Cost-effectiveness of hepatitis C–positive donor kidney transplantation for hepatitis C–negative recipients with concomitant direct-acting antiviral therapy
Abstract
Pilot studies suggest that transplanting hepatitis C virus (HCV)–positive donor (D+) kidneys into HCV-negative renal transplant (RT) recipients (R−), then treating HCV with direct-acting antivirals (DAA) is clinically feasible. To determine whether this is a cost-effective approach, a decision tree model was developed to analyze costs and effectiveness over a 5-year time frame between 2 choices: RT using a D+/R− strategy compared to continuing dialysis and waiting for a HCV-negative donor (D−/R−). The strategy of accepting a HCV+ organ then treating HCV was slightly more effective and substantially less expensive and resulted in an expected 4.8 years of life (YOL) with a cost of ≈$138 000 compared to an expected 4.7 YOL with a cost of ≈$329 000 for the D−/R− strategy. The D+/R− strategy remained dominant after sensitivity analyses including the difference in RT death probabilities or acute rejection probabilities between using D+ vs D− kidney; time that D−/R− patients waited for RT; dialysis death probabilities while waitlisted for RT in the D−/R− strategy; DAA therapy expected cure rate; costs of transplant, immunosuppressives, DAA therapy, dialysis, or acute rejection. The D+/R− strategy followed by treatment with DAA is less costly and slightly more effective compared to the D−/R− strategy.
Abbreviations
-
- Ab
-
- donor antibody
-
- CPI
-
- Consumer Price Index
-
- D+/-
-
- positive/negative donor
-
- DAA
-
- direct-acting antivirals
-
- DRG 652
-
- diagnosis-related group
-
- ESRD
-
- end-stage renal disease
-
- HCV
-
- hepatitis C
-
- NAT
-
- nucleic acid test
-
- OPTN
-
- Organ Procurement and Transplantation Network
-
- R+/−
-
- positive/negative recipients
-
- RT
-
- renal transplantation
-
- SRTR
-
- Scientific Registry of Transplant Recipients
-
- SVR
-
- sustained virologic response
-
- YOL
-
- years of life
1 INTRODUCTION
The continuing disparity between demand and supply for kidney transplants has exerted pressure on the transplant community to utilize donors at increased risk of transmission of bloodborne infections such as hepatitis B, human immunodeficiency virus, and hepatitis C virus (HCV). HCV donor antibody (Ab) +/nucleic acid test (NAT) + positive donor (D+) kidneys are now increasingly being offered to HCV NAT+ recipients due to published data that HCV NAT+ kidney transplant recipients (positive recipients [R+]) can significantly cut short their waiting time if they are willing to accept HCV D+ kidneys.1, 2 The shorter waiting time can result in an overall improved mortality without any significant worsening of hepatitis C–related-liver disease.3 Unfortunately, a large proportion of D+ kidneys might never be used due to the unavailability of R+ candidates. Reese et al reported that nearly 65% (out of a total 6546) of all D+ kidneys were discarded between the years 2005 and 2014.4
Traditionally, HCV D+ kidneys have not been offered to HCV− (R−) recipients due to the concerns of viral transmission. This was due to the fact that early small studies reported in the 1990 s suggested a very high transmission rate. A study from the New England Organ Bank on 29 organ transplants (from 13 HCV Ab+ donors; 19 kidneys) showed that chronic hepatitis C developed in 55% of all transplants. After transplant, 67% (of 29) of patients tested positive for HCV by antibody testing and 96% by NAT-based testing.5-7 Nevertheless, our recent analysis of the national Organ Procurement and Transplantation Network (OPTN) data suggested that this practice continued sporadically and ≈700 such HCV Ab+ kidneys were transplanted from 1994 to 2014. Interestingly, even in this era HCV D+/R− kidney transplant patients have improved mortality compared with matched controls on the waitlist who never received a transplant.8 The generalizability of these findings was limited by the fact that HCV NAT testing was not available for a large majority of donors. Thus, it is conceivable that many donor kidneys could have been HCV Ab+/NAT−, resulting in a minimal infectious transmission risk.
The development of direct-acting antiviral (DAA) drugs for the treatment of hepatitis C has changed the landscape of HCV therapy with high rates of sustained virologic response (SVR) even for patients with kidney transplants.9-12 With these advances in therapy, there is a potential of preemptive treatment with DAAs allowing for safe transplantation of D+ kidneys into needy R− recipients to minimize any risk of viral transmission. Early data from some pilot studies suggest that this is feasible.13, 14 While data on safety and efficacy are becoming available, the cost-effectiveness of such a strategy is less clear. Thus, it is necessary and important to assess the cost-effectiveness of this approach, given the fact that DAAs are extremely expensive.
To address this gap in knowledge, we performed a cost-effectiveness analysis comparing 2 strategies: (a) renal transplantation (RT) from a HCV+ donor (D+) into HCV− recipients (R−) followed by immediate DAA therapy vs (b) HCV− recipients (R−) continuing dialysis and waiting for RT from a HCV− donor (D−).
2 METHODS
The clinical and research activities reported here are consistent with the Principles of the Declaration of Istanbul as outlined in the “Declaration of Istanbul on Organ Trafficking and Transplant Tourism.”
This study was conducted from the perspective of a third-party payer. The hypothetical population studied consisted of adult end-stage renal disease (ESRD) patients who were hepatitis C negative (R−) and on the waiting list for a deceased donor kidney. A decision tree model was developed to analyze costs and effectiveness over a 5-year time frame between 2 choices: RT using D+ kidney followed by DAA treatment compared to continuing dialysis and waiting for RT with a D− kidney. The model was developed in Microsoft Excel 2016.
Posttransplant cumulative survival probabilities of ESRD adult patients completing RT were extracted from the OPTN/Scientific Registry of Transplant Recipients (SRTR) 2016 Annual Report.15 All data were the latest available records for a 2011 ESRD incident patient cohort followed for 5 years. The same data source was used for obtaining cumulative death probabilities of ESRD adult patients undergoing dialysis while waitlisted for RT. These data came from the latest available records of a 2013 ESRD incident patient cohort followed for 3 years, plus an estimation of another 2 years follow-up to fit our 5-year time frame. We used an exponential model to estimate the rates for the last 2 years for 2 reasons: (1) the literature indicates that the annual death rates increase over time for waitlisted dialysis patients16; (2) the exponential model provides an average annual mortality rate of ≈5% over the 5-year frame. This is consistent with expert opinion and literature.15 We then took the cumulative death probabilities and calculated corresponding annual death probabilities (see Supporting Information).
For D+/R− patients, DAA therapy was initiated immediately after the HCV-infected kidney transplant. If initial DAA therapy failed, a second round of treatment was implemented. D−/R− patients remained on dialysis until a HCV-negative (D−) kidney became available. We assumed that the probability of getting a D− kidney was 0 in year (Y) 1, 25% in Y2, 50% in Y3, 75% in Y4%, and 100% by the end of Y5. Base case probabilities used in the model are listed in Table 1.
| Variables | Base case probability | Reference |
|---|---|---|
| DAAa cure rate for initial treatment | 94% | 9-13, 23 |
| DAA cure rate after initial DAA treatment failure | 89% | 24-26 |
| Death within 5 y of RTa | 13% | 15 |
| Death within 3 y of RTa | 7% | |
| Death within 2 y of RTa | 5% | |
| Death within 1 y of RTa | 3% | |
| Death within 2 y of dialysis; waitlisted for RTa | 6% | |
| Death in the third y on dialysis; waitlisted for RTa | 2% | |
| Death in the fourth y on dialysis; waitlisted for RTa | 8% | |
| Death in the fifth y on dialysis; waitlisted for RTa | 12% | |
| Get a HCV− kidney at the end of y 2 for D−/R− patient | 25% | Expert opinion |
| Get a HCV− kidney at the end of y 3 for D−/R− patient | 50% | |
| Get a HCV− kidney at the end of y 4 for D−/R− patient | 75% | |
| Get a HCV− kidney at the end of y 4 for D−/R− patient | 100% | |
| Additional acute rejection probability for transplant using a HCV+ kidney compared to that using a HCV− kidney | 10% | Expert opinion |
- D−, negative donor; DAA, direct-acting antiviral hepatitis C medication; HCV, hepatitis C virus; R−, negative recipient; RT, renal transplantation.
- a See Supporting Information for details about calculation of death probabilities.
Effectiveness was measured in expected years of life (YOL) using data from the ESRD incident patients from the OPTN/SRTR 2016 Annual Report. Cost data were collected from public data sources (Table 2). National Average Drug Acquisition Costs were used to estimate DAA costs.17 Costs for other services were based on Medicare reimbursement rates, extracted from either the United States Renal Data System 2016 Annual Report or the Centers for Medicare and Medicaid Services payment database.18-20 Costs included direct expenditures for RT and immediate care post-RT, immunosuppressive therapy, dialysis, and associated medication costs while awaiting RT, and DAA therapy for D+/R− patients. Expenditures for RT and immediate care post-RT were calculated as the sum of Medicare reimbursements for the kidney transplant Diagnosis-Related Group (DRG 652) and corresponding provider service costs. All cost data were adjusted to 2017 US dollars, using the Consumer Price Index (CPI).21, 22 Specifically, the medicinal drugs CPI was used to adjust immunosuppressive medication and dialysis-related medication costs. The inpatient hospital service CPI was used to adjust the RT DRG cost, while the physician’s services CPI was used to adjust costs of corresponding provider services related to RT. The medical care services by other medical professionals CPI were used to adjust dialysis cost. Indirect and intangible costs were not modeled.
| Cost item | Base case ($)a: Unit cost × quantityb | Reference |
|---|---|---|
| Total kidney transplant cost | 29 555 | |
|
Cost per RT DRG = 652 |
26 675 | 18 |
|
Provider service per RT (initial hospital inpatient care, typically 70 min per d) HCPCS = 99 223 |
163 | 20 |
|
Provider service per RT (ultrasound of transplanted kidney) HCPCS = 76 776 |
30 | |
|
Provider service per RT (radiograph of chest, 1 view, front) HCPCS = 71 010 |
7 × 2 | |
|
Provider service per RT (subsequent hospital inpatient care, typically 25 min per d) HCPCS = 99 232 |
58 × 6 | |
| Provider service per RT (preparation of donor kidney for transplantation)HCPCS = 50 323 | 87 | |
|
Provider service per RT (transplantation of donor kidney) HCPCS = 50 360 |
1639 | |
|
Provider service per RT (insertion of central venous catheter for infusion, patient 5 y or older) HCPCS = 36 556 |
100 | |
|
Provider service per RT (routine electrocardiogram using at least 12 leads with interpretation and report) HCPCS = 93 010 |
7 × 2 | |
|
Provider service per RT (hospital discharge day management, 30 min or less) HCPCS = 99 238 |
59 | |
|
Provider service per RT (surgical pathology consultation and report) HCPCS = 88 321 |
68 | |
|
Provider service per RT (anesthesia for kidney transplant) HCPCS = 00 868 |
360 | |
| Immunosuppressive drug cost | 5218 | |
| Standard immunosuppressive drug therapy per patient per y | 5218 | 19 |
| Total DAA therapy cost | 54 733 | |
| Cost per DAA therapy per patient per course of treatment | 53 276 | 17 |
| Donor HCV genotyping at baseline per patient | 287 | Dept. Internal Medicine. Virginia Commonwealth University School of Medicine. Data access on Nov. 17, 2017. |
| Donor quant RT PCR per patient | 1170 | |
| Total acute rejection treatment cost | 30 457 | |
| Standard acute rejection treatment per patient per y | 30 457 | 32 |
| Total dialysis cost | 95 729 | |
| Cost of dialysis per patient per y | 89 404 | 19 |
| Cost of dialysis-related medications per patient per y | 6325 |
- DAA, direct-acting antiviral hepatitis C medication; DRG, diagnosis-related group; HCV, hepatitis C virus; HCPCS, Healthcare Common Procedure Coding System; PCR, polymerase chain reaction; RT, renal transplantation.
- a All listed base values have been Consumer Price Index adjusted to 2017 values and rounded to integers.
- b When quantity equals 1, it is omitted.
- HCV− and HCV+ transplant patients received the same surgical procedure and the same posttransplant care (ie, hospitalization, follow-up monitoring, and follow-up blood tests).
- The immunosuppressive therapy is the same for patients receiving HCV− and HCV+ kidneys.
- To fit a parsimonious model, graft failures and other complications or adverse events (eg, infections or malignancies) associated with immunosuppressive therapy were not modeled. We made a conservative assumption that the D+/R− group would have a higher acute rejection rate than the D−/R− group. To simplify the model, rather than modeling the acute rejection rate for each group, we modeled the difference in acute rejection rates between the groups. We modeled this as a 10% acute rejection rate in the D+/R− group and a 0% acute rejection rate in the D−/R− group.
- All D+/R− patients were infected with HCV (NAT+) and received DAA treatment.
- DAA consisted of 12 weeks of therapy with grazoprevir/elbasvir 50/100 mg (Zepatier; Merck, NJ), 1 tablet daily for 12 weeks. DAA treatment resulted in HCV cures for 94% of D+/R− patients. This assumption is supported by previous studies using DAA to treat HCV after RT.9-13, 23 The remaining 6% of patients received a second round of DAA therapy. After the second round of DAA therapy, 89% of patients were cured based upon recent studies in the general population with or without kidney disease.24-26 We used a conservative estimate of the lowest SVR12 (89%) published for patients with a primary treatment failure as our base case. The remaining 11% of patients were assumed to have similar clinical outcomes over the 5-year time frame of our study. Sensitivity analyses were conducted using a lower cure probability (75% for the initial round of therapy and 71% for the second round of therapy) and a 20% higher death probability (25%) for patients who failed the second round of DAA therapy. Additional sensitivity analyses were performed using other available DAAs on the market including sofusbuvir/ledipasvir (Harvoni; Gilead, CA), sofosbuvir/velpatasvir (Epclusa; Gilead, CA), sofosbuvir/velpatasvir/voxilaprevir (Vosevi; Gilead, CA), and glecaprevir/pibrentasvir (Mavyret; Abbvie, IL). The upper end of our sensitivity analysis range was the highest National Average Drug Acquisition Cost price of these 5 DAA therapies in the current market. The lower end of our sensitivity analysis range was the least expensive 340B price of the same 5 DAA therapies at our health system.
- Cumulative death probabilities after RT were the same for D+/R− and D−/R− patients. We did additional sensitivity analysis assuming a 20% higher probability of death in D+/R− patients compared to D−/R− patients.
- D+/R− patients received a transplant within the first 3 months of the study’s 5-year time frame. Sensitivity analysis was performed assuming waiting times from 0 to 24 months.
- Patients waiting for an HCV+ kidney did not die within the first 3 months of the 5-year time frame.
- For patients remaining on dialysis, the probabilities of getting a HCV− kidney were 0% during year (Y)1, 25% by the end of Y2, 50% by the end of Y3, 75% by the end of Y4%, and 100% by the end of Y5. Sensitivity analysis was conducted assuming the probabilities of getting an HCV− kidney were 25% by the end of Y1, 50% by the end of Y2, 75% at the end by Y3%, and 100% by the end of Y4.
- Patients who died during a given year were assumed to have died at the end of the year (rather than at some other time within that year).
- The outcomes of patients remaining on dialysis were evaluated at the end of years 2, 3, and 4, as the probability of getting a D− kidney increased each year. Year 1 outcomes were built into the year 2 assessment, because we assumed there is 0% probability of getting a HCV− kidney at the end of year 1. Year 5 outcomes were excluded to avoid inflated costs because we assumed there was a 100% probability of getting a HCV− kidney by the end of year 5. Including year 5 outcomes would have included the costs of transplants done in year 5 but would not have reflected improved lifespan because the model did not include outcomes of YOL beyond 5 years. Sensitivity analysis was conducted that included the costs of RT in Year 5.
- Mortality probabilities for the study patient cohort were same as for the patient cohort included in the 2016 OPTN/SRTR Annual Report. Additional sensitivity analyses were performed using both higher and lower mortality rates for both posttransplant and waitlisted dialysis patients.
3 RESULTS
The decision tree is shown in Figure 1. For those accepting a HCV NAT+ organ, the cost of care (transplant, DAA, immune suppression, and provider care for up to 5 years) and YOL ranged from $123 092 to $218 196 and 3.1 to 5 years, respectively. Costs were lowest in those who did not survive the 5 years and highest in those who survived and required a second course of DAA. Using an expected 94% SVR rate with DAA and published 5-year survival rates (87%), the cost of the D+/R− strategy was ≈$138 000. In comparison, for those who remained on dialysis while waiting for a HCV-negative organ, the costs and YOL ranged from $143 594 and 1.5 years, respectively, in those who died within 2 years while on dialysis to $478 645 and 5 YOL, respectively, for those who were still waiting for an organ on dialysis after 5 years. Because of the cost of dialysis, the shorter the wait time for a HCV-negative organ the lower the cost: $230 927 to $236 667 if transplanted in year 2 compared to $417 689 if transplanted in year 4. The baseline cost-effectiveness analysis found that the D+/R− strategy resulted in an expected 4.8 YOL with an expected total cost of ≈$138 000 compared to 4.7 YOL and ≈$329 000 for the D−/R− strategy.
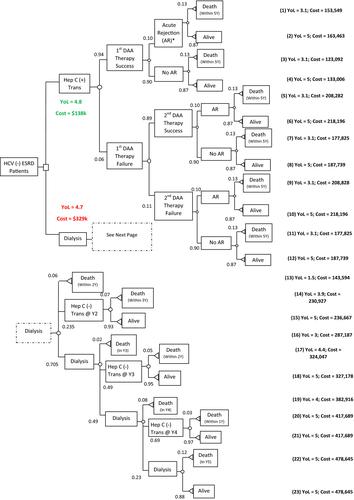
As shown in Table 3, the D+/R− strategy remained dominant in all 1-way sensitivity analyses including adjustment for RT cumulative death probability (D+/R− was equivalent to or 20% higher than D−/R−), difference in acute rejection probability between strategies (D+/R− was 5%-20% higher than D−/R−), DAA therapy cure rate (75%-94% for the first round; 71%-89% for the second round), death probability after the second round of DAA therapy failure (failure group was equivalent to or 20% higher than the success group), waiting time on dialysis in the D+/R− strategy (0-24 months), cost of kidney transplant ($19 579-$142 913), cost for treating acute rejection ($11 192-$45 686), immunosuppressive medication costs ($4174-$6262), dialysis costs ($76 217-$114 325), DAA treatment costs ($18 692-$93 582), probability of dialysis patients getting a RT in the D−/R− strategy by year (25% at Y1, 50% at Y2, 75% at Y3%, and 100% at Y4 vs base case of 0% at Y1, 25% at Y2, 50% at Y3, 75% at Y4%, and 100% at Y5), including the cost of Y5 RT (vs excluding it in base case), kidney transplant annual death probability (±20% of base value), and dialysis annual death probability while waitlisted for RT in the D−/R− strategy (±20% of base value). All sensitivity analyses resulted in the same or slightly higher YOL with the D+/R− strategy compared to the D−/R− strategy.
| D+/R− strategy | D−/R− strategya | |||
|---|---|---|---|---|
| Expected YOL | Expected cost (2017 USD) | Expected YOL | Expected cost (2017 USD) | |
| Base case | 4.8 | 138 047 | 4.7 | 329 329 |
| Probability of death with D+ is 20% greater than with D− (Base case is equal probabilities of survival) | 4.7 | 137 749 | 4.7 | 329 329 |
| Cure probability with first round of DAA therapy is 75% (Base case is 94%) | 4.8 | 148 446 | 4.7 | 329 329 |
| Cure probability with second round of DAA therapy is 71% (Base case is 89%) | 4.8 | 138 047 | 4.7 | 329 329 |
|
Probability of death with second round of DAA therapy failure is 20% higher than with second round of DAA therapy success (Base case is equal probabilities of death) |
4.8 | 138 045 | 4.7 | 329 329 |
| Wait time for D+ | ||||
| 0 mo | 4.8 | 115 419 | 4.7 | 329 329 |
| 12 mo | 4.7 | 202 915 | 4.7 | 329 329 |
| 24 mo | 4.7 | 287 826 | 4.7 | 329 329 |
| (Base case is 3-mo wait) | ||||
| Additional acute rejection probability with D+ | ||||
| 20% (increased 100%) | 4.8 | 141 093 | 4.7 | 329 329 |
| 5% (decreased 50%) | 4.8 | 136 524 | 4.7 | 329 329 |
| (Base case is 10%) | ||||
| Cost of total renal transplant | ||||
| $19 579 | 4.8 | 128 071 | 4.7 | 321 161 |
| $142 913 | 4.8 | 251 405 | 4.7 | 422 148 |
| (Base case is $29 555 USD) | ||||
| Cost of immunosuppressive drug | ||||
| $6262 (increased 20%) | 4.8 | 142 748 | 4.7 | 331 006 |
| $4174 (reduced 20%) | 4.8 | 133 346 | 4.7 | 327 653 |
| (Base case is $5218 USD) | ||||
| Cost of total dialysis | ||||
| $114 875 (increased 20%) | 4.8 | 142 833 | 4.7 | 388 680 |
| $76 583 (reduced 20%) | 4.8 | 133 260 | 4.7 | 269 979 |
| (Base case is $95 729 USD) | ||||
| Cost of total DAA therapy | ||||
| $93 582a | 4.8 | 179 227 | 4.7 | 329 329 |
| $18 692a | 4.8 | 99 843 | 4.7 | 329 329 |
| (Base case is $54 733 USD) | ||||
| Cost of total acute rejection treatment32 | ||||
| $45 686 (increased 50%) | 4.8 | 139 570 | 4.7 | 329 329 |
| $11 192 (decreased 50%) | 4.8 | 136 120 | 4.7 | 329 329 |
| (Base case is $30 457) | ||||
|
Cost of total renal transplant included by the end of year 5 (Base case has it excluded) |
4.8 | 138 047 | 4.7 | 331 396 |
| Reduced wait time for D−/R−b | 4.8 | 138 047 | 4.7 | 248 963 |
| Annual probability death on kidney transplantc | ||||
| Decreased 20% | 4.8 | 138 305 | 4.7 | 329 359 |
| Increased 20% | 4.7 | 137 789 | 4.7 | 329 300 |
| (Base case: Y 1 = 3%, Y 2 = 2%, Y 3 = 2%, Y 4 = 3%, Y 5 = 3%) | ||||
| Annual probability of death on dialysis and waitlisted for kidney transplantc | ||||
| Decreased 20% | 4.8 | 138 047 | 4.8 | 332 238 |
| Increased 20% | 4.8 | 138 047 | 4.7 | 326 434 |
| (Base case: Y 1 = 3%, Y 2 = 3%, Y 3 = 2%, Y 4 = 8%, Y 5 = 12%) | ||||
- D+, hepatitis C virus–positive kidney; R−, hepatitis C virus–negative recipient; D−, hepatitis C virus–negative kidney; DAA, direct-acting antiviral hepatitis C medication; NADAC, National Average Drug Acquisition Cost; USD, US dollars; Y, year; YOL, years of life.
- a The upper end of our sensitivity analysis range is the highest NADAC price of the 5 most commonly used DAA therapies in the current market: Zepatier, Mavyret, Vosevi, Harvoni, and Epclusa. The lower end of our sensitivity analysis range is the least expensive 340B price of the same 5 DAA therapies at our health system.
- b Wait time in base case was: 25% of patients would get a D− kidney by the end of 2 y, 50% by the end of 3 y, 75% by the end of 4 y, and 100% by the end of 5 y. Reduced wait time was: 25% of patients would get a D− kidney by the end of 1 y, 50% by the end of 2 y, 75% by the end of 3 y, and 100% by the end of 4 y.
- c See Supporting Information for calculation of death probabilities.
The difference in cost between the 2 strategies, however, was substantial in all sensitivity analyses, with R+/D− strategy being cost-saving. The cost-saving for each sensitivity analysis was compared to the base case cost-savings. The variables with the largest impact on cost-savings between the 2 strategies in the sensitivity analyses were expected waiting time for a D+ kidney, reduced wait time for a D− kidney, and total cost of dialysis. In the base case, the expected cost of the D+/R− strategy was $191 282 less than that for the D−/R− strategy. Compared to the base case, the D+/R− group would have an additional saving of around $55 000 compared to the D−/R− group if total dialysis costs were 20% higher than the base case. As the expected waiting time for a D+ kidney increased, the cost-savings for the D+/R− strategy decreased: it was about $65 000 lower if waiting time was 1 year and almost $150 000 lower if waiting time was 2 years. On the other hand, a 1-year reduction in wait time for D−/R− patients to get a D− kidney decreased the cost advantage of the D+/R− strategy by about $80 000. For all sensitivity analyses, however, the cost of the D+/R− strategy was less than that of the D−/R− strategy.
The difference between total expected costs of the D+/R− strategy and that of the D−/R− strategy decreased as the cost of RT or DAA therapy increased. Therefore, additional analysis was conducted to determine the breakeven cost of RT and DAA therapy. The results indicated that, all other factors being equal, the total cost of RT would have to be $1 085 258 or the cost of DAA therapy $235 188 to make the expected costs of the D+/R− and D−/R− strategies equal. The breakeven costs are far higher than our baseline estimates of the costs of these treatments.
4 DISCUSSION
The advent of DAA therapy has created a new paradigm for the therapy of hepatitis C. In addition to providing a cure for a large majority of patients infected with HCV, these drugs have opened a pathway for the use of HCV viremic donor organs into HCV-negative recipients followed by DAA therapy to eliminate the risk of donor-derived HCV disease. Early pilot studies suggest that this is clinically feasible and excellent sustained virologic responses can be achieved both for kidney as well as other solid-organ transplants. In addition, it was reported that between 29% and 82% of the potential HCV− kidney transplant recipients on the waiting list might be willing to accept HCV+ kidneys under certain scenarios.27 In an analysis using contemporaneous cost data from Medicare (in US dollars), we now report that in addition to providing extra years of life for patients, this technique results in lower costs.
When compared to staying on dialysis and waiting for an HCV-negative donor kidney, the baseline cost-effectiveness analysis found that the D+/R− strategy resulted in an average of about one tenth of an additional year of life with a cost-saving of approximately $190 000 per patient. The D+/R− strategy remained dominant even after sensitivity analyses (Table 3) for a variety of variables. It should be noted that the use of yearly waitlist mortality in our study (done using only a 5-year time frame) does not capture the long-term benefits of transplantation extending beyond 5 years. In addition, it also does not capture the mortality of patients who were never waitlisted (or removed from the waitlist after waiting a few years) because they were considered too sick for transplantation. Thus, we believe that in the long-term the HCV D+/R− strategy might result in additional YOL than suggested by our results.
As expected, the difference between total calculated costs of the D+/R− strategy and that of the D−/R− strategy decreased as the cost of RT or DAA therapy increased. Since an important focus of the D+/R− strategy is the cost of DAA therapy, we took a very conservative approach in our sensitivity analysis. Even an increase in the cost of DAA therapy by 50% did not change the dominance of the D+/R− strategy. Our results indicate that, all other factors being equal, the cost of RT would have to be about a million USD or the cost of DAA therapy about $235 000 USD to make the expected costs of the D+/R− and D−/R− strategies equal. In fact in the actual market, it is currently apparent that the new DAA therapy combinations are less costly than the older ones and even the older ones have higher rebates than available previously.
The findings in our study corroborate that of the previous expert opinions as well as the data published from the United Kingdom recently.28 Trotter et al reported that while the use of DAA (sofusbuvir/ledipasvir) therapy increased the cumulative cost of transplantation at 5 years by £38 979 per patient, transplantation remained cost-effective compared to hemodialysis at 5 years by approximately £20 000 per patient.29 The primary mechanism for the realization of these cost-savings is by reduction in dialysis time. For example, Sawinski et al assessed HCV-positive recipients who received either a HCV D+ or a D− kidney.30 They reported that on average, wait times went down nearly by half (485 days vs 969 days) in case HCV kidney recipients received D+/R+ transplants as opposed to waiting for a D−/R+ transplant. This resulted in a cost-saving of $38 000 to $386 000 per patient, depending upon local wait times.
There are limitations to this analysis, as we used several assumptions for the derivation of our cost-effectiveness models. We assumed that survival probabilities after RT were the same for D+/R− and D−/R− patients. While there is scant clinical evidence to support or refute this assumption, we did do an additional sensitivity analysis assuming a 20% higher probability of cumulative death in D+/R− patients compared to D−/R− patients. This did not change our results significantly (Table 3). In addition, the 2 recently reported HCV D+/R− pilot trials did not report an increase in acute rejection, increased infections, early graft loss, or patient mortality in recipients of HCV D+/R− kidneys, thus supporting our assumption.13, 14 In fact, since HCV+ donors are much more likely to be younger, it is also possible that long-term graft survival might be better for this population than estimated.1, 4 It should also be noted that our data were limited to patients insured by Medicare, and thus findings may not be generalizable to patients with private health insurance. However, there is no reason to believe that these data would not be applicable to those with non-Medicare coverage.
Our analyses only included costs directly related to the transplant or to dialysis. That is, we did not include other health care costs—such as the cost of managing hypertension, diabetes, bone disease, anemia, access complications, etc—incurred by patients on dialysis or after transplant. If such costs were substantially higher for transplant patients than for dialysis patients, then our cost-savings might be overestimates. However, our sensitivity analyses indicate that these differences would have to be very large—around $190 000 per patient in most cases—to eliminate the cost-savings of the D+/R− strategy.
For the purpose of this analysis, we conservatively assumed that all patients would get HCV infection. Infection transmission has ranged anywhere from 0% to 100% based upon the previous data accumulated over the last 3 decades.5, 7, 31 Thus, it is possible that some patients can avoid HCV transmission and might not require DAA therapy. This could potentially result in additional cost-savings. We also assumed that DAA treatment would result in cures for 94% of patients. This assumption is supported by previous studies using DAA to treat HCV after RT.8, 9, 16 Further sensitivity analyses using a lower cure probability and a higher death probability for patients who failed the second round of DAA therapy did not alter the results significantly (Table 3). Finally, we assumed that 100% of HCV D+/R− patients will receive a kidney transplant between 3 and 24 months from listing. Although these data are corroborated by previous evidence as well as our own ongoing study on HCV D+/R− kidney transplants (# NCT03249194), it might not be true for some parts of the country.1 On the other hand, we did use a conservative assumption that all HCV D−/R− patients will receive a kidney transplant by the end of the fifth year on the waiting list. Since wait times can be much longer in many parts of the country, this could have resulted in an underestimation of the cost-effectiveness of the HCV D+/R− approach. Finally, this is a population-based study in which we looked at averages across the dialysis population rather than at specific patient subtypes. It was not our intention to provide estimates for specific patient types, nor do we have the data to support this.
In conclusion, our analysis performed using a third-party payer’s perspective suggests that utilization of HCV donor–positive kidney transplants for HCV-negative recipients is cost-effective. This strategy provides for equal or slightly reduced mortality and substantially reduced costs by limiting dialysis time.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to declare as described by the American Journal of Transplantation.




