Cost-effectiveness of using kidneys from hepatitis C nucleic acid test–positive donors for transplantation in hepatitis C–negative recipients
See also: Kiberd et al, Chascsa et al, and Sawinski
Abstract
Kidneys from deceased donors who are hepatitis C virus (HCV) nucleic acid test positive are infrequently used for transplantation in HCV-negative patients due to concerns about disease transmission. With the development of direct-acting antivirals (DAAs) for HCV, there is now potential to use these kidneys in HCV-negative candidates. However, the high cost of DAAs poses a challenge to adoption of this strategy. We created a Markov model to examine the cost-effectiveness of using deceased donors infected with HCV for kidney transplantation in uninfected waitlist candidates. In the primary analysis, this strategy was cost saving and improved health outcomes compared to remaining on the waitlist for an additional 2 or more years to receive a HCV-negative transplant. The strategy was also cost-effective with an incremental cost-effectiveness ratio of $56 018 per quality-adjusted life year (QALY) from the payer perspective, and $4647 per QALY from the societal perspective, compared to remaining on the waitlist for 1 additional year. The results were consistent in 1-way and probabilistic sensitivity analyses. We conclude that the use of kidneys from deceased donors with HCV infection is likely to lead to improved clinical outcomes at reduced cost for HCV-negative transplant candidates.
Abbreviations
-
- DAAs
-
- direct-acting antivirals
-
- HCV
-
- hepatitis C virus
-
- ICER
-
- incremental cost-effectiveness ratio
-
- NAT
-
- nucleic acid test
-
- QALY
-
- quality-adjusted life year
1 INTRODUCTION
Kidney transplantation is the treatment of choice for patients with end-stage kidney disease as it is associated with increased life expectancy and quality of life, and is less costly compared to treatment with dialysis.1, 2 As in the United States, the need for kidney transplantation in Canada exceeds the supply of transplantable kidneys. The median wait-time of deceased donor kidney transplant recipients in Canada from the time of dialysis initiation is currently 3.96 years (compared to 3.9 years in the United States), and novel strategies to increase the supply of kidneys available for transplantation are desperately needed.3, 4
Kidneys from deceased donors with active hepatitis C virus (HCV) infection are rarely utilized for transplantation because of the risk of transmitting infection to the recipient. The development of direct-acting antivirals (DAAs) against HCV raises the possibility of using kidneys from deceased donors who are HCV RNA nucleic acid test (NAT) positive for transplantation in HCV-negative wait-listed patients. A recent consensus conference sponsored by the American Society of Transplantation and an accompanying editorial endorsed this approach in the United States within the context of clinical studies to ensure appropriate patient consent, and necessary clinical expertise to manage the new DAA drug regimens, potential drug interactions with immunosuppressant and nonimmunosuppressant medications, viral relapse, viral resistance, and infection with multiple viral strains.5, 6 Although clinical trials are under way in the United States and preliminary findings are reassuring, there is consensus that larger clinical experience and patient follow-up are needed before the use of HCV NAT-positive donor kidneys in HCV-negative recipients is routinely adopted into clinical transplant practice.7, 8 A significant challenge is the need for rapid posttransplant treatment with DAAs. This necessitates a commitment from health insurers to provide coverage for DAAs prior to transplantation (typical market cost of DAA regimens is $45 000 to $100 000 Canadian dollars).9 DAAs are not approved nor routinely covered by insurance providers to support the use of HCV-positive donor organs for transplantation in HCV-negative recipients, and information about the cost effectiveness of using DAA for this indication may be useful in helping advance clinical trials to better understand this strategy.
In our review of the literature, only 1 study has examined the cost of using HCV NA-positive kidneys for transplantation.10 This study projected that there would be cost-savings if there was a >4-year reduction in the waiting time for transplantation by using a HCV NAT-positive kidney.10 This study did not consider the quality of life of patients, survival, or the potential need for a second course of DAA treatment. The current study is motivated by the need to inform researchers, payers, and decision makers regarding the use of HCV NAT-positive donor kidneys in HCV-negative wait-list candidates. This cost-effectiveness analysis examines the purposeful use of HCV NAT-positive donor kidneys for transplantation in previously uninfected waitlist candidates followed by treatment with DAAs compared to the alternative of remaining on the kidney transplant waitlist from a HCV NAT-negative kidney transplant.
2 MATERIALS AND METHODS
2.1 Study design
In this cost-effectiveness analysis, we used Markov models to simulate a fixed cohort of nondiabetic, dialysis-treated patients wait-listed for kidney transplantation in the age range of 18 to 65 years. The primary model compares the strategy of transplanting a HCV-NAT positive deceased donor kidney and shortening the need for further dialysis treatment for a period of 1, 2, 3, 4, or 5 years, compared to a strategy of remaining on the waitlist for a kidney transplant from an HCV NAT-negative donor. The primary study outcome is the incremental cost-effectiveness ratio (ICER) in dollars per quality-adjusted life year (QALY). The model was constructed from the health-care payer perspective. A second model from the societal perspective including out-of-pocket expenses associated with dialysis, as well as the potential improvement in employment with a functioning kidney transplant was also developed. The secondary study outcome was the budgetary impact of the strategy from the health-care payer. Discounting of 1.5% was applied to both cost and utility as per current guidelines.11 A 10-year time horizon was set with a 1-year cycle length. The Markov model was created using TreeAge Pro© 2017 (TreeAge Software , Williamstown, MA).12
2.2 Health states and outcomes
Possible patient health states in the models include dialysis dependent, alive with a functioning kidney transplant, and deceased. The potential transitions between these health states are depicted in Figure 1. For patients who remained on the waitlist for a HCV NAT-negative kidney transplant, patients remained in the dialysis-dependent state for their projected waitlist duration. After their predicted waitlist time was completed, all patients on dialysis then transitioned to the health state of alive with a functioning transplant. Patients who received a HCV NAT-positive kidney transplant started in the model with a functioning transplant. If transplant failure occurred in either patient cohort, the patient would transition from transplantation to the dialysis-dependent state. Patients could transition to death from any other health state. It was assumed that a patient would not receive a repeat transplant after transplant failure within the 10-year model time horizon.
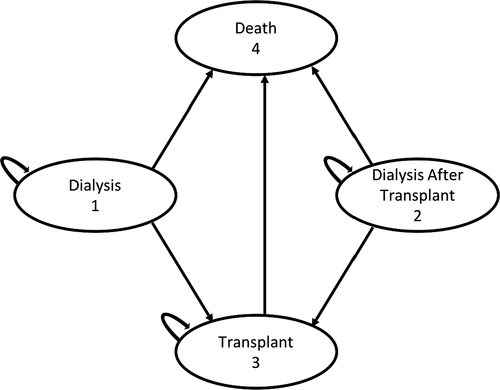
In the primary models, all patients receiving a kidney from a HCV NAT-positive donor would receive DAA therapy. Based on the treatment regimens used in prior studies, a 12-week course of elbasvir/grazoprevir was used as the first course of therapy in the model.7, 8 It was assumed that up to 4% of patients would require further therapy with an alternate DAA treatment regimen based on published sustained viral response rates.13-15 Given that the second DAA treatment regimen may vary depending on viral genotype and prescriber preference, the cost of a second course of therapy was set as equivalent to the cost of the first DAA drug regimen.
2.3 Cost, utility, and probability estimates
The model was informed by previous estimates of cost and utility for dialysis and transplant (Table 1). The cost and utility estimates were derived from the published literature with costs adjusted for inflation to 2017 Canadian dollars. Health-care payers have negotiated a lower cost for DAAs; however, the negotiated cost of these drugs is frequently confidential; therefore, market value was used in this analysis and a lower negotiated price was explored in a sensitivity analysis.9 The cost of specialist consultation and follow-up included a pretransplant assessment of cirrhosis with transient elastography (required to rule out pre-existing cirrhosis), HCV viral monitoring posttransplantation, and HCV drug susceptibility testing, derived from locally obtained fee lists and previously published estimates.16-18 The cost of a year of dialysis therapy was derived from a Canadian study of the cost of peritoneal, in-center hemodialysis, and home hemodialysis.19 A final cost estimate for a year of dialysis therapy assumed a ratio of in-center hemodialysis to home hemodialysis to peritoneal dialysis of 0.75:0.05:0.20 as per the current prevalence of each therapy for treatment of kidney failure in Canada.3 The cost of the first, second, and third year of care posttransplant was obtained from a previously published Canadian study.20 The estimate of utility of a year of dialysis therapy and a year of transplant therapy was acquired from a systematic review that provides QALY estimates adjusted for the utility elicitation method for both dialysis and transplantation. Transition probabilities were derived from reported survival of waitlisted dialysis and transplant patients in Canada.3, 21 Patient out-of-pocket expenses were extracted from an as yet unpublished report of the economic burden of end-stage kidney disease in Alberta (S. Klarenbach, unpublished data, 2017). Estimates of the potential improvement in employment following transplantation and subsequent income generation were derived from the literature.22-24
| Estimate | Value assigned | Plausible range | References |
|---|---|---|---|
| Cost (Canadian dollars) | |||
| 1 y of dialysis therapy | 88 811 | 81 929-95 692 | 19, 30 |
| First y of care with transplant | 109 427 | 107 078-111 776 | 20 |
| Second y of care with transplant | 26 595 | 23 950-29 250 | 20 |
| Third y of care with transplant | 24 297 | 21 850-26 700 | 20 |
| Direct-acting antivirals for treatment of hepatitis C (elbasvir/grazoprevir as prototype) | 60 300 | 48 240-60 300 | 31 |
| Cost of specialist consultation, additional investigations and follow-up for hepatitis C | 1075 | 975-1175 | 16, 17, 32 |
| Monthly out-of-pocket expenses for patients on hemodialysis | $158.80 | N/A | 33 a |
| Monthly out-of-pocket expenses for patients on peritoneal dialysis | $118.10 | N/A | 33 a |
| Canadian national mean annual salary | $32 099 | N/A | 34 |
| Utility (QALYs) | |||
| 1 y of transplantation | 0.82 | 0.78-0.86 | 1, 35 |
| 1 y of dialysis therapy | 0.71 | 0.67-0.74 | 1, 35 |
| Probabilities | |||
| Annual probability of death on the waitlist | 0.05 | 0.03-0.07 | 3, 36 |
| Probability of death after dialysis re-initiation | Varies by duration of transplant survival (see Supplemental Table S1-S3) | 3, 21 | |
| Probability of graft failure following transplant | |||
| Probability of death with function following transplant | |||
| Probability of requiring second course of treatment | 0.04 | 0-0.1 | 13-15, 36 |
| Absolute increase in probability of employment after transplant | 0.20 | 0.18-0.22 | 22-24 |
| Probability of HCV transmission from donor to recipient | 1.0 | 0.75-1.0 | 25 |
| Discounting | |||
| Discounting for cost | 0.015 | 0.00-0.05 | 11 |
| Discounting for utility | 0.015 | 0.00-0.05 | 11 |
- HCV, hepatitis C virus; N/A, not applicable; QALYs, quality-adjusted life years.
- a S. Klarenbach, unpublished data, 2017.
2.4 Model assumptions and limitations
The study model assumes that the time to transplantation for waitlist candidates who do not accept a HCV NAT-positive kidney is fixed (ranging from 1 to 5 years) and that the patient would not receive a living donor transplant. In this model, a patient receiving a HCV NAT-positive kidney transplant will not affect the wait-time of other individuals on the waitlist. The risk of HCV infection after receiving an organ from an HCV NAT-positive donor is 100%. The model assumes that the cure of HCV (as defined by achievement of a sustained viral response after 12 weeks of DAA therapy) would be 96% and that 4% of patients would require a second course of treatment.7, 8, 13-15 The model assumes that treatment with DAAs would not substantially impact patient quality of life and that after the HCV infection in a recipient was cured, the HCV infection would have no future impact on transplant or patient survival. The probability of death after return to dialysis in the models was equal to the probability of death in non–wait-listed dialysis patients.
2.5 Sensitivity analysis
To ensure robustness and external validity of the model outputs to health-care settings beyond Canada including the United States and other countries, we performed a variety of sensitivity analyses. Uncertainty of the model parameters was determined using the plausible ranges in Table 1. The plausible range for costs of 1 year of dialysis and transplant treatment was calculated, assuming a 10% range in the cost estimate. The plausible range for cost of DAAs was produced by assuming a potential 20% discount obtained by health-care payers. Additionally, there is evidence to suggest that the risk of HCV transmission from donor to recipient may not be 100% in donors with low levels of viremia.25 We therefore performed a sensitivity analysis exploring the possibility that some recipients may not be infected with HCV and therefore may not require DAA therapy. A 10% range was used for the transition probabilities where the probabilities varied with survival time. A 1-way sensitivity analysis was performed for all the parameters in Table 1.
The long-term survival of previously uninfected patients who received a kidney transplant from a HCV NAT-positive donor is unknown. Several publications with short term but ongoing follow-up have documented that DAAs are safe in kidney transplant recipients and do not increase the risk of transplant failure.13, 14 However, it is possible that DAAs may increase the risk of rejection because of drug interactions with immunosuppressant medications. Therefore, we performed a sensitivity analysis exploring the impact of 10% increase in overall relative risk of death-censored transplant failure.
We also conducted a probabilistic sensitivity analysis using probability distributions to replace the estimates for the parameters included in the model (ie, cost, utility, and survival probabilities). In this way, uncertainty in the model is represented by each of these distributions. We then analyzed the model repeatedly, with various combinations of parameter estimates that are drawn randomly from the probability distributions. In this case, we repeated the analysis 10 000 times. The results of these analyses are presented in cost-effectiveness acceptability curves with thresholds of willingness-to-pay varying from $0 to $100 000 per QALY to include the range of internationally accepted willingness-to-pay thresholds.
2.6 Budget impact assessment
Cumulative cost as a function of time was calculated using the cost estimates produced from the Markov models. The cost and cost-savings were calculated by subtracting the projected cumulative cost of using a HCV NAT-positive donor kidney from the projected cumulative cost of remaining on the waitlist. This was then multiplied by a theoretical number of HCV NAT-positive kidneys that may become available in a single center in 1 year to determine the budget impact over a 5- and 10-year period of time from the health-care payer perspective.
3 RESULTS
Kidney transplantation using a HCV NAT-positive kidney increased the projected QALYs compared to remaining on the waitlist for a HCV NAT-negative kidney, and this finding was consistent in scenarios where the use of a HCV NAT-positive kidney decreased the waiting-time for transplantation by 1, 2, 3, 4, or 5 years (Figure 2). Using a kidney from a HCV NAT-positive kidney donor dominated (ie, resulted in increased QALYs at lower cost) remaining on the waitlist for 2, 3, 4, and 5 additional years prior to transplantation. In addition, with a willingness-to-pay threshold of $50 000/QALY, using a HCV NAT-positive kidney was cost-effective compared to remaining on the waitlist for 1 additional year for a HCV NAT-negative kidney with an ICER of $56 018/QALY and an incremental cost of $9188.26, 27
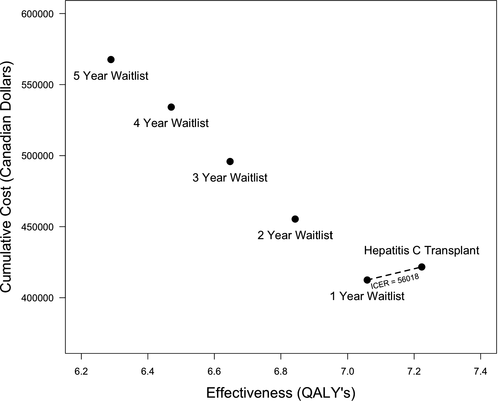
When modeled from the societal perspective, using a HCV NAT-positive kidney donor dominated remaining on the waitlist for 2 or more years. The ICER for using a HCV NAT-positive kidney donor compared to remaining on the waitlist for 1 year was $4647/QALY with an incremental cost of $762.
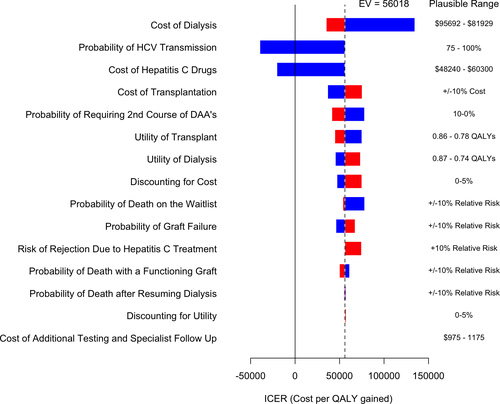
The Markov model results were robust in all 1-way sensitivity analyses that compared receipt of HCV NAT-positive kidney to remaining on the waitlist for an additional 2, 3, 4, and 5 years and demonstrated cost-savings in all scenarios. When comparing receipt of a HCV NAT-positive kidney to remaining on the waitlist for 1 additional year, the sensitivity analysis was robust, with the exception of the estimates of cost of DAAs and the annual cost of dialysis therapy (Figure 3). When the cost of DAAs decreased to 80% of the market price, using a HCV NAT-positive kidney was cost-saving from the health-care payer perspective. The strategy of using a HCV NAT-positive kidney in a HCV-negative recipient was cost neutral when the probability of disease transmission was 85%, and cost-saving when the probability for disease transmission was 75% (Figure 3). Conversely, as the annual cost of dialysis therapy decreased, the incremental cost of using a HCV NAT-positive kidney compared to remaining on the wait-list for 1 further year was increased. We specifically evaluated a scenario where the overall relative risk of death-censored transplant failure was increased by 10% with the use of DAAs. This scenario still dominated remaining on the waitlist for 2 or more years and when compared to remaining on the waitlist for 1 additional year, the ICER was still within a plausible willingness-to-pay threshold of $74 653/QALY.
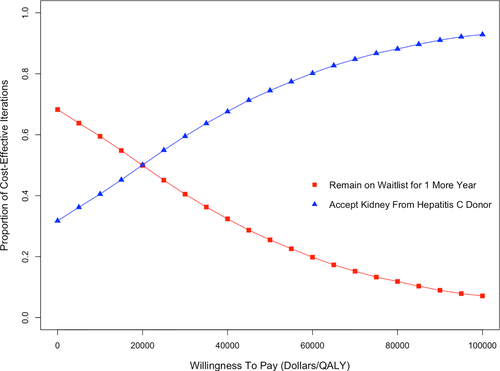
We further explored the model comparing receipt of a HCV NAT-positive kidney to remaining on the waitlist for 1 year with a probabilistic analysis. Using a HCV NAT-positive kidney was cost-effective using a willingness-to-pay threshold of $50 000 in 58% of the iterations. If the willingness-to-pay threshold was increased to $100 000/QALY, using a HCV NAT-positive kidney was cost-effective in 93% of the iterations (Figure 4).
The cumulative health-payer cost at 5 years after using a HCV NAT-positive kidney for transplantation was $310 099 compared with a cumulative cost of $343 637, $387 322, $429 287, and $469 306 for remaining on the waitlist for 2, 3, 4, and 5 years, respectively. The cumulative cost after 10 years of using a HCV NAT-positive kidney was $421 656, compared with a cumulative cost of $455 365, $495 872, $534 161, and $567 627 for remaining on the waitlist for 2, 3, 4, and 5 years, respectively. Assuming 20 HCV NAT-positive kidneys were used in patients to shorten their wait-time by 2 to 5 years, this would potentially save the health-care payer between $674 000 and $2.9 million over 10 years despite the increased upfront cost of providing DAAs.
4 DISCUSSION
The use of HCV NAT-positive kidneys followed by DAA treatment in HCV-negative recipients is a cost-saving strategy if it shortens the wait-time for transplant candidates by 2 or more years. This strategy is cost-effective with an ICER of $56 018/QALY if it shortens the wait-time by 1 year. The projected cost-savings to the health-care payer could approach $2.9 million over 10 years, assuming 20 such transplants were performed even considering the cost of DAAs at market price. Additionally, with a 20% reduction in cost of DAAs, the cost savings are even greater such that it would be cost-saving to use HCV NAT-positive kidneys even if this strategy only reduced the patient waiting time for transplantation by 1 year. The cost-effectiveness of this strategy is more pronounced if a societal perspective is considered.
Although this model was informed by Canadian cost estimates, the Medicare-based cost estimates for dialysis, transplantation, and treatment with DAAs in the United States are well within the ranges of our sensitivity analyses, making the results of this model generalizable to the United States.4, 28, 29 Of note, the estimate for the annual cost of health care for individual dialysis patients in the United States ranges from $88 750 to $250 000.4, 5 If the higher end of this range is the true estimate, the cost-savings of using kidneys from HCV NAT-positive donors is accentuated. Additionally, dialysis costs are only 1 component of caring for waitlisted patients. For example, our estimates do not include the costs of monitoring and maintaining the waitlist fitness of transplant candidates. A prospective economic evaluation would be required to truly evaluate the cost-effectiveness of using kidneys from HCV NAT-positive donors for transplantation in HCV NAT-negative recipients.
This model likely underestimates the potential economic benefit of using kidneys from HCV NAT-positive kidneys for several reasons. First, the model does not account for the impact of removing patients from the waitlist by utilizing HCV NAT-positive kidneys, which would reduce the wait-time for other wait-list candidates. The sensitivity analysis assumed the negotiated government price for DAAs may be as low as 20% below the market price: It is quite possible the actual negotiated price of DAAs is substantially lower than the 80% market price.9
Our analysis found that using kidneys from HCV NAT-positive donors in HCV NAT-negative recipients is likely to result in substantially more cost-savings than the previously published estimates.10 To our knowledge, this is the first detailed cost-effectiveness analysis of this strategy. Although the results are promising, there are several assumptions informing this analysis and significant clinical questions that remain to be answered, namely, the long-term outcomes and patient acceptance of using HCV NAT-positive kidneys in HCV NAT-negative recipients.
In summary, we found that the use of HCV NAT-positive kidneys for transplantation in HCV-negative recipients followed by treatment with DAAs is likely to lead to improved patient outcomes, with significant cost-savings from both a health-care payer and societal perspective. The findings indicate that it is cost-saving to consider the use of HCV NAT-positive kidneys for wait-list candidates in whom the waiting time for transplantation would be shortened by 2 years in the context of a clinical study. Prospective adequately powered trials evaluating the use of HCV NAT-positive kidneys are required before this strategy can be considered in routine practice. The findings may encourage insurance providers to make the upfront investment in DAAs required to advance knowledge about this strategy that has significant potential to increase access to lifesaving kidney transplantation.
ACKNOWLEDGMENTS
M. Kadatz is funded though the Clinician Investigator Program at the University of British Columbia, Vancouver, Canada. S. Klarenbach is funded by the Kidney Health Research Chair and the Division of Nephrology at the University of Alberta, Edmonton, Canada. J. Gill is funded by a Scholar Award from the Michael Smith Foundation for Health Research. J. S. Gill is funded by a Foundation Award from the Canadian Institutes of Health Research, and the costs related to study are funded by this award.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.




