document a significant increase in the percentage and/or absolute number of CD3− CD56+ NK cells according to age.46, 47 We could also identify the presence of CD49a+CXCR6+ NK cells in the aged kidney. Both CD49a and CXCR6 have been originally described as markers of murine and human liver NK cells, the latter being critical for their long-term homeostasis.48, 49 These liver CXCR6+ NK cells can mediate intense skin inflammation, suggesting that CXCR6+ NK cells possess a memory potential.48 As NK cells are emerging as powerful drivers of immune-mediated kidney allograft rejection,50-53 future studies will be necessary to determine which defined NK cell subpopulation might be responsible for these effects. In summary, our findings document that the aging renal compartment is characterized by a defined composition of lymphocytes, which is not necessarily reflected in spleen or lymph nodes. Thus, inflamm-aging is an organ-specific characteristic, which needs to be considered in future studies addressing senescence.
The interaction between senescent cells and the intra-renal immune milieu post-transplantation is complex. Potential targets of recipient-derived graft-infiltrating cells including PTECs or gECs were characterized by significantly higher expression of activating and co-stimulatory markers indicating a premature aging phenotype. Although it can be assumed that senescence can also be triggered actively when cells are exposed to excessive inflammatory stimuli, such as IFNγ, aged versus young PTECs did not demonstrate a significant difference in their expression of MHC class II or CD80 in vitro. Similarly, we did not detect a difference in the stimulatory capacity of young versus aged renal-derived DCs towards allo-sensitized T cells. Thus, these data suggest that a subtle chronic inflammatory status shapes and maintains a senescent intra-renal micromilieu in vivo resulting in a mature phenotype of PTECs and gECs, which is difficult to mimic in vitro, in contrast to recently published data within a heart transplantation setting.54 With the exception of infiltrating CD4+ and CD8+ T cells acquiring a Trm phenotype,55 we did not detect dramatic differences in lymphocyte frequencies infiltrating a young versus an aged organ. However, recipient-derived T cells isolated from aged donor grafts were characterized by an inflammatory phenotype. Based on the observation that a higher activation status was still observed for old gECs on day 7 post kidney transplantion, we postulate that recipient-derived T cells will become activated by the higher stimulatory capacity of the local endothelium/epithelium present in aged grafts.
Murine studies have shown that depletion of senescent cells delays age-associated disease.56 We therefore pre-treated aged kidneys by the direct inhibition of the pro-survival Bcl-2 pathway applying ABT-263 to donor animals. This resulted in a depletion of senescent PTECs and gECs as well as in a reduction of activated graft-resident CD4+ and CD8+ T cells. Thus, it can be assumed that in naïve aged kidneys, activated PTECs and gECs maintain a pro-inflammatory phenotype of resident T cells. Interestingly, a reduction of allostimulatory gECs as a consequence of ABT-263 donor-pretreatment was still detected on day 7 post kidney transplantation. Targeting of renal senescent cells resulted in reduced IFNγ and IL-10 production by graft-infiltrating lymphocyte subsets, although graft histology and renal function were not influenced. Similar observations were made for day 28, however, an improvement of graft function could be observed for the ABT-263 pretreamtent group.
The application of senolytics has already been demonstrated to ameliorate numerous conditions in experimental models, including diabetes, cardiac dysfunction or acute kidney injury32, 34, 57 as well as experimental heart transplantation.58 Here, we demonstrate that targeting of renal senescence might also be a valuable approach to improve donor organ function, bearing the potential to reduce mainteneance immunosuppression.
We are aware that our study has several limitations. First, it remains unclear why p16INK4a mRNA expression is not lower in ABT-263 pre-treated kidneys compared to controls, although this has been shown e.g. in lungs of aged ABT-263 treated mice.32 As p16INK4A is not found in all senescent cells and can also be expressed in some non-senescent cells, the induced expression remains to be clarified.59, 60 Although the effectiveness of alternative senolytics, such as Dasatinib and Quercetin has been proven in experimental models as well as in clinical studies,54, 61-63 it needs to be evaluated whether their application might result in comparable—or even better results. Second, we failed with potential co-culture assays to understand the direct interaction between recipient-derived graft-infiltrating cells and potential target cells explaining their enhanced effector functions. Finally, it remains to be determined how very subtle changes in tissue integrity could be uncovered by advanced histological techniques.
Our current understanding of the influence of age on the size of the memory compartment entirely relies on studies of peripheral blood and lacks information on resident memory cells. It is therefore mandatory to better understand the molecular and cellular pathways that are responsible for linking the aging immune system with the kidney. On that background, pre-conditioning of senescent organs, as demonstrated here, bears a high potential for clinical translation due to its regulation of the inflammatory cellular response and improvement of organ function.
ACKNOWLEDGMENTS
A.H., A.SAR., L.M.L.T., J.S., A.S., M.I.A., V.P., C.S., T.D., Y.B., P.V.R., S.E., K.W.H., E.G.S., R.D.B., and P.B. carried out experiments; A.H., A.SAR., L.M.L.T., and A.S. analyzed the data; made the figures; A.H., A.SAR., and K.K. drafted and revised the paper; K.K. designed the study; all authors approved the final version of the manuscript. Open Access funding enabled and organized by Projekt DEAL.
FUNDING INFORMATION
This work was funded by grants from Deutsche Forschungsgemeinschaft (DFG-Ko2270/4-1, DFG-Ko2270/5-1), Sonnenfeldstiftung and Sanofi Genzyme GmbH to K.K. and A.H. is funded by a grant from Chinese Scholarship Council (CSC). P.B. is supported by the Deutsche Forschungsgemeinschaft (Project-IDs 322900939, 454024652, 432698239, and 445703531 and 445703531), European Research Council (ERC) Consolidator Grant AIM.imaging.CKD (no. 101001791), and the Federal Ministry of Education and Research (STOP-FSGS-01GM1901A). EGS is supported by Deutsche Forschungsgemeinschaft (STA 1657/2-1).
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.