Connexins in endothelial cells as a therapeutic target for solid organ transplantation
Dinesh Jaishankar and Kristen M. Quinn contributed equally to the manuscript.
Abstract
Connexins are a class of membrane proteins widely distributed throughout the body and have various functions based on their location and levels of expression. More specifically, connexin proteins expressed in endothelial cells (ECs) have unique roles in maintaining EC barrier integrity and function—a highly regulated process that is critical for pro-inflammatory and pro-coagulant reactions. In this minireview, we discuss the regulatory influence connexin proteins have in maintaining EC barrier integrity and their role in ischemia–reperfusion injury as it relates to organ transplantation. It is evident that certain isoforms of the connexin protein family are uniquely positioned to have far-reaching effects on preserving organ function; however, there is still much to be learned of their roles in transplant immunology and the application of this knowledge to the development of targeted therapeutics.
Abbreviations
-
- aCT1
-
- alpha-connexin carboxyl-terminal 1
-
- AKI
-
- acute kidney injury
-
- ALI
-
- acute lung injury
-
- APC
-
- antigen-presenting cell
-
- Ca2+
-
- calcium ion
-
- cAMP
-
- cyclic adenosine monophosphate
-
- Cx
-
- connexins
-
- EC
-
- endothelial cells
-
- eNOS
-
- endothelial nitric oxide synthase
-
- ER
-
- endoplasmic reticulum
-
- HUVEC
-
- human umbilical vein EC
-
- ICAM-1
-
- intercellular cell adhesion molecule-1
-
- IRI
-
- ischemia–reperfusion injury
-
- LPS
-
- lipopolysaccharide
-
- NF-κB
-
- nuclear factor-kappa B
-
- NO
-
- nitric oxide
-
- ROS
-
- reactive oxygen species
-
- TNF-α
-
- tumor necrosis factor-alpha
-
- Tx
-
- solid organ transplantation
-
- VCAM-1
-
- vascular cell adhesion molecule-1
-
- ZO-1
-
- zonula occludens-1
1 BACKGROUND
Solid organ transplantation (Tx) is the mainstay therapy for patients with end-stage organ failure. Ischemia–reperfusion injury (IRI) is an inevitable physiologic and pathologic process that occurs during Tx and can predispose the graft to and exacerbate acute and chronic graft rejection.1 Although significant strides have been made in preventing acute graft rejection after solid organ Tx, a solution to chronic rejection remains elusive. Chronic graft rejection of solid organs is defined as the loss of allograft function several months to years after transplantation.2 By 5 years posttransplant, the prevalence of chronic rejection is highest in the lung (50%), followed by the heart (29%), and kidney (17%–28%).3-6 Consequently, these patients may require retransplantation, which adds further pressure to the long wait times for life-saving organs.
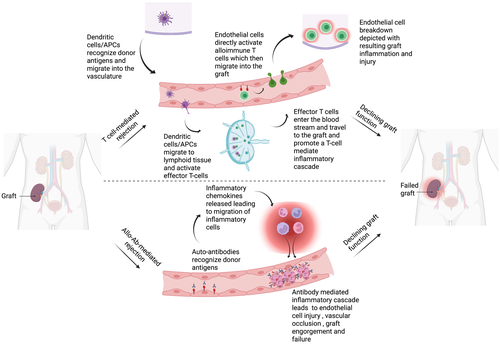
During organ transplantation, three phases can delineate the complex logistic and physiologic process that culminates with the restoration of blood flow, that is, reperfusion, to an allograft, namely: (1) donor organ procurement, (2) organ preservation, and (3) organ implantation. During phase 1, various injurious events can target the donor organ's endothelium. Specifically, donor brain death exacerbates the release of harmful cytokines within the endothelial lining of the donor's vasculature.7 Additionally, warm ischemia resulting from organ procurement from both standard criteria donors and those after circulatory death further damage the endothelial lining.8 In phase 2, whether through pulsatile hypothermic perfusion or static cold storage, cold ischemia results in additive insults to the endothelial cells (ECs).9 Finally, in phase 3, reconstruction of the vascular anastomoses and handling of the organ upon implantation causes mechanical and shear stress on the ECs which is only worsened by subsequent reperfusion.10 Reperfusion injury results in swelling and detachment of ECs from the basal membrane, immune cell infiltration, and generation of pro-inflammatory/pro-coagulative factors, leading to vasoconstriction of graft vessel lumens and reduction of blood supply to the organ.11, 12 This ultimately results in vascular intimal fibrosis and ischemia, which sets the stage for eventual graft dysfunction. Thus, in solid organ transplantation, disruption of the EC barrier contributes to both acute and chronic rejection through alterations in vascular permeability, leukocyte adhesion, transmigration, and the release of various inflammatory cytokines9, 13 (Figure 1).
The EC barrier is composed of an intricate network of adherens, tight junctions, and gap junctions. Together, they modulate cell-to-cell communication and signaling pathways to regulate the essential functions of ECs. In recent years, the role of connexin proteins in the context of organ Tx has gained significant interest, and these gap junction proteins are being targeted to develop therapeutics to preserve graft function and prevent transplant-related complications. This minireview summarizes the current knowledge of the role of connexin proteins in organ transplantation and EC function.
2 INTRODUCTION TO CONNEXINS
Connexins are integral membrane proteins and are widely distributed throughout the body. They are synthesized in the endoplasmic reticulum (ER) where they form a hexameric structure, called a connexon or hemichannel, and are transported via microtubules to the plasma membrane.14 Through a highly regulated process, they dock themselves on the cell surface and act as channels of communication between the cytoplasm and the extracellular milieu.14 Near other cells, hemichannels can fuse with adjacent hemichannels to form gap junctions that are regulated by the tight junction protein zonula occludens-1 (ZO-1).15, 16 Connexin gap junctions create direct channels from the cytoplasm of one cell to that of a neighboring cell. The opening and closing of channels allow for cellular communication through the exchange of ions such as calcium (Ca2+), metabolites, second messengers such as cyclic adenosine monophosphate (cAMP), and other small molecules, a process known as gap junctional intercellular communication (GJIC).17 The location of connexins dynamically affects cell function. In cardiac tissues, connexins facilitate electrical current to stimulate cardiomyocyte contraction, and in the brain, they facilitate communication between neurons.18
Twenty-one highly conserved isoforms of human connexin proteins have been identified, all of which share the same topology: they contain four transmembrane spanning motifs and two extracellular loop domains with both the amino (N) and carboxyl (C) tails located in the cytoplasm.19, 20 The isoforms differ based on their molecular weights and the ability of the C-terminus to interact with other proteins. Posttranslational modifications to the C-terminus of connexins such as phosphorylation, acetylation, and ubiquitination enable them to play key roles in regulating gap junctions and their associated functions. For example, phosphorylation regulates the trafficking and docking of connexons into the gap junction.14 Additionally, the posttranslational modifications to connexins facilitate interactions with proteins involved in various signaling pathways, cell cycle control, and cytoskeletal elements, demonstrating their crucial roles in coordinating cellular functions.14
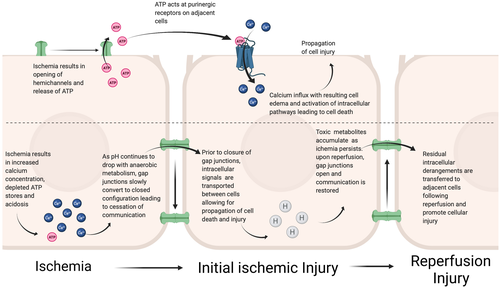
The expression and activity of connexins vary based on age, gender, and disease state. Under pathological conditions like IRI, connexin turnover is increased because of posttranslational modifications of the C-terminal tail resulting in endocytosis and eventual degradation in lysosomes.14 Consequently, both the hemichannel and the gap junction activity of connexins are affected. During ischemic conditions, the response of hemichannels and gap junctions varies and affects cell injury and death. Johansen et al. showed that following exposure to ischemia, rat heart myofibroblasts preferentially closed intracellular Cx43 gap junctions and opened Cx43 hemichannels.21 Further, IRI has been shown to promote the extrusion of adenosine triphosphate (ATP) from the intracellular space via connexin hemichannels.22 Extracellular ATP can then act at purinergic receptors on adjacent cells leading to calcium influx, cellular edema, and upregulation of pathways important for cell death and injury.22 On the other hand, connexin-based gap junctions slowly close in response to depleted intracellular ATP, acidosis, and calcium influx—conditions that occur during IRI.22 This temporarily halts communication between cells; however, prior to closure intracellular signals pass from cell to cell and propagate cell injury and death.22 Upon reperfusion, gap junctions reopen allowing residual intracellular derangements to be transferred to neighboring cells, resulting in further cellular injury22 (Figure 2).
3 CONNEXINS IN ECS
Five connexin proteins have been identified in ECs (connexin 32 [Cx32], connexin 37 [Cx37], connexin 40 [Cx40], connexin 43 [Cx43] and connexin 45 [Cx45]), and their expression in ECs depends on the type of the vessel.23 Cx37 and Cx40 are expressed in venous ECs, whereas Cx43 is expressed in microvascular ECs.24 Physical stress from blood flow and external stimuli from neighboring cells induces the secretion of molecules from ECs that regulate vascular homeostasis and barrier integrity.25 Connexins in ECs contribute to this process by participating in the exchange of ions, metabolites, and signaling molecules to regulate GJIC. Cx37 has been identified as a critical protein that controls the intracellular levels of Ca2+ in ECs, as dysfunction of Cx37 has been shown to promote the development of atherosclerosis by allowing monocyte adhesion to ECs and transmigration.26 In addition to participating in GJIC, connexins also contribute to EC function, binding to cytoskeleton proteins and regulating EC stiffness to control the EC barrier permeability.27 Nitric oxide (NO) in ECs regulates vascular homeostasis and is generated by the endothelial nitric oxide synthase (eNOS) enzyme.28 Cx37 and Cx40 have been shown to bind to eNOS to regulate the generation of NO thereby influencing EC function.29 These studies illustrate the important roles connexins in ECs play in regulating various EC functions and EC interactions with the extracellular compartment.
4 CONNEXINS IN EC BARRIER INJURY AND INFLAMMATION
Endothelial cells are important regulators of barrier integrity, inflammation, and maintaining vascular homeostasis. Under pathological conditions, inflammatory stimuli affect the expression and function of EC connexins. Lipopolysaccharide (LPS), an endotoxin found in the cell wall of gram-negative bacteria, has been found to reduce GJIC between microvascular ECs in both rat and mouse skeletal muscle through the phosphorylation of Cx43 and dephosphorylation of Cx40.30 Exposure to tumor necrosis factor-alpha (TNF-α) not only reduced the expression of Cx32, Cx37, and Cx40 in human umbilical vein ECs (HUVEC) but also altered their gap junction functions.31 In models of acid- and LPS-induced acute lung injury (ALI), Yin et al. identified that Cx40 increased the vascular permeability of pulmonary ECs.32 Using Cx40−/− mice, lung injury and edema were significantly attenuated indicating the importance of Cx40 in the pathogenies of ALI. A study by O'Donnell et al. also identified the role of Cx43 in increasing lung-vascular permeability.33 Upon various inflammatory stimuli tested in the study, the authors found increased expression of Cx43 in pulmonary ECs potentially contributing to vascular permeability.
The expression and function of connexins in ECs can also contribute to inflammation in other disease processes. In a mouse model of atherosclerosis, Chadjichristos et al. demonstrated that EC-specific deletion of Cx40 accelerated atherosclerosis by observing an increase in the vascular cell adhesion molecule-1 (VCAM-1) protein expression in aortic ECs and observing an increase in CD73-dependent leukocyte recruitment and adhesion.34 Moreover, in a follow-up study from this group, the authors demonstrated that the Cx40–CD73 axis also contributed to IRI by observing an increase in myocardial infarct size and neutrophil infiltration in EC-specific Cx40 deletion.35 A single-dose administration of methotrexate to inhibit CD73 activation significantly reduced IRI signifying the importance of EC Cx40 in regulating inflammation. Liao et al. generated EC-specific Cx43 knockout mice and observed that these mice were hypotensive and bradycardic.36 Although the underlying mechanism was not investigated, the authors found elevated levels of plasma NO in these mice suggesting the important role of EC Cx43 in maintaining vascular homeostasis. The effect of modulating Cx43 expression in ECs to regulate inflammation has also been studied. Through genetic and pharmacologic manipulation of Cx43 in HUVECs, Yuan et al. observed that upregulating the expression of Cx43 in HUVECs resulted in an increased expression of VCAM-1 and intercellular cell adhesion molecule-1 (ICAM-1) proteins which consequently enhanced monocyte-EC adhesion.37 Conversely, downregulating Cx43 in HUVECs exerted a protective effect on HUVECs. The mechanism of Cx43 upregulation on adhesion protein expression in HUVECs was not investigated, but the authors speculate a role of the nuclear factor-kappa B (NF-κB) transcription factor in this process. On the contrary, Okamoto et al. showed that blocking the Cx32 gap junction function in HUVECs resulted in higher levels of VCAM-1 and ICAM-1 transcript levels consequently leading to EC activation.38 Thus, connexins in ECs have distinct and varied roles that are critical in regulating barrier integrity and inflammation.
5 CONNEXINS AS THERAPEUTIC TARGETS IN ORGAN TRANSPLANTATION
Beyond ECs, connexins play a vital role in inflammation and many investigators have turned their attention to connexins as potential targets in transplantation, summarized in Table 1. Cx32 is widely expressed in both the kidney and liver and its modulation has been investigated in liver transplant-related acute kidney injury (AKI) and hepatic IRI. Propofol, a nonselective connexin inhibitor, has been shown to reduce postischemic oxidative stress and attenuate myocardial IRI, with one study demonstrating that it decreased X-ray-induced cellular toxicity through Cx32 inhibition.39, 43, 44 Luo et al. investigated propofol-induced manipulation of Cx32 expression on postliver transplantation AKI in a rat autologous orthotopic liver transplantation model. They demonstrated that a 15 μM concentration of propofol significantly inhibited Cx32 expression in HeLa cells that stably expressed Cx32.39 This inhibition of Cx32 led to a reduction of posthypoxic reactive oxygen species and reductions in posttransplant serum creatinine and blood urea nitrogen.39 Szymanska et al. used the small peptide, Gap27, to selectively inhibit connexin hemichannels following uterine explantation and studied the levels of cell death in various storage conditions (warm vs. cold).42 Their data illustrated that connexin inhibition under cold storage conditions resulted in a fourfold decrease in apoptotic cell counts, whereas under warm conditions, there was no such effect observed.42
Cx43 has been the focus in the development of novel, targeted therapeutics as it is widely expressed throughout the body. The inhibition of Cx43 with heptanol was demonstrated to protect against AKI following liver transplantation in a rat autologous orthotopic liver transplant model through attenuation of reactive oxygen species (ROS) transmission between neighboring cells leading to a reduction in necrotopsis.40 Pederson et al. reported on the protective effects of the drug in the peri-revascularization period and the reduction of IRI-specific endothelial damage and the downstream pathologic consequences of IRI.45 Alpha-connexin carboxyl terminal (α-CT1) is a newer peptide therapeutic that targets gap junctions by inhibiting the interaction between the C-terminus of Cx43 and ZO-1 (tight junction-associated protein). Disruption of this interaction leads to the cellular redistribution of Cx43 away from cell surface hemichannels and into gap junctions. Specifically related to organ transplantation, pretreatment with aCT1 has been shown to decrease IRI in a mouse cardiac transplantation model as measured by reduced serum troponin, histological analysis, and reduced graft infiltration by neutrophils and macrophages.41 Rotigaptide is a hexapeptide drug that was originally developed as an antiarrhythmic agent but has been found to act at Cx43.46 While the exact mechanism of action is not completely understood, it is thought to promote electrical coupling in the heart between ventricular myocytes by increasing gap junction conductance, potentially through alterations in the phosphorylation status of Cx43 and increased expression of gap junctions in ischemic myocardium.45, 46 Although Rotigaptide has not been used in the context of organ transplantation, by increasing gap junction conductance during ischemia, it can potentially be used as a therapeutic for organ transplantation.
6 FUTURE OF CONNEXIN-TARGETED THERAPEUTICS IN TRANSPLANTATION
Connexins play integral and dynamic roles in cell-to-cell communication, cell metabolism, and immune response, yet their variability makes them difficult to mechanistically understand. Pharmacological modulation agents lack specificity and antibodies directed against extracellular portions of connexin proteins can be nonselective and block hemichannel formation as well as function.4 Connexin mimetic peptides show promise and may present a novel opportunity for therapeutic targeting as they have greater specificity in regulating hemichannel function. Allograft ECs are uniquely susceptible to the negative effects of IRI given their location in the donor vasculature, and as such, protection of these cells through the manipulation of gap junctions and hemichannels could provide a means by which to mitigate immunological insults to the graft and ultimately prevent graft rejection. Cx43-based channels are of particular interest as their release of Ca2+ ions, cyclic adenosine monophosphate, and adenosine triphosphate at the immunological synapse acts as a second messenger for many immunological processes, including regulatory T cell–mediated immune suppression.47 We are far from fully understanding the temporality and fluctuating roles of these proteins; however, with further research our understanding of connexin function in ECs' immunogenicity will only continue to expand, enabling the utilization of these proteins as novel therapies in organ transplantation.
7 CONCLUSION
Solid organ transplantation is the cure for organ failure and saves lives. Extending the viability of the transplanted organ is crucially important. Graft dysfunction and failure have been linked to IRI, during which ECs play a critical role. The response of ECs to IRI is mediated not only at the individual cellular level but also through the maintenance of the EC membrane barrier. To better understand and interrupt this injurious cycle, the focus should be placed on a key component of EC barrier integrity—connexin gap junction proteins. Connexins’ integral roles in EC response to injury and their ability to create bystander effects on neighboring cells puts them in a primary position to have far-reaching effects on organ health. In summary, connexins play a dynamic and formative role in organ transplant immunology and IRI, that if better understood, could represent an impactful target for therapeutics.
FUNDING INFORMATION
This study was supported by the National Institute of Health (NIAID R01 AI142079) awarded to Dr. Satish N. Nadig.
DISCLOSURE
Dr. Satish N. Nadig holds a patent—Donor organ pretreatment formulation, US2016/039315. Dr. Nadig currently serves as the Chief Medical Advisor for Pandorum Technologies, Pvt Ltd.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing does not apply to this article as no new data were created or analyzed in this minireview. Data sharing does not apply to this minireview as no data sets were generated or analyzed.