Monitoring of alphatorquevirus DNA levels for the prediction of immunosuppression-related complications after kidney transplantation
Abstract
The replication kinetics of nonpathogenic anelloviruses belonging to the Alphatorquevirus genus (such as torque teno virus) might reflect the overall state of posttransplant immunosuppression. We analyzed 221 kidney transplant (KT) recipients in whom plasma alphatorquevirus DNA load was quantified by real-time polymerase chain reaction at baseline and regularly through the first 12 posttransplant months. Study outcomes included posttransplant infection and a composite of opportunistic infection and/or de novo malignancy (immunosuppression-related adverse event [iRAE]). Alphatorquevirus DNA loads at month 1 were higher among patients who subsequently developed posttransplant infection (P = .023) or iRAE (P = .009). Likewise, those with iRAE beyond months 3 and 6 also exhibited higher peak viral loads over the preceding periods. Areas under the curve for log10 alphatorquevirus DNAemia estimated by months 1 or 6 were significantly higher in patients experiencing study outcomes. Alphatorquevirus DNA loads above 3.15 and 4.56 log10 copies/mL at month 1 predicted the occurrence of posttransplant infection (adjusted hazard ratio [aHR]: 2.88; 95% confidence interval [CI]: 1.13-7.36; P = .027) and iRAE (aHR: 5.17; 95% CI: 2.01-13.33; P = .001). In conclusion, posttransplant monitoring of plasma alphatorquevirus DNA kinetics may be useful to identify KT recipients at increased risk of immunosuppression-related complications.
Abbreviations
-
- ATG
-
- antithymocyte globulin
-
- AUC
-
- area under curve
-
- auROC
-
- area under receiver operating characteristics curve
-
- BKVAN
-
- polyomavirus-associated nephropathy
-
- CI
-
- confidence interval
-
- CMV
-
- cytomegalovirus
-
- ESRD
-
- end-stage renal disease
-
- HIV
-
- human immunodeficiency virus
-
- HR
-
- hazard ratio
-
- IFI
-
- invasive fungal infection
-
- IQR
-
- interquartile range
-
- iRAE
-
- immunosuppression-related adverse event
-
- KT
-
- kidney transplantation
-
- LLoD
-
- lower limit of detection
-
- LT
-
- lung transplantation
-
- SD
-
- standard deviation
-
- SOT
-
- solid organ transplantation
-
- TTV
-
- torque teno virus
1 INTRODUCTION
Kidney transplantation (KT) remains the best therapeutic option for end-stage renal disease (ESRD),1 although middle- and long-term outcomes are still threatened by the development of adverse events attributable to excessive immunosuppression. Therapeutic drug monitoring currently stands as the only strategy to assess the status of immunocompetence after KT.2 However, this unidimensional approach remains limited to certain immunosuppressive drugs.3 As a consequence, various biomarkers with or without antigenic specificity have been proposed, with most of them not having yet reached clinical maturity.4
The widespread use of high-throughput sequencing methods has provided a revolutionary insight into the composition of human blood virome.5 Anelloviruses are small, nonenveloped, single-stranded DNA viruses with a circular, negative-sense genome. They are ubiquitously distributed, exhibit remarkable genetic variability, and occupy the largest fraction (≈70%) of blood virome in healthy subjects.6 Torque teno virus (TTV), first isolated in 1997 from a Japanese patient with posttransfusion hepatitis,7 constitutes the prototype member within the Anelloviridae family. TTV belongs to the Alphatorquevirus genus, which comprises at least 29 different genospecies according to the 2011 report of the International Committee on the Taxonomy of Viruses. TTV infection is thought to be acquired in childhood via multiple routes (transplacental, respiratory, fecal-oral, or transfusion) and reaches a prevalence as high as 90% among the adult population.8, 9 Anelloviruses such as TTV are still considered “orphan” viruses, with no evidence so far supporting a direct causal association with specific clinical manifestations.6, 8
Regardless of the nonpathogenic nature of chronic alphatorquevirus infection, increasing interest is being shown in its potential application as a functional marker of immune status.6 Plasma alphatorquevirus DNA levels in nonimmunocompromised subjects reflect a long-term steady-state balance between virus replication and the host's immune response, resulting from a high daily turnover rate.10, 11 Immunosuppressive therapy for solid organ transplantation (SOT),12-15 postengraftment phase after allogeneic hematopoietic stem-cell transplantation, 16-19 or CD4+ T cell depletion in human immunodeficiency virus (HIV) infection 20, 21 lead to an evident increase in alphatorquevirus DNA loads that would in turn correlate with the overall amount of immunosuppression.
It has been suggested that replication kinetics for alphatorquevirus might be a useful tool to identify SOT recipients at increased risk of graft rejection (ie, those with lower DNA loads).22-24 In addition, some authors have reported a direct association between higher DNA loads and posttransplant infection.25 Nevertheless, most of these studies had small sample sizes or were exclusively focused on graft rejection as study outcome. We investigated in a large cohort of KT recipients the clinical value of monitoring alphatorquevirus DNA kinetics to predict the occurrence not only of posttransplant infection, but also of a constellation of complications, such as opportunistic infection or de novo malignancy, whose pathogenesis may be more specifically attributed to excessive immunosuppression (ie, immunosuppression-related adverse events [iRAEs]).
2 MATERIALS AND METHODS
2.1 Study population and setting
The present prospective, observational, cohort study was performed between November 2014 and December 2016 at the Hospital Universitario “12 de Octubre.” All adult patients with ESRD undergoing KT during this period and providing informed consent were deemed eligible for inclusion. Double organ recipients were excluded. This study was performed in accordance with the ethical standards laid down in the Declarations of Helsinki and Istanbul. The local Clinical Research Ethics Committee approved the study protocol.
2.2 Study design
Participants were enrolled at the time of KT and followed up for at least 12 months, unless graft loss (retransplantation or permanent return to dialysis) or death occurred earlier. Scheduled follow-up visits were carried out at baseline, every 2 weeks during the first 3 months, and monthly thereafter, as well as whenever clinically indicated. A number of pretransplant, perioperative, and posttransplant variables were recorded by using a standardized case report form. Plasma alphatorquevirus DNA load was quantified at baseline (ie, within 6 hours prior to the transplant procedure), day 7, and months 1, 3, 6, and 12 by a polymerase chain reaction (PCR)-based quantitative nucleic acid amplification test. Descriptions of immunosuppression and prophylaxis regimens are detailed in Supplementary Methods.
The study outcome was the occurrence of overall posttransplant serious infection (defined by the requirement of hospitalization and intravenous antimicrobial therapy) and iRAE, with the latter encompassing the occurrence of opportunistic infection (as defined below) and/or posttransplant de novo malignancy.26
2.3 Plasma alphatorquevirus DNA load quantification
Blood samples were immediately centrifuged and plasma specimens were preserved at −80°C, with aliquots thawed for the first time for the present analyses. DNA was extracted from 200 μL of plasma with the NucliSENS® easyMAG® automated system (bioMérieux, Marcy-l’Étoile, France), following the manufacturer's instructions. DNA loads were quantified by means of the TTV R-gene® kit (ARGENE® range, bioMérieux), a real-time PCR assay targeting a highly conserved segment of the 5′ untranslated region of the viral genome with >90% identity across isolates.6 Analytical performances of this assay in terms of precision (reproducibility and repeatability), correlation with the in-house PCR assay developed by Maggi et al,27 inclusivity and exclusivity, and linearity have been recently reported 28 and are detailed in Supplementary Methods. PCR amplification and amplicon detection was performed on an ABI Prism 7500 system (PE Biosystems, Foster City, CA). The viral load (in copy numbers per mL) was determined using a standard curve with known copy numbers and log10-transformed for statistical analyses. The lower limit of detection (LLoD) was 167 copies/mL (95% confidence interval [CI]: 92-581) or 2.2 log10 copies/mL (95% CI: 2.0-2.8), with DNA quantitation in the linear range from 2.1 × 102 to 2.1 × 107 copies/mL. Specimens with undetectable DNA loads were assigned a value of 0.01 (−2.0 log10) copies/mL for analysis purposes. All samples from each patient were simultaneously assayed in singlets. Although the taxonomic classification and nomenclature of the Anelloviridae family are still discussed, there is general consensus to recommend the term “alphatorquevirus” instead of “TTV” in order to encompass the different genospecies within this genus. Therefore, results of the PCR assay were referred to as plasma alphatorquevirus DNA load.
2.4 Study definitions
We defined opportunistic infection as that due to intracellular bacteria (mycobacteria, Listeria monocytogenes, and Nocardia spp.), herpesviruses (cytomegalovirus [CMV], herpes simplex virus, and varicella-zoster virus), polyomaviruses (biopsy-proven BK polyomavirus-associated nephropathy [BKVAN]), yeasts (Candida spp. and Cryptococcus spp.), molds (invasive aspergillosis and mucormycosis), and parasites (Toxoplasma gondii, Pneumocystis jirovecii, and Leishmania spp.).29 We also included the diagnosis of presumptive BKVAN (high-level polyomavirus BK replication [plasma viral load >4 log10 copies/mL] at 2 time points 3 or more weeks apart) even in the absence of biopsy-proven nephropathy.30 Surgical site and urinary tract infections due to Candida spp. were excluded since such complications are usually related to previous invasive procedures, mucosal breakdown, and indwelling urinary catheters rather than to the patient's immune status. The diagnosis of CMV disease required the demonstration of CMV replication and the presence of attributable symptoms, and was categorized as viral syndrome or end-organ disease according to consensus definitions.31 Proven or probable invasive fungal infection (IFI) was defined by the European Organization on Research and Treatment in Cancer and the Mycoses Study Group criteria.32 Further study definitions are detailed in Supplementary Methods.
2.5 Statistical analysis
Quantitative data were shown as the mean ± standard deviation (SD) or the median with interquartile ranges (IQR). Qualitative variables were expressed as absolute and relative frequencies. Categorical variables were compared using the χ2 test. Student t test or Mann-Whitney U test were applied for continuous variables. Repeated measures were compared with the Student t test for paired samples or the Wilcoxon test. Pearson's correlation coefficient or Spearman's rank correlation coefficient were used to investigate the correlation between continuous variables.
The optimal cutoff values (ie, that with the highest value for the combined sensitivity and specificity) of plasma alphatorquevirus DNA loads to predict study outcomes were identified by the Youden's index 33 in the area under receiver operating characteristics (auROC) curve. In the absence of an external validation cohort, the selected cutoff values were evaluated by bootstrap simulation, which estimated how good the predictive performance of the test (ie, having a plasma DNA load below or above the threshold) would be on a hypothetical set of new patients. To this aim, 1000 bootstrap samples of equal size were generated from the study population by sampling with replacement. Time-to-first-event curves were plotted by the Kaplan-Meier method. Intergroup differences in cumulative incidence curves were compared with the log-rank test, and multivariate Cox regression models were used to evaluate the association between plasma DNA loads and study outcomes. Multicollinearity was assessed using variance inflation factors. Associations were given as hazard ratios (HRs) and 95% CIs.
Plasma alphatorquevirus DNA load area under curve (AUC) was calculated by means of the trapezoid rule.34 As previously described,15, 35 we estimated the DNA load doubling time according to the formula: dt = (t2-t1) × [Ln2/Ln(q2/q1)], where q1 and q2 represent the plasma loads (in copies/mL) at the time of the first and second positive PCR result, respectively, and (t2-t1) the interval (in days) between both dates. Only episodes in which there was an increase between the first and second alphatorquevirus DNA load values of >3-fold were considered for analysis.
All the significance tests were 2-tailed. The threshold for significance was set at a P < .05. Statistical analysis was performed using SPSS version 20.0 (IBM Corp., Armonk, NY) and Prism version 6.0 (GraphPad Software Inc., La Jolla, CA).
3 RESULTS
3.1 Characteristics of the study population
We included 221 KT recipients, whose clinical characteristics are shown in Table 1. The median follow-up was 494 days (IQR: 434-542). Five patients (2.3%) experienced graft loss after a median posttransplant interval of 41 days (IQR: 18-260.5), whereas 2 patients (0.9%) died (1-year survival rate: 98.0%). These patients were censored for follow-up at these points.
| Variable | Value |
|---|---|
| Age of recipient, y (mean ± SD) | 53.9 ± 15.7 |
| Sex of recipient (male) (n [%]) | 160 (72.4) |
| Prior or current smoking history (n [%]) | 90 (40.7) |
| BMI at transplantation, kg/m2 [mean ± SD]a | 25.2 ± 4.1 |
| Pretransplant chronic comorbidities (n [%]) | |
| Hypertension | 188 (85.1) |
| Diabetes mellitus | 70 (31.7) |
| Coronary heart disease | 22 (10.0) |
| Other chronic heart disease | 39 (17.6) |
| Peripheral arterial disease | 21 (9.5) |
| Cerebrovascular disease | 18 (8.1) |
| Chronic obstructive pulmonary disease | 6 (2.7) |
| Previous solid organ transplantation (n [%]) | 29 (13.1) |
| Underlying end-stage renal disease (n [%]) | |
| Glomerulonephritis | 50 (22.6) |
| Diabetic nephropathy | 45 (20.4) |
| Polycystosis | 26 (11.8) |
| Nephroangiosclerosis | 20 (9.0) |
| Chronic interstitial nephropathy | 12 (5.4) |
| Congenital nephropathy | 10 (4.5) |
| Reflux nephropathy | 6 (2.7) |
| Vasculitis | 5 (2.3) |
| Lupus nephropathy | 4 (1.8) |
| Unknown | 26 (11.8) |
| Other | 17 (7.7) |
| CMV serostatus (n [%]) | |
| D+/R+ | 157 (71.0) |
| D-/R+ | 24 (10.9) |
| D+/R- | 28 (12.7) |
| D-/R- | 8 (3.6) |
| D unknown/R+ | 4 (1.8) |
| Positive EBV serostatus (anti-EBNA IgG) (n [%]) | 200 (90.5) |
| Positive HCV serostatus [n (%)] | 17 (7.7) |
| Positive HBsAg status (n [%]) | 8 (3.6) |
| Positive HIV serostatus (n [%]) | 2 (0.9) |
| Pretransplant renal replacement therapy [n (%)] | 194 (87.8) |
| Hemodialysis | 159 (82.0) |
| Continuous ambulatory peritoneal dialysis | 35 (18.0) |
| Time on dialysis, days (median [IQR]) | 536 (284-1074) |
| Highly sensitized recipient (n [%]) | 21 (8.0) |
| Age of donor, y [mean ± SD] | 52.5 ± 16.1 |
| Gender of donor (male) (n [%]) | 117 (52.9) |
| Type of donor (n [%]) | |
| DBD donor | 144 (65.2) |
| DCD donor | 47 (21.3) |
| Living donor | 29 (13.1) |
| Cold ischemia time, h (mean ± SD) | 15.9 ± 8.0 |
| Number of HLA mismatches (median [IQR]) | 4 (3-5) |
| Induction therapy (n [%]) | |
| ATG | 106 (48.0) |
| Total dose, mg (mean ± SD) | 5.4 ± 2.2 |
| Basiliximab | 85 (38.5) |
| None | 30 (13.6) |
| Primary immunosuppression regimen (n [%]) | |
| Steroids | 220 (99.5) |
| Tacrolimus | 221 (100.0) |
| Mycophenolate mofetil/mycophenolic acid | 210 (95.0) |
| Azathioprine | 10 (4.5) |
| Anti-CMV prophylaxis (n [%]) | 125 (56.6) |
| Duration of prophylaxis, days (median [IQR]) | 103.5 (91-148.5) |
| Posttransplant complications (n [%]) | |
| Delayed graft function | 102 (46.2) |
| Number of dialysis sessions (median [IQR]) | 2 (1-3) |
| Reintervention within the first month | 24 (10.9) |
| NODAT | 19 (12.6) |
| Renal artery stenosis | 43 (19.5) |
| Acute graft rejectionb | 25 (11.4) |
| Time from transplantation, days (mean ± SD) | 112.8 ± 105.1 |
| T cell–mediated acute rejection | 13 (5.9) |
| Antibody-mediated acute rejection | 6 (2.7) |
- ATG, antithymocyte globulin; BMI, body mass index; CMV, cytomegalovirus; D, donor; DBD, donation after brain death; DCD, donation after circulatory death; EBV, Epstein-Barr virus; EBNA, EBV nuclear antigen; HLA, human leukocyte antigen; HBsAg, hepatitis B virus surface antigen; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range; NODAT, new-onset diabetes after transplantation; R, recipient; SD, standard deviation.
- a Data on BMI not available for 18 patients.
- b Includes 4 patients with borderline acute rejection and 2 with empirically treated episodes not confirmed by biopsy.
Regarding the occurrence of study outcomes, 128 patients (57.9%) developed a total of 287 episodes of posttransplant infection (incidence rate: 2.67 episodes per 1000 transplant-days). The clinical and microbiological characteristics are detailed in Supplementary Results (Table S1). The median interval from transplantation to the first episode was 37.5 days (IQR: 14-99.3). Fifty-one patients (23.1%) had 65 episodes of iRAE (incidence rate: 0.61 episodes per 1000 transplant-days) (Table 2). The median interval to the first episode was 78 days (IQR: 39-235).
| Clinical syndrome | N (%) |
|---|---|
| Opportunistic infection | 54 (83.1) |
| CMV viral syndrome | 25 (38.4) |
| CMV colitis | 4 (6.1) |
| HSV mucocutaneous infection | 9 (13.8) |
| Herpes zoster | 6 (9.2) |
| Significant BK viremiaa | 3 (4.6) |
| Nocardiosis | 1 (1.6) |
| Invasive aspergillosis | 2 (3.1) |
| Mucormycosis | 1 (1.6) |
| Cryptococcosis | 1 (1.6) |
| IFI due to Trichosporon spp. | 1 (1.6) |
| Visceral leishmaniasis | 1 (1.6) |
| Posttransplant de novo malignancy | 11 (16.9) |
| Solid organ cancerb | 7 (10.8) |
| Nonmelanoma skin cancerc | 4 (6.1) |
- CMV, cytomegalovirus; IFI, invasive fungal infection; iRAE, immunosuppression-related adverse event; HSV, herpes simplex virus.
- a Plasma viral load >4 log10 copies/mL at 2 different time points 3 or more weeks apart.
- b Includes colon adenocarcinoma (3 cases), renal cell carcinoma (2 cases), rectal carcinoma, gastric adenocarcinoma, and prostate adenocarcinoma (1 case each).
- c Includes basal cell and squamous cell carcinoma (2 cases each).
3.2 Kinetics of plasma alphatorquevirus DNA load
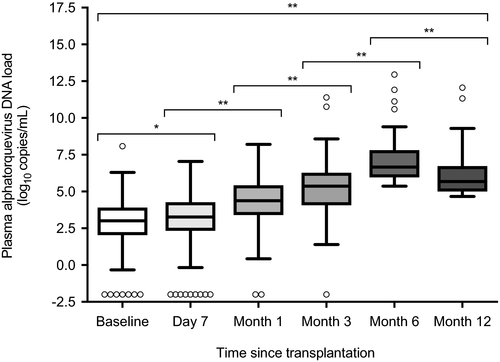
The total number of monitoring points for plasma alphatorquevirus DNA was 997 (median of 5 points per patient [IQR: 4-5]). Only 2.4% (24/997) samples were below the LLoD (ie, undetectable load), with rates ranging from 3.7% (7/187) at baseline to 0.6% (1/177) at month 6. There was a progressive increase in viral load from baseline (2.9 ± 1.6 log10 copies/mL), to peak at month 6 (5.7 ± 1.9 log10 copies/mL) and slightly decreased at month 12 (5.0 ± 2.1 log10 copies/mL) (P < .05 for all paired comparisons) (Figure 1). Accordingly, viral loads peaked in most patients at month 6 (51.6% [114/221]).
We found a significant although weak direct correlation between recipient age and plasma alphatorquevirus DNA loads at baseline (r = 0.263; P = .0002) and day 7 (r = 0.151; P = .029), but not thereafter. CMV-seronegative recipients had lower alphatorquevirus loads as compared to those who were seropositive (2.3 ± 1.6 vs 2.9 ± 1.6 log10 copies/mL; P = .028). Regarding posttransplant factors, induction therapy with anti-thymocyte globulin (ATG) was associated with higher plasma alphatorquevirus DNA loads through the first 6 posttransplant months, with significant differences at month 3 (5.5 ± 1.8 vs 4.8 ± 1.9 log10 copies/mL; P = .018) (Figure S1). We found no impact of the administration of anti-CMV prophylaxis or the primary immunosuppression regimen. Twenty-two patients (10.0%) were converted to an mTOR inhibitor after a median interval of 217 days (IQR: 117.5-306.8). Plasma alphatorquevirus DNA loads did not differ between this subgroup of patients and the rest of the cohort either (data not shown).
3.3 Correlation between alphatorquevirus DNA loads and immune parameters
In order to better characterize the potential of alphatorquevirus DNA kinetics as a surrogate marker for immunosuppression, we analyzed the relationship between this parameter and absolute counts of peripheral blood lymphocyte populations. At posttransplant month 1, there were significant inverse correlations between the plasma alphatorquevirus DNA load and concurrently measured CD3+ (r = −0.238; P = .017), CD4+ (r = −0.241; P = .015), and CD8+ T cell counts (r = −0.240; P = .016). Such correlations were even more evident by month 3, particularly for CD3+ (r = −0.347; P < .0001) and CD4+ T cell counts (r = −0.330; P < .001) (Figure S2).
3.4 Alphatorquevirus DNA loads at single points and outcomes
First, we explored the association between plasma alphatorquevirus DNA loads at discrete time points and study outcomes. We found no significant differences in baseline loads between patients with or without posttransplant infection (2.9 ± 1.6 vs 2.8 ± 1.6 log10 copies/mL; P = .854) or iRAE (3.1 ± 1.5 vs 2.8 ± 1.6 log10 copies/mL; P = .369). Such lack of significance persisted when plasma loads were measured at day 7, either for infection (3.2 ± 1.7 vs 3.1 ± 1.7 log10 copies/mL; P = .697) or iRAE (3.5 ± 1.6 vs 3.0 ± 1.7 log10 copies/mL; P = .148).
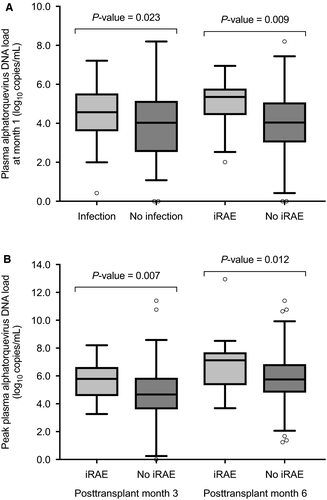
Nevertheless, differences emerged thereafter, when most recipients have reached a stable level of immunosuppression. Plasma alphatorquevirus DNA loads at month 1 were higher among patients who subsequently developed posttransplant infection as compared to those remaining free from this complication (4.6 ± 1.3 vs 3.8 ± 1.9 log10 copies/mL; P = .023). Such a difference was also observed between patients developing or not developing iRAE (4.9 ± 1.2 vs 3.9 ± 1.8 log10 copies/mL; P = .009) (Figure 2A). Likewise, peak DNA loads during the preceding periods were significantly higher in patients who developed iRAE beyond month 3 (5.6 ± 1.3 vs 4.7 ± 1.8 log10 copies/mL; P = .007) or month 6 (6.8 ± 2.0 vs 5.8 ± 1.7 log10 copies/mL; P = .012) (Figure 2B), with a nonsignificant trend at the latter point for posttransplant infection (6.3 ± 1.9 vs 5.8 ± 1.7 log10 copies/mL; P = .097).
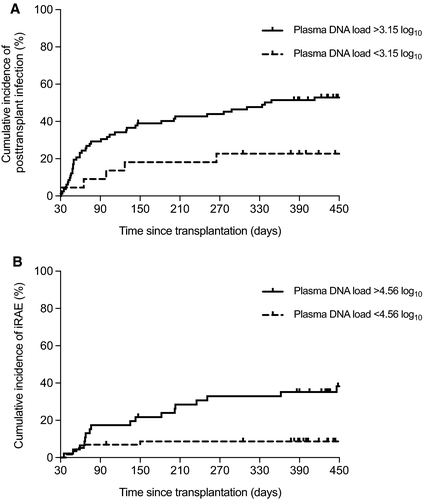
In view of its potential utility for guiding clinical decisions early after transplantation, we further analyzed the discriminative value of alphatorquevirus DNA loads at month 1. The auROCs for predicting infection and iRAE were 0.624 (95% CI: 0.517-0.732; P = .029) and 0.704 (95% CI: 0.588-0.820; P = .002), with optimal cutoff values set at 3.15 and 4.56 log10 copies/mL, respectively. The presence of plasma alphatorquevirus DNA loads above these thresholds was associated with higher cumulative incidences of infection (log-rank P = .009) and iRAE (log-rank P = .0006) (Figure 3). The predictive performance of both cutoff values estimated through 1000 bootstrap samples is detailed in Table 3. Such associations remained significant after multivariate adjustment, both for posttransplant infection (adjusted HR: 2.88; 95% CI: 1.13-7.36; P = .027) (Table S2) and iRAE (adjusted HR: 5.17; 95% CI: 2.01-13.33; P = .001) (Table S3).
| Cutoff value | Predicted posttransplant event | Sensitivity (95% CI)a | Specificity (95% CI)a | PPV (95% CI)a | NPV (95% CI)a |
|---|---|---|---|---|---|
| Plasma load >3.15 log10 copies/mL | Infection beyond mo 1 | 89.8% (79.6-98.0) | 30.9% (18.2-43.6) | 53.8% (42.7-64.6) | 77.3% (59.1-95.5) |
| Plasma load >4.56 log10 copies/mL | iRAE beyond mo 1 | 76.0% (60.0-92.0) | 65.8% (55.7-75.9) | 41.3% (28.3-54.3) | 89.7% (81.0-96.6) |
- CI, confidence interval; iRAE, immunosuppression-related adverse event; NPV, negative predictive value; PPV, positive predictive value.
- a Mean and 95% bootstrap CI.
3.5 Areas under curve for plasma alphatorquevirus DNA and outcomes
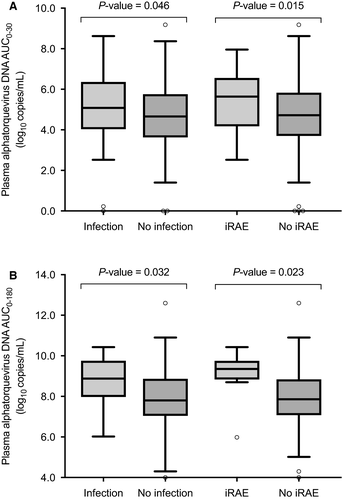
We explored the correlation between the cumulative magnitude of alphatorquevirus DNAemia, estimated through the AUC for log10 plasma DNA load, and study outcomes. The AUCs between baseline and month 1 (AUC0-30) were significantly higher among patients with posttransplant infection (5.1 ± 1.7 vs 4.6 ± 1.7 log10 copies/mL; P = .046) or iRAE (5.4 ± 1.4 vs 4.7 ± 1.7 log10 copies/mL; P = .015) beyond that point. Likewise, the AUCs to month 6 (AUC0-180) were also higher among patients subsequently developing posttransplant infection (8.8 ± 1.3 vs 7.9 ± 1.6 log10 copies/mL; P = .032) or iRAE (9.1 ± 1.2 vs 7.9 ± 1.5 log10 copies/mL; P = .023) (Figure 4).
3.6 Kinetics of alphatorquevirus DNA loads and outcomes
Previous studies have suggested that TTV replication kinetics mirrors more accurately the state of immunosuppression than the viral load at a given point.15, 36 Thus, we investigated whether dynamic changes in alphatorquevirus loads correlates with posttransplant outcomes by separately analyzing the trajectory (ascending or nonascending [ie, stable or decreasing] slope) and magnitude (viral doubling time) of change in plasma alphatorquevirus DNA loads between 2 consecutive monitoring points.
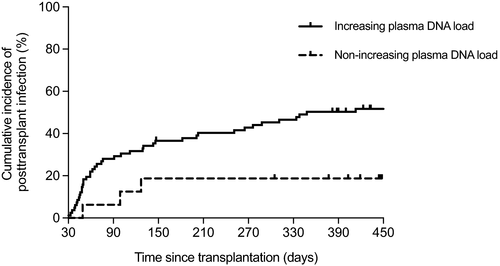
Patients showing an increasing slope of change in alphatorquevirus DNA loads between day 7 and month 1 were more likely to subsequently develop posttransplant infection compared to those with nonascending kinetics (57.3% [47/82] vs 18.8% [3/16]; P = .005). A similar nonsignificant trend was also observed for iRAE (26.8% [22/82] vs 6.2% [1/16]; P = .108). Increasing kinetics of alphatorquevirus DNA load between both points acted as an independent predictor for posttransplant infection (adjusted HR: 4.29; 95% CI: 1.32-14.04; P = .016) (Table S4), with significant differences in terms of cumulative incidence (log-rank P = .013) (Figure 5). No comparable associations were observed for any of the remaining time intervals, including that immediately after transplantation (ie, from baseline to day 7). This finding is concordant with the sigmoidal-shaped model proposed for TTV DNA kinetics in lung transplant (LT) recipients, in which the increase in viral load exhibits a delay of ≈15 days after the initiation of immunosuppression, followed by an almost linear increase between days 15 and 45 and a progressive stabilization thereafter.15 Figure S3 depicts illustrative examples of increasing dynamics of alphatorquevirus DNA loads and relevant posttransplant events.
The lowest doubling time for alphatorquevirus DNA load across different time intervals was observed between day 7 and month 1 (median: 4.9 days [IQR: 3.3-7.6]) (Table S5), in accordance with the aforementioned sigmoidal-shaped course. Doubling times through the first month were lower among patients who received ATG induction, either between baseline and day 7 (4.0 [IQR: 2.1-6.5] vs 7.1 [IQR: 4.3-17.1] days; P < .0001) or between day 7 and month 1 (4.0 [IQR: 2.8-6.1] vs 6.3 [IQR: 3.6-9.1] days; P = .020) (Figure S4). In view of this significant interaction, we separately analyzed alphatorquevirus doubling times according to the type of induction therapy. There were no differences among ATG-treated patients who did or did not develop posttransplant infection or iRAE. However, doubling times between day 7 and month 1 were lower for patients who did not receive ATG and developed posttransplant infection as compared to those remaining free from this complication (5.5 [IQR: 3.5-8.4] vs 7.3 [IQR: 5.3-22.4] days; P = .070) (Figure S5).
3.7 Alphatorquevirus DNA loads and graft rejection
Finally, we analyzed the correlation between plasma alphatorquevirus DNA loads and graft rejection. In concordance with the presumed nature of this variable as a marker of immunosuppression, baseline loads were lower (suggesting a higher level of immunocompetence) among patients who developed acute rejection during the first 90 posttransplant days (1.7 ± 2.3 vs 2.9 ± 1.6 log10 copies/mL; P = .035). In addition, the cumulative incidence of rejection was significantly higher among patients with undetectable DNA at baseline (28.6% [2/7] vs 3.3% [6/180]; P = .030). After multivariate adjustment, higher plasma alphatorquevirus DNA loads at baseline remained as a protective factor for the development of acute graft rejection (adjusted HR [per 1-log10 copies/mL increase]: 0.69; 95% CI: 0.49 - 0.97; P = .034) (Table S6).
4 DISCUSSION
In the present cohort of KT recipients, a clear association between plasma alphatorquevirus DNA kinetics and posttransplant complications was observed. Patients who developed posttransplant infection or iRAE—a composite outcome encompassing opportunistic infection and de novo cancer—beyond the first month exhibited higher alphatorquevirus DNA loads over the preceding months, either measured at single time points or expressed through the AUC as a proxy to the cumulative magnitude of DNAemia. Such associations still persisted for late-onset events. The demonstration of increasing plasma DNA loads during the first month was found to be particularly informative. On the other hand, recipients with higher pretransplant loads were less likely to experience acute graft rejection. Collectively, these results reinforce the hypothesis linking anellovirus replication kinetics and posttransplant immunocompetence.
Our experience provides additional insight into the epidemiology of alphatorquevirus infection among ESRD patients. First, alphatorquevirus DNA was detectable in 96.3% of plasma specimens collected at baseline, suggesting that the prevalence in this specific population is higher than that reported for healthy individuals in other European countries,9, 37, 38 but similar to the rates found among other chronic patient groups such as HIV- or hepatitis-infected subjects20, 38, 39 or those with end-stage pulmonary or liver disease.14, 39-41 We observed a direct correlation, although weak, between recipient age and plasma DNA loads, in line with previous studies performed in healthy adults.9, 37, 38 In addition, CMV seropositivity was associated with higher baseline alphatorquevirus loads, as also reported by other authors.9 Both findings would support the impact of age- and CMV-associated immunosenescence on the host control of chronic alphatorquevirus infection.42
More relevantly for the potential usefulness of alphatorquevirus DNA kinetics as surrogate marker of posttransplant immunosuppression, we observed a negative correlation between plasma loads and CD3+, CD4+, and CD8+ T cell counts assessed at months 1 and 3. It is commonly believed that alphatorquevirus replication mostly occurs in T lymphocytes, since some authors found a sharp drop in TTV viremia among liver transplant recipients treated with ATG that was not observed in those receiving non–T-cell-depleting induction regimens (ie, basiliximab).19, 36 We did not replicate, however, such findings since the use of ATG as induction therapy in our cohort was associated with higher viral loads and lower doubling viral times during the first posttransplant months. The exact nature of replication-competent cells for alphatorquevirus remains largely unknown.6 The ATG-induced decay in DNA load reported in previous studies was only evident very early (ie, first 2 weeks) after transplantation.36 It is plausible that such an effect could be attenuated in the midterm due to partial T cell repopulation. In addition, it has been shown that ATG induction increases the frequency of late-stage differentiated T cells and accelerates immunosenescence,43 potentially impairing alphatorquevirus-specific immune response. Differences in ATG dosing regimens and concurrent immunosuppression may also account for this discrepancy between studies.
The replication kinetics observed in our cohort was similar to that previously reported in LT recipients, with a rapid increase in viral load from days 7 to 30, which was followed by a less pronounced slope thereafter, to peak beyond month 3 in most patients.15, 44 It should be noted that the alphatorquevirus doubling time was higher during the first posttransplant week than in the subsequent period until day 30, pointing to a certain delay in the triggering effect of iatrogenic immunosuppression on viral replication and shaping a sigmoid-like curve, which has also been observed after LT.15
Recent studies have reported an association between low or undetectable alphatorquevirus viremia and an increased risk of graft rejection after lung,24 kidney, 23 and liver transplantation.22 Similarly to our experience, data from the Swiss Transplantation Cohort Study showed that 1-year cumulative incidence of rejection among liver transplant recipients with undetectable TTV DNA loads at transplantation were significantly higher than in patients with detectable titers.22 Nevertheless, available information remains scarce regarding the predictive value of high DNA loads to anticipate the occurrence of posttransplant infection, with contradictory results. Görzer et al reported than a cutoff level of 9.3 log10 copies/mL for TTV DNA was predictive for the development of infection after LT, although the monitoring time largely varied from 89 to 364 posttransplant days.25 More recently, Nordén et al failed to find a relationship between alphatorquevirus kinetics and the risk of infectious complications also in LT recipients,44 although outcome definitions were somewhat discordant between both studies.
We observed that the magnitude of alphatorquevirus replication (estimated through cross-sectional viral load measurements or AUCs) was directly correlated with the subsequent risk of infection and iRAE, and identified 2 specific thresholds at month 1 for each of these outcomes (3.15 and 4.56 log10 copies/mL, respectively). Since the positive predictive value of a diagnostic test is directly proportional to the prevalence of the condition being detected, the bootstrap estimates were limited by the low cumulative incidence rates observed beyond posttransplant month 1 for infection or iRAE. On the other hand, the negative predictive values were substantially higher, particularly for iRAE, suggesting that those patients with DNA loads below such thresholds have a very low risk of developing complications attributable to over-immunosuppression. Interestingly, an ascending slope of change (ie, increasing kinetics) in DNA load during the first month acted as an independent predictor for posttransplant infection. It is likely that such replication kinetics identifies a subgroup of recipients particularly unable to control chronic alphatorquevirus infection due to the functional immune impairment associated with posttransplant immunosuppression. In accordance with this notion, viral doubling times between days 7 and 30 were lower—indicating a more explosive viral kinetics—among patients who developed posttransplant infection beyond this latter point, although the difference did not reach statistical significance and disappeared in the presence of ATG induction.
By using overall posttransplant infection and iRAE as separate outcomes, we aimed at investigating the role of alphatorquevirus DNAemia from 2 complementary perspectives. The first outcome constituted a sensitive measure of the recipient's susceptibility to infection since it encompasses a multiplicity of factors, in addition to immunosuppressive therapy, contributing to the occurrence of this complication (ie, surgical and invasive procedures or environmental exposures). On the other hand, the working concept of iRAE was conceived to capture more precisely the distinctive role played by the net state of immunosuppression after transplantation. In support of this hypothesis, we found that the differences in alphatorquevirus DNA loads between patients with or without iRAE were more marked than those according to the occurrence of posttransplant infection.
Although we lacked a formal control group composed of nonimmunocompromised subjects, the aforementioned study by Kulifaj et al included 31 healthy volunteers in whom plasma DNA loads were measured by means of the same PCR assay used in the present research28, thus allowing valid comparisons. Interestingly, the mean alphatorquevirus DNA load in this nonimmunocompromised group (2.8 ± 1.1 log10 copies/mL) was virtually identical to that observed by us at the pretransplant (baseline) assessment (2.9 ± 1.6 log10). Both selected cutoff values at month 1 were notably higher than the DNA load expected for healthy individuals or ESRD patients prior to transplantation. Such a difference was particularly evident (approaching 2 log10) for the iRAE-oriented threshold, in accordance with the ability of this variable to more accurately reflect the net state of immunosuppression.
The present study has limitations, including its single-center design that compromises external generalizability. The real-time PCR assay used did not allow us to investigate the genetic diversity or genogroup distribution within alphatorquevirus-infected patients, which has been shown to evolve with posttransplant immunosuppression.13 The number of iRAE episodes analyzed was low, particularly for posttransplant cancer. The accuracy of doubling time estimates could have been limited by the time elapsed between consecutive DNA load measurements. Finally, the proposed interpretation linking alphatorquevirus viremia and posttransplant complications, although biologically plausible and supported by previous studies, should be taken as merely hypothesis-generating. Our study was exploratory in nature and primarily aimed at investigating the potential application of alphatorquevirus DNA load as a surrogate marker for immunosuppression, rather than proposing a diagnostic tool for clinical decision-making. Since the same dataset was used to both establish and evaluate the cutoff values, the predictive performance of the test may have been overestimated. The external validation would first require international standardization of PCR primers, protocols, blood compartments, and reporting units to enable comparability across different laboratories. It is likely that by assembling larger multicenter cohorts or by performing a patient-level meta-analysis of existing studies, the identification of more specific outcomes (ie, viral opportunistic infection) or at-risk subgroups (ie, patients treated with T cell–depleting agents) could refine the predictive value of alphatorquevirus DNAemia measured early after transplantation.
In conclusion, by means of a large cohort of prospectively followed KT recipients with multiple monitoring points, the present study suggests that plasma alphatorquevirus DNA kinetics may be useful to predict the development not only of posttransplant infection but also of other complications attributable to over-immunosuppression. Alphatorquevirus viremia is emerging as a feasible, comprehensive surrogate biomarker for the overall state of immunosuppression after SOT. The recent introduction of commercial real-time PCR assays, although still pending on technical harmonization and clinical validation, would open the way for implementing serial measurements of alphatorquevirus DNA into the decision-making process involving KT recipients.
ACKNOWLEDGMENTS
The authors thank bioMérieux for kindly providing the reagents for the measurement of alphatorquevirus DNA load (DNA extraction system and real-time PCR assay) free of charge. This research was supported by “Plan Nacional de I+D+I” and Instituto de Salud Carlos III (Fondo de Investigaciones Sanitarias [FIS] 15/01953 and Proyecto Integrado de Excelencia [PIE] 13/00045), Subdirección General de Redes y Centros de Investigación Cooperativa, Spanish Ministry of Economy and Competitiveness, Spanish Network for Research in Infectious Diseases (REIPI RD16/0016) co-financed by the European Development Regional Fund (EDRF) “A way to achieve Europe.” M.F.R. has held a clinical research contract “Juan Rodés” (JR14/00036) from the Instituto de Salud Carlos III, Spanish Ministry of Economy and Competitiveness.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. D.N. has received research funds from bioMérieux. The other authors have no conflicts of interest to disclose.




