Danger, Metabolism and T Cell Memory
Abstract
Extracellular ATP promotes durable CD8+ T cell memory by signaling through the purinergic receptor P2RX7.
, , , et al. The purinergic receptor P2RX7 directs metabolic fitness of long-lived memory CD8+ T cells. Nature. 2018; 559: 264-268.
Summary and Analysis
It seems that all research roads lead to metabolism these days. The study by Borges da Silva et al. demonstrates how a danger signal, extracellular ATP (eATP), is essential to drive durable central (TCM) and tissue-resident (TRM) CD8+ T cell memory, not by changing gene expression, but by modifying the cells’ mitochondrial metabolism.
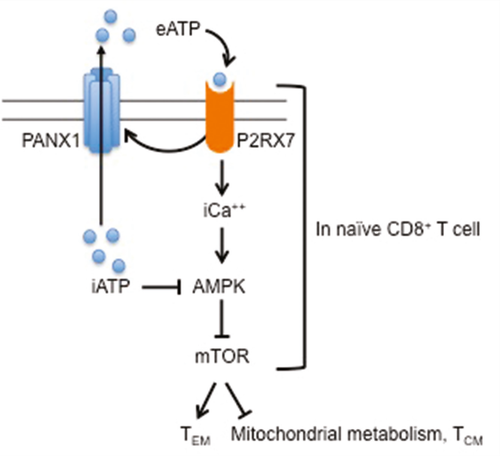
The purinergic receptor P2RX7 senses eATP and is known to activate innate immunity and pain responses. P2RX7 inhibitors are currently being tested in the clinic to reduce neuropathic pain. High levels of eATP occur during cell damage and tissue inflammation and induce P2RX7-mediated ion transport, including Ca2+ influx. Borges da Silva and colleagues investigated the role of P2RX7 in the development of T cell memory by crossing P14 transgenic mice, in which T cells are specific for a peptide of the lymphocytic choriomeningitis virus (LCMV), with P2rx7-/- mice. Co-transfer of wild-type (WT) and P2rx7-/- P14 T cells into congenic mice infected with acute LCMV preserved the development of short-lived effector T cells (SLECs) and effector memory T (TEM) cells in P2rx7-/- T cells. However, this co-transfer was associated with a profound defect in the development of CD62L+ TCM cells and CD69+CD103+ TRM cells and impaired recall responses to systemic or vaginal antigen restimulation. Early memory precursor effector cells (MPECs) were generated successfully in P2rx7-/- T cells, but their numbers declined over time. While RNA-seq revealed minimal differences between WT and P2rx7-/- cells, an analysis of metabolic processes after LCMV infection indicated that d8 P2rx7-/- MPECs and d14 TCM cells had reduced mitochondrial mass, mitochondrial membrane potential, glucose uptake, oxygen-consumption rate and respiratory capacity, whereas aerobic glycolysis was mostly preserved. These metabolic defects were associated with increased cell death. Notably, treatment with a P2RX7 inhibitor during CD8+ T cell activation recapitulated the dysregulated metabolism in both mouse and human T cells, suggesting translatability. Whether similar pathways are at play in CD4+ T cells remains to be determined.
ATP is released by dying cells and exported extracellularly by activated T cells through Pannexin 1 (PANX1) channels that are activated by P2RX7 signaling. Inhibition of PANX1 during T cell activation recapitulated the metabolic defects. Consistent with these findings, P2rx7-/- T cells had elevated intracellular ATP, which can inhibit activation of the adenosine monophosphate (AMP)-activated protein kinase (AMPK) that restrains the activity of mechanistic target of rapamycin (mTOR), an enzyme known to favor effector rather than memory differentiation. Indeed, pharmacological activation of AMPK restored metabolism, survival and TCM/TRM generation, and transient blockade of mTOR enabled TCM development by P2rx7-/- CD8+ T cells.
Most importantly, blockade of P2RX7 in vivo in a model of spared nerve injury combined with LCMV infection reduced nerve sensitivity as expected, but also diminished TCM generation and resulted in the loss of pre-existing memory CD8+ TCM cells. Thus, P2RX7 signals appear to promote the maintenance of CD8+ T cell central memory in addition to its induction. The authors caution that P2RX7 inhibition to reduce chronic pain in the clinic may inadvertently attenuate T cell memory.
The dependence on P2RX7 for the maintenance of T cell memory is exciting for the transplant field, because pre-existing T cell memory to donor antigens (whether via previous infections that generate heterologous immunity, or via prior transplantation, pregnancies or transfusions) is deleterious to transplant outcomes. Pharmacological inhibition of P2RX7 could, therefore, not only limit eATP-mediated danger signals from tissue injury following ischemia/reperfusion, but also destabilize CD8+ T cell memory, which could be attractive in sensitized hosts. Testing these concepts in models of pre-existing anti–donor memory may be worthwhile, as such animals are normally resistant to transplantation tolerance. These studies will help determine whether inhibition of P2RX7 prior to or at the time of transplantation can help promote graft acceptance and tolerance and how this intervention affects alloreactive T and B cell memory.
Dr. Alegre is a professor in the Department of Medicine at the University of Chicago. She is also section editor of “Literature Watch.”