High tacrolimus trough level variability is associated with rejections after heart transplant
Abstract
Tacrolimus, the major immunosuppressant after heart transplant (HTx) therapy, is a narrow therapeutic index drug. Hence, achieving stable therapeutic steady state plasma concentrations is essential to ensure efficacy while avoiding toxicity. Whether high variability in steady state concentrations is associated with poor outcomes is unknown. We investigated the association between tacrolimus trough level variability during the first year post-HTx and outcomes during and beyond the first postoperative year. Overall, 72 patients were analyzed for mortality, of whom 65 and 61 were available for rejection analysis during and beyond the first year post-HTx, respectively. Patients were divided into high (median >28.8%) and low tacrolimus level variability (<28.8%) groups. Mean tacrolimus levels did not differ between the groups (12.7 ± 3.4 ng/mL vs 12.8 ± 2.4 ng/mL, P = .930). Patients in the high variability group exhibited higher long-term rejection rate (median total rejection score: 0.33 vs 0, P = .04) with no difference in rejection scores within the first year post-HTx. Multivariate analysis showed that high tacrolimus trough level variability was associated with >8-fold increased risk for any rejection beyond the first year post-HTx (P = .011). Mortality was associated only with cardiovascular complications (P = .018), with no effect of tacrolimus through level variability.
Abbreviations
-
- ATG
-
- antithymocyte globulin
-
- BMI
-
- body mass index
-
- CAV
-
- cardiac allograft vasculopathy
-
- CMV
-
- cytomegalovirus
-
- CrCl
-
- Cockcroft-Gault equation for creatinine clearance
-
- CV
-
- coefficient of variation
-
- CYP
-
- cytochrome P450
-
- DM
-
- diabetes mellitus
-
- dnDSA
-
- de novo donor-specific antibody
-
- EMB
-
- endomyocardial biopsy
-
- ESRF
-
- end-stage renal failure
-
- HF
-
- heart failure
-
- HTN
-
- hypertension
-
- HTx
-
- heart transplantation
-
- IHD
-
- ischemic heart disease
-
- ISHLT
-
- International Society of Heart and Lung Transplantation
-
- LVAD
-
- left ventricular assist device
-
- MMF
-
- mycophenolate mofetil
-
- TAC
-
- tacrolimus
-
- TRS
-
- total rejection score
1 INTRODUCTION
Heart transplantation (HTx) remains the gold standard treatment for end-stage heart failure. Since the first HTx in 1967,1 survival has improved considerably with increment of the median survival of adult HTx to 11 years.2 Improved immunosuppression therapy is one of the factors that has contributed significantly to better results. Tacrolimus (TAC), the primary immunosuppressive drug for HTx recipients, prevents T cell activation and proliferation by forming a complex with immunophilin, which interacts with intracellular calcineurin that inhibits the expression of genes coding for proinflammatory cytokines.3, 4 TAC, a narrow therapeutic index drug, demonstrates large pharmacokinetic interindividual variability, partially due to presystemic metabolism by the intestinal cytochrome P450 (CYP)3A system, which may be affected by other CYP3A substrates, inducers, or inhibitors.3, 5 High variability in TAC trough levels has been related to worse outcomes in both adult and pediatric kidney transplant recipients.6-15 However, the impact of such variability after HTx has been defined only in preliminary reports.16, 17 Our objectives were to investigate the influence of TAC trough level variability on long-term outcomes and rejections, during and beyond the first year post-HTx.
2 METHODS
2.1 Patient population
Our study included patients transplanted at a single center between January 2006 and July 2017. All patients were treated with triple-drug immunosuppression (TAC, mycophenolate mofetil [MMF], and corticosteroids) with induction therapy consisting of antithymocyte globulin (ATG). TAC was started in the early postoperative period, when renal function started to normalize, usually by the third postoperative day. Institutional review board approval was obtained for the study.
2.2 Definitions and endpoints
2.2.1 TAC trough level assays
Whole blood trough levels were measured by using the Architect Tacrolimus Reagent Kit (Abbott, Abbott Park, IL). The assay is a chemiluminescent microparticle immunoassay routinely used in the clinical practice for quantitative determination of TAC trough levels.18
2.2.2 TAC trough levels and coefficient of variation
For each patient, whole blood TAC steady state (at least 48 hours [ie, 4 half-lives] on fixed-dose regimen) trough levels at 3-12 months post-HTx were documented. TAC trough level coefficient of variation (CV) was calculated as the ratio: (TAC trough level standard deviation)/(mean) multiplied by 100 (Figure 1). Only patients with ≥2 TAC trough level measurements were included. Based on variability in the entire cohort, patients were divided into high TAC variability group (above median CV) and low TAC variability (below median CV) group. Absolute TAC trough levels between 8 and 12 ng/mL for 3-6 months post-HTx and between 5 and 10 ng/mL in stable patients beyond 6 months after HTx were considered therapeutic.4
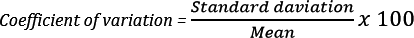
2.2.3 De novo donor-specific antibodies
HLA antibody specificities and mean fluorescence intensities (MFIs) were determined using the Luminex single-antigen bead assay.
2.2.4 Outcome measures
Data for each patient included demographic information, clinical characteristics, and follow-up data. Primary endpoints were histologic rejections and mortality. Rejections were analyzed in 2 separate time frames: (1) 3-12 months post-HTx and (2) from the end of the first year post-HTx. This is done to avoid a prothopathic bias, that is, changes in daily tacrolimus dose in response to rejection, which may increase trough level variability.
2.2.5 Rejections, surveillance, and classification
Rejections were diagnosed through use of the routine institutional follow-up protocol or, if clinically indicated, endomyocardial biopsy (EMB) and were classified according to the revised International Society of Heart and Lung Transplantation (ISHLT) classification system for rejection.19 Routine EMBs were performed every week for the first 4 weeks after HTx, twice a month in the second and third months, once a month for the next 3 months, and thereafter every 3 months until the end of the first year. From the end of the first year until the end of the third to fifth years, biopsies were performed annually. Major rejection was defined as cellular grade ≥2R, or any symptomatic acute rejection regardless of the ISHLT EMB grade. The diagnosis of antibody-mediated rejection (AMR) was based on cardiac dysfunction with lack of cellular infiltrates on the heart biopsy together with histologic evidence of acute capillary injury.20 For each patient we calculated total rejection score (TRS) as 0R = 0, 1R = 1, 2R = 2, and 3R = 3, by dividing the summed scores by the total number of biopsy specimens taken during the study period.21
For multivariate logistic regression analysis, patients were further classified into 2 groups: (1) the no-rejection group: in which ≥50% of biopsy specimens demonstrated ISHLT 0R grade and no histologic evidence for major rejection during the entire follow-up period, and (2) the any-rejection group: in which ≥50% of biopsy specimens demonstrated ≥ISHLT 1R grade, or ≥1 biopsy specimens revealed major rejection.
2.2.6 Cardiac allograft vasculopathy
The institutional posttransplant care protocol includes an annual invasive coronary angiography for the first 5 years after HTx along with echocardiogram and right-sided heart catheterization. Cardiac allograft vasculopathy (CAV) was diagnosed with the use of coronary angiography, and invasive hemodynamic assessment was performed annually, along with clinical assessment and echocardiography, combined according to the recommended nomenclature for CAV of the ISHLT consensus statement.22
2.3 Statistical analysis
Data are presented as mean ± SD or median with IQR as appropriate for continuous variables and proportions for categorical variables. Comparison between groups was conducted by using independent t test and Mann–Whitney U test for parametric and nonparametric analyses, respectively. Categorical variables were compared by using χ2 or Fisher exact tests. Linear regression was used to determine the association between TAC trough levels CV and TRS. Logistic regression analysis was used to examine the association between related variables and any rejection group as previously defined. Cox regression analysis was used to evaluate the relative effect of selected covariates on mortality, including TAC-related toxicity (ie, creatinine raise ≥50% baseline, malignancy development, and infections requiring hospitalization) and cardiovascular complications (post-HTx heart failure, myocardial infarction, stroke, and CAV).
All analyses were 2-tailed and P ≤ .05 was considered significant. Statistical analyses were performed by using SPSS software (version 21 IBM, SPSS Inc).
3 RESULTS
3.1 Study cohort
Between January 2006 and July 2017, a total of 76 HTx recipients were treated de novo with TAC. Four patients were excluded from the analysis: 2 patients had a single TAC level between 3 and 12 months and 2 additional patients had their first documented TAC level 1 year after HTx. The mean follow-up period was 51.1 (± 34.9) months, during which 4 patients developed CAV and 10 patients died. Patients’ and donors’ characteristics are presented in Table 1.
| Variable | Low TAC trough level variability group (n = 36) | High TAC trough level variability group (n = 36) | P value |
|---|---|---|---|
| Recipient baseline characteristics | |||
| Sex, female, No. (%) | 10/36 (27.8) | 8/36 (22.2) | .786 |
| Age at HTx, y (IQR) | 51.5 (33.7-58.7) | 50 (28.0-56.0) | .401 |
| Recipient BMI, kg/m2 (SD) | 23.6 (4.6) | 23.3 (5.2) | .827 |
| Ischemic etiology, No. (%) | 11/36 (30.6) | 17/36 (47.2) | .227 |
| HTN before HTx, No. (%) | 10/36 (27.8) | 12/36 (33.3) | .798 |
| DM before HTx, No. (%) | 4/36 (11.1) | 7/36 (19.4) | .514 |
| Past smoker, No. (%) | 12/36 (33.3) | 12/36 (33.3) | 1.0 |
| Family history for IHD, No. (%) | 12/36 (33.3) | 17/36 (47.2) | .337 |
| Recipient baseline CrCl, mL/min (IQR) | 77.6 (64.3-91.9) | 64.7 (48.1-103.8) | .323 |
| Recipient baseline bilirubin, mg/dL (IQR) | 1.0 (0.6-1.3) | 1.07 (0.7-1.55) | .309 |
| PAS, mm Hg (SD) | 45.5 (19.3) | 48.3 (16.5) | .551 |
| PAD, mm Hg (SD) | 20.3 (10.1) | 22.0 (10.1) | .531 |
| Mean PAP, mm Hg (SD) | 29.9 (13.1) | 32.4 (11.6) | .443 |
| PCWP, mm Hg (SD) | 21.4 (11.5) | 22.7 (9.6) | .643 |
| CO, L/min (SD) | 3.3 (1.3) | 3.6 (1.3) | .426 |
| PRA >30%, No. (%) | 0/33 (0) | 1/35 (2.9) | 1.0 |
| Donor-related characteristics | |||
| Donor age, y (SD) | 32.68 (11.4) | 25.51 (12.2) | .018 |
| Donor BMI, kg/m2 (SD) | 24.61 (2.6) | 24.38 (4.5) | .813 |
| Recipient-donor–related variables | |||
| Sex mismatch, No. (%) | 5/31 (16.1) | 8/33 (24.2) | .539 |
| CMV mismatch, No. (%) | 8/22 (36.4) | 6/12 (50) | .487 |
| Perioperative variables | |||
| Ischemic time, min (SD) | 156.2 (42.7) | 167.0 (41.7) | .320 |
| Early complications, No. (%) | 22/36 (48.9) | 23/36 (51.1) | 1.0 |
| Prolonged inotropic support, No. (%) | 16/36 (44.4) | 24/36 (66.7) | .096 |
| Days from admission to discharge (IQR) | 27 (15.6, 63.3) | 46 (23.6, 85.0) | .075 |
| Days from HTx to discharge (IQR) | 17 (12, 25.8) | 25 (14.5, 36.3) | .046 |
| Follow-up period | |||
| Follow-up duration, mo (IQR) | 39.3 (13.0-72.3) | 58.8 (23.7-87.5) | .083 |
| HTN post-HTx, No. (%) | 11/36 (30.6) | 20/35 (57.1) | .032 |
| DM post-HTx, No. (%) | 8/36 (22.2) | 12/35 (34.3) | .300 |
| Statins post-HTx, No. (%) | 30/36 (83.3) | 31/35 (88.6) | .735 |
| HF during follow-up, No. (%) | 0/36 (0) | 2/35 (5.7) | .239 |
| Infections requiring hospitalization, No. (%) | 11/36 (30.6) | 20/36 (55.6) | .056 |
| Stroke, No. (%) | 2/36 (5.6) | 5/35 (14.3) | .260 |
| CAV, No. (%) | 2/36 (5.6) | 2/25 (5.7) | 1.0 |
| Malignancy, No. (%) | 1/36 (2.8) | 2/35 (5.7) | .614 |
| ESRF, No. (%) | 2/36 (5.6) | 1/35 (2.9) | 1.0 |
| Creatinine rise ≥50% of baseline, No. (%) | 15/31 (48.1) | 21/32 (65.6) | .130 |
| dnDSAs, No. (%) | 4/36 (11.1) | 4/36 (11.1) | 1.0 |
| HLA class I only | 2/36 (5.6) | 1/36 (2.8) | 1.0 |
| HLA class II only | 0/36 (0) | 0/36 (0) | — |
| HLA class I and II | 2/36 (5.6) | 3/36 (8.3) | 1.0 |
- Continuous variables are expressed as mean (SD) or median (IQR) as indicated, and proportions as percentages.
- TAC, tacrolimus; HTx, heart transplantation; BMI, body mass index; HTN, hypertension; DM, diabetes mellitus; IHD, ischemic heart disease; CrCl, Cockcroft-Gault equation for creatinine clearance; CMV, cytomegalovirus; dnDSA, de novo donor-specific antibody; LVAD, left ventricular assist device; ESRF, end-stage renal failure; HF, heart failure; CAV, cardiac allograft vasculopathy.
- The bold values are those with significant p value (less than/equal to 0.05)
3.2 TAC trough level and CV
The mean number of TAC trough levels between 3 and 12 months post-HTx was 13.5 (± 9.6, range: 2-58) with a mean concentration of 12.7 (± 2.9) ng/mL. The median TAC CV was 28.8%. Accordingly, patients were divided into high and low CV groups, with 36 patients in each group. The high TAC trough level variability group consisted of more patients with ≥1 measurement below the therapeutic range (77.8% vs 36.1%, P ≤ .001). Mean TAC trough level did not differ between groups (12.7 ± 3.4 ng/mL vs 12.8 ± 2.4 ng/mL for the high and low variability groups, respectively; P = .93). The high TAC trough level variability group was characterized by a younger donor age (25.5 ± 12.3 vs 32.7 ± 11.4 years, P = .018, respectively), higher rate of post-HTx hypertension (57.1% vs 30.6%, P = .032), and longer post-HTx hospital stay (25 vs 17 days, P = .046). TAC-related variables are presented in Table 2.
| Variable | Low TAC trough level variability group (n = 36) | High TAC trough level variability group (n = 36) | P value |
|---|---|---|---|
| Mean tacrolimus level, ng/mL (SD) | 12.8 (2.4) | 12.7 (3.4) | .930 |
| No. of measurements (IQR) | 10.0 (7-14.5) | 13.0 (9-16.7) | .060 |
| Median CV between 3 and 12 m, % (IQR) | 19.9 (13.7-25.4) | 39.0 (3.5-49.3) | ≤.001 |
| No. of patients with ≥1 measurement <8 ng/mL (%) | 13/36 (36.1) | 28/36 (77.8) | ≤.001 |
| Percent of measurements <8 ng/mL (IQR) | 0 (0-7.6) | 16.0 (5.8-34.8) | ≤.001 |
| Percent of measurements >12 ng/mL (IQR) | 60.6 (15.7-80.0) | 50.0 (27.14-66.7) | .433 |
| Daily tacrolimus dose, mg (IQR) | 6.1 (4.7-8.7) | 5.7 (4.8-7.8) | .922 |
| Tacrolimus dose variability, % (IQR) | 10.5 (3.2-17.8) | 14.5 (4.70-29.6) | .310 |
| Daily MMF dose, g (SD) | 2.1 (0.7) | 1.7 (0.8) | .028 |
- Continuous variables expressed as mean (SD) or median (IQR) where indicated, and proportions as percentages.
- TAC, tacrolimus; CV, coefficient of variation; MMF, mycophenolate mofetil.
- The bold values are those with significant p value (less than/equal to 0.05)
3.3 Rejection analysis
Of the 72 patients analyzed, 2 patients died within the first postoperative year, and 9 were followed for <1 year, of whom 7 were followed for <3 months. Hence, 65 and 61 patients were included for rejection analysis between 3 and 12 months and beyond the first year post-HTx, respectively (Table 3).
| Outcome | Time from HTx | Low TAC trough level variability group | High TAC trough level variability group | P value |
|---|---|---|---|---|
| No. of biopsies (IQR) | 3-12 mo | 4 (4-5) | 3 (3-4) | .001 |
| ≥1 y | 2 (1-3) | 3 (2-4) | .168 | |
| Median time to biopsy, mo (IQR) | 3-12 mo | 5.4 (5.1-5.75) | 5.5 (4.8-6.17) | .857 |
| ≥1 y | 25.83 (16.25-36.25) | 30.60 (19.50-47.67) | .030 | |
| Patients with ≥80% of biopsies scored ISHLT 0R (%) | 3-12 mo | 17/35 (48.6) | 13/30 (43.3) | .804 |
| ≥1 y | 19/30 (63.3) | 11/31 (35.5) | .041 | |
| Patients with ≤50% of biopsies scored ISHLT 0R (%) | 3-12 mo | 11/35 (31.4) | 9/30 (30) | 1.0 |
| ≥1 y | 6/30 (20.0) | 14/31 (45.2) | .056 | |
| TRS (IQR) | 3-12 mo | 0.25 (0-0.5) | 0.25 (0-0.25) | .83 |
| ≥1 y | 0 (0-0.3) | 0.33 (0- 0.7) | .035 | |
| Antibody-mediated rejection (%) | 3-12 mo | 0/35 (0) | 0/30 (0) | — |
| ≥1 y | 0/30 (0) | 3/31 (9.7) | .238 | |
| Mortality, n (%) | 3-12 mo | 0/36 (0) | 2/36 (5.6) | .493 |
| ≥1 y | 1/36 (2.8) | 7/34 (20.6) | .026 |
- HTx, heart transplantation; TAC, tacrolimus; ISHLT, International Society of Heart and Lung Transplantation; TRS, total rejection score.
- The bold values are those with significant p value (less than/equal to 0.05)
3.4 Rejection analysis: 3-12 months post-HTx
The TRS did not differ between the high and low TAC variability groups (median score of 0.25 for both groups, P = .83), with similar rates of patients of whom the majority of biopsy specimens (≥80%) demonstrated ISHLT 0R (48.6% vs 43.4%, P = .80). Multivariate logistic regression did not demonstrate significant association between any rejection and all other variables (Table S1).
3.5 Rejection analysis from the end of the first postoperative year
Patients in the higher TAC trough level variability group had higher median TRS compared with the low TAC trough level variability group (0.33 vs 0, P = .035). Furthermore, compared with the high TAC trough level variability group, the low TAC group had significantly more patients, with the majority of biopsy specimens (≥80%) scoring ISHLT 0R (63.3% vs 35.5%, P = .041). Rejections ≥2R occurred in 2 patients, both from the high TAC trough level CV group. Importantly, TRS did not differ between patients with ≥1 measurement below the therapeutic range and those with all measurements above therapeutic level (median of 0.25 vs 0, P = .345). Further comparison between the latter 2 groups demonstrated similar proportions where all biopsy specimens scored ISHLT 0R (15/35 [42.9%] vs 14/26 [53.8%], P = .445).
We found a significant association between TRS and TAC trough level variability (R2 = 0.174, P = .001). Multivariate logistic analysis showed that any rejection (as previously defined) was significantly associated with high TAC trough level variability (odds ratio 8.52, 95% confidence interval 1.63-44.53, P = .011; Table 4). Recipient age, donor age, MMF daily dose, AMR, and mean time from HTx to biopsy did not demonstrate statistical significance and were excluded from the final multivariate model. An inverse association between hypertension during the follow-up period and risk for rejection was also demonstrated.
| Outcome | Risk factor | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|---|
| Any rejection ≥1 y post-HTx | ||||
| Recipient sex, female | 0.06 | 0.01-0.47 | .007 | |
| Hypertension post-HTx period (yes) | 0.07 | 0.01-0.4 | .003 | |
| High tacrolimus trough level CV group | 8.52 | 1.63-44.53 | .011 | |
| Mortality | ||||
| Age at HTx (y) | 1.04 | 0.98-1.09 | .192 | |
| Cardiovascular complicationa | 7.05 | 1.41-35.33 | .018 | |
| Tacrolimus-related toxicityb | 3.14 | 0.36-27.59 | .302 | |
| High tacrolimus trough level CV group | 1.04 | 0.988-1.10 | .129 | |
- HTx, heart transplantation; MMF, mycophenolate mofetil; CV, coefficient of variation.
- a Stroke, heart failure, acute myocardial infarction, CAV.
- b Infections requiring hospitalization, creatinine ≥50% of baseline, malignancy.
- The bold values are those with significant p value (less than/equal to 0.05)
3.6 Mortality analysis
Ten patients died during a median follow-up of 29.8 (15.5-76.2) months. Mortality was associated with a higher rate of cardiovascular complications during follow-up (5/62 [8.1%] vs 5/9 [55.6%], P = .002), of which the development of heart failure (P = .014) and stroke (P = .039) were the main contributors. Cox regression demonstrated that only cardiovascular complications were significant variables affecting mortality (P = .018) (Table 4).
4 DISCUSSION
In recent decades, there have been significant developments in immunosuppression therapies leading to improved outcomes after HTx. Reducing complications associated with immunosuppression without affecting efficacy is the goal of posttransplant management. Because TAC is the cornerstone of these regimens, improving its management is of utmost importance. This study is the first to address the impact of TAC trough level variability after HTx on long-term outcomes. Our main finding shows that high TAC trough level variability during the first postoperative year was associated with 8-fold increased risk for long-term rejections, highlighting the importance of detecting and neutralizing factors that may increase this variability.
TAC has become the calcineurin inhibitor of first choice after HTx. However, given its narrow therapeutic index, it is recommended to monitor through levels in an attempt to avoid toxicity and improve efficacy.4 Frequent monitoring of TAC trough levels together with its high intrapatient variability enabled us to examine the impact of trough level variation overtime on clinical outcomes such as rejection and mortality.
In our cohort, a follow-up period of 3-12 months post-HTx with a mean number of 13.4 TAC measurements yielded a median TAC trough level CV of 28.8%. This relatively high CV in our study compared with that of renal transplant patients may stem from a higher number of TAC measurements in our patients.6, 15 This is in line with a previous report of HTx patients that supports high CV, but data have yet to be published.16 Indeed, the high TAC trough level variability group in our cohort had more TAC measurements, but this did not reach statistical significance.
High TAC trough level variability has been shown in our study to be associated with a lower rate of biopsy specimens that were scored ISHLT 0R and a >8-fold increased risk for any rejection in the long term. These findings are in line with previous studies showing that high TAC trough level variability was associated with renal graft rejections.9-12 Univariate analysis showed that the high TAC CV group had a lower daily MMF dose. However, when adjusting for additional confounders, MMF daily dose lost its statistical significance.
Conversely, no association was demonstrated between TAC trough level variability between 3 and 12 months post-HTx and rejection rates during the corresponding period. This lack of association might be a result of a protopathic bias and, thus, should be interpreted with caution.
Our findings demonstrate that high TAC trough level variability is only partially attributed to trough concentrations below the therapeutic window. It is important to note that in addition to similar mean TAC trough level between the groups, neither TRS nor rates of ISHLT 0R scores differed between patients with measurements below therapeutic range and those without. This suggests that a higher rejection risk is not only due to low TAC through level per se. Of note, high TAC trough level variability was mainly attributed to TAC concentrations below therapeutic level with no differences in above therapeutic concentrations. This is probably reflected in the similar rates of outcome associated with TAC toxicity such as infections requiring hospitalizations, rise in serum creatinine, and rates of malignancies. Additional prospective and controlled studies are needed to further strengthen and confirm these findings.
Some studies suggest that once-daily sustained-release preparations of TAC might be associated with reduced intraindividual trough level variability. A prospective study, aiming to investigate the change in within-patient variability among stable kidney transplant recipients after conversion from twice-daily regimen to the same daily dose of once-daily TAC (Advagraf prolonged-release hard capsules; Astellas Pharma US, Inc, Northbrook, IL) showed significantly lower within-patient variability of TAC after conversion.23 Similarly, conversion of renal transplant patients also supported reduced intraindividual variability in a 24-h exposure for TAC daily compared with TAC twice daily (10.9% ± 14.1%; P = .012), especially among CYP3A5*1/3 genotype patients.24 Noteworthy is a recent head-to-head comparison between Advagraf and Envarsus XR (TAC extended-release tablets; Veloxis Pharmaceuticals A/S, Cary, NC), a different daily formula, which demonstrated significant differences in pharmacokinetic parameters that might also affect long-term outcomes.25 These may also contribute to improved adherence as suggested previously.26-28 Future studies should define the effect of once-daily preparations of tacrolimus on its trough level variations, adherence, and clinical implications among HTx patients.
Our findings suggest that reduced intraindividual TAC trough level variability may result in reduction of rejections rates after HTx. Several practical measures may assist in reducing TAC level variability: (1) strict therapeutic drug monitoring with dose adjustment (while noting that within-patient changes between laboratories are common), (2) avoiding changes in concurrent treatments and habits that may affect CYP3A4/5 activity and TAC pharmacokinetics, and (3) improving drug adherence, through empowering knowledge, supervision, and financial regulations.
Two strengths are noteworthy: the first is the relatively high number of TAC trough level measurements during the first year post-HTx, which enabled to demonstrate higher trough level CV than previously reported. Moreover, in the present study, rejections were analyzed in 2 different time periods. This approach enabled us to isolate the effect of TAC variability per se. During the first 3 months, post-HTx patients were treated with high-dose corticosteroids and were exposed to perioperative ATG. These actions could have masked the true effect of TAC variability on rejection rates and, accordingly, that initial post-HTx period was excluded from the analysis. Additionally, by separately analyzing rejections that occurred beyond the first year we avoided a protopathic bias in which the treating physician changes the TAC daily dose (thus varying trough levels) secondary to rejections and not vice versa. This potential bias might also explain the lack of difference between the high and low variability groups in TRS during this period (3-12 months post-HTx).
Several limitations of our study should be noted. Its retrospective nature prevented the recording of and adjusting for all possible confounders, including measurement bias of nontrough samples. Additionally, effects of acute exceptional changes such as temporary concomitant medication, intercurrent illness, or intervention could not be excluded. Compliance was only indirectly evaluated based on patient adherence with recommended follow-up protocols. However, pill count and other formal methods for adherence assessment were not available. Finally, the relatively small sample size, and hence the wide confidence intervals, limits our ability to make definitive conclusions; this is particularly true regarding rejection ≥2R, as in our cohort the latter included only 2 patients. Thus, any future conclusions to be drawn from the data warrant a larger sample size and a prospective multicenter design.
4.1 Conclusions and clinical implications
This is the first study to examine TAC trough level variability on HTx outcomes. The results of the present study are in line with previous observations that have been reported in non-HTx populations, demonstrating a significant association between higher tacrolimus trough level variability and higher rates of graft rejection.7, 9, 10, 12 Our findings suggest that high TAC trough level variability during the first year is associated with increased risk of rejections after HTx. The data show the necessity to identify those patients who demonstrate high TAC trough level variability, especially for patients with nontherapeutic concentrations, emphasizing the need for closer monitoring of these patients.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.




