Hypertension after kidney donation: Incidence, predictors, and correlates
Abstract
Incidence of postdonation hypertension, risk factors associated with its development, and impact of type of treatment received on renal outcomes were determined in 3700 kidney donors. Using Cox proportional hazard model, adjusted hazard ratios (HRs) for cardiovascular disease (CVD); estimated glomerular filtration rate (eGFR) <60, <45, <30 mL/min/1.73m2; end stage renal disease (ESRD); and death in hypertensive donors were determined. After a mean (standard deviation [SD]) of 16.6 (11.9) years of follow-up, 1126 (26.8%) donors developed hypertension and 894 with known antihypertensive medications. Hypertension developed in 4%, 10%, and 51% at 5, 10, and 40 years, respectively, and was associated with proteinuria, eGFR < 30, 45, and 60 mL/min/1.73m2, CVD, and death. Blood pressure was <140/90 mm Hg at last follow-up in 75% of hypertensive donors. Use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (compared to other antihypertensive agents) was associated with a lower risk for eGFR <45 mL/min/1.73m², HR 0.64 (95% confidence interval [CI] 0.45-0.9), P = .01, and also less ESRD; HR 0.03 (95% CI 0.001-0.20), P = .004. In this predominantly Caucasian cohort, hypertension is common after donation, well controlled in most donors, and factors associated with its development are similar to those in the general population.
Abbreviations
-
- ACEI
-
- angiotensin-converting enzyme inhibitor
-
- ARB
-
- angiotensin-receptor blocker
-
- ARIC
-
- Atherosclerosis Risk in Communities
-
- BUN
-
- blood urea nitrogen
-
- CKD
-
- chronic kidney disease
-
- CVD
-
- cardiovascular disease
-
- DBP
-
- diastolic blood pressure
-
- eGFR
-
- estimated glomerular filtration rate
-
- ESRD
-
- end-stage renal disease
-
- HR
-
- hazard ratio
-
- KDIGO
-
- Kidney Disease Improving Global Kidney Outcomes
-
- NHANES
-
- National Health and Nutrition Examination Survey
-
- RAAS
-
- renin-angiotensin-aldosterone system
-
- SBP
-
- systolic blood pressure
1 INTRODUCTION
Reduction in renal mass and function are associated with a progressive increase in blood pressure and the development of systemic hypertension in animal models and humans with low nephron number.1, 2
Studies addressing changes in blood pressure and the development of new-onset hypertension following kidney donation have been generally small and with short follow-up. One meta-analysis reported that systolic blood pressure (SBP) increased by 1.1 mm Hg per decade, whereas diastolic blood pressure (DBP) did not change and there was no difference in the prevalence of hypertension between donors and controls.3 This study included donors (60%) and nondonors who underwent uninephrectomy for disease or had renal agenesis.3 In a meta-analysis that specifically addressed hypertension in kidney donors, Boudville et al reported that mean SBP and DBP were 6 and 4 mm Hg higher, respectively, in kidney donors than in controls.4 The risk of incident hypertension, however, could not be accurately determined due to the inability to pool results from the 6 studies comparing donor to controls due to statistical heterogeneity.4 Understanding hypertension after donation is important, as it appears to be a leading cause of end-stage renal disease (ESRD), particularly, late after donation.5, 6 Attributing ESRD to hypertension is problematic, as almost none of these cases are biopsy proven. This is very important as the proportion of ESRD attributed to hypertension is overestimated, as evidenced from case series where patients whose ESRD is “caused” by hypertension do not exhibit a histological pattern of benign nephrosclerosis.7 Moreover, the evidence linking hypertension to chronic kidney disease (CKD) is far from convincing, as hypertension may actually be a result of underlying kidney disease rather than it causing.8 Very few data exist on how well hypertension is treated in donors and with what agents. This is highly significant, as most clinicians believe that agents that interrupt the renin-angiotensin-aldosterone system (RAAS) would be beneficial due to their excellent antihypertensive properties, with the added benefit of ameliorating hyperfiltration, which is related to the reduction in renal mass from uninephrectomy. This hyperfiltration is not driven by a rise in intraglomerular pressure.9 The Kidney Disease Improving Global Kidney Outcomes (KDIGO) Clinical Practice Guideline on the Evaluation and Follow-up Care of Living Kidney Donors states: “There is a need for well-designed studies to quantify the impact of live kidney donation on hypertension risk, as well as the impact of hypertension before and after donation on clinical outcomes including lifetime ESRD incidence.”10
The aims of this analysis are, therefore, to determine the incidence and risk factors for hypertension after donation, describe how hypertension is treated, and to assess its association with the development of reduced estimated glomerular filtration rate (eGFR), proteinuria, ESRD, cardiovascular disease (CVD), and death.
2 MATERIALS AND METHODS
2.1 Study population
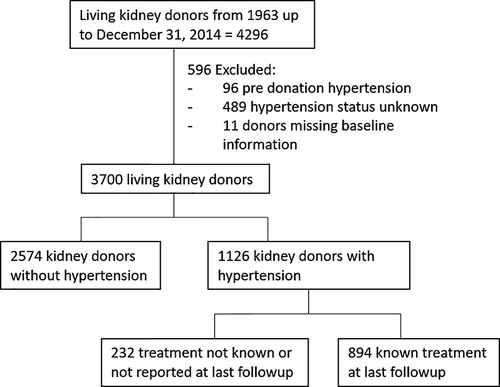
This is a longitudinal follow-up study of kidney donors who have donated between 1963 and December 31, 2014 (n = 4286), at the University of Minnesota. Of these, 96 were excluded only from the incidence analysis because of pre-donation hypertension, 489 were also excluded because there were no records of their hypertension status (ie, surveys not returned or missing answers on the surveys they returned), and 11 donors had missing information at time of donation (Figure 1). The remaining 3700 kidney donors in whom hypertension status was known, 1126 developed postdonation hypertension, for 894 of them antihypertensive treatment was known. The type and date of initiation of antihypertensive medication was reported (Figure 1). We also studied the outcomes of the 96 donors who were hypertensive at the time of donation. Donors provided written informed consent and all procedures were performed in accordance with the Declaration of Helsinki and approved by the University of Minnesota Institutional Review Board (HSC #0301M39762).
2.2 Data-gathering methods
Laboratory and demographic variables are entered into our database at the time of donation. Starting in 2003, donors are contacted at 6, 12, and 24 months, and then every 3 years indefinitely as described previously.11 Most donors, 87.5%, returned at least one survey. At each contact, donors are asked about hypertension requiring treatment. Donors are also asked to provide recent laboratory test results and copies of records (or, if not done, to have these tests); alternatively, with donors’ permission, we contact their local clinics for recent medical history, physical examination notes, and laboratory test results, including serum creatinine, glucose, urinalysis, and urinary protein measurements. Blood pressure measurements are obtained at each patient clinic site following routine care procedures. Blood pressure measurements were reported at the time of evaluation and date of last follow-up. In addition, blood pressure measurements were obtained from clinical records and from patient surveys at varied time points, and these data were used to assess the progression of blood pressure from time of donation to last follow-up.
2.3 Exposures and outcomes
Hypertension was defined by receipt of antihypertensive medications. Donors who had a diagnosis of hypertension were asked to provide the date of initial diagnosis and provided the name and start date for each antihypertensive agent. Antihypertensive agents were grouped as follows: angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blocker (ARB) vs other classes. Proteinuria was defined as a urinary albumin excretion >30 mg/g creatinine, 24-hour urinary protein >200 mg/day or ≥2+ on urine dipstick. End-stage renal disease (or ESRD) was defined by needing dialysis, undergoing kidney transplantation, or being placed on the deceased donor waitlist for a transplant. To calculate serial eGFR, we used the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.12 Hyperlipidemia was defined in the survey as high cholesterol treated by diet or medication.
2.4 Statistical analyses
Continuous data with normal distributions are presented as mean (standard deviation, SD) and categorical variables using frequencies and percentages. Differences between groups were assessed using student t-test and chi-square for continuous and categorical variables, respectively. The progression of blood pressure by time was determined using mixed models analysis for repeated measures with unequally spaced time points with an unstructured covariance structure. The estimated mean blood pressure values were plotted as a function of time since donation. A main effect by hypertension status and by time since donation was determined. A hypertension status × time since donation interaction term was considered to determine if progression of blood pressure was different between groups. Cox proportional hazard model multivariate stepwise procedure was used to determine covariates associated with the development of hypertension. Variables entered in the model included the following: sex, age, race, relationship to recipient, family history of hypertension, and the following variables at time of donation (BMI, serum glucose, eGFR, SBP and DBP, hyperlipidemia, and smoking status). A significance level of 0.15 and 0.20 was required to allow a variable for entry and stay in the model, respectively. Time of censoring was the date of last follow-up, and death was modeled as a competing risk factor for incident hypertension and for all clinical outcomes. Cox proportional hazard models were used to estimate hazard ratios (HRs) for incident hypertension by quintiles of age at time of donation adjusted for same variables as described earlier. Kaplan-Meir cumulative incident curves were developed by quintiles of age at time of donation and categories of risk factors. Difference among quintiles of age and categories of risk factors were assessed using the Log-rank test. Risk factors for incident hypertension chosen were the following: male sex, age at time of donation >49.6 years, family history of hypertension, BMI >25 kg/m2, SBP and/or DBP ≥130/85 mm Hg, and hyperlipidemia. Based on the number of risks factors, donors were placed in 4 different categories: no risk factors, and 1, 2, or more than 3 risk factors. Cox proportional hazard models were used to assess unadjusted HRs for incident hypertension by categories of risk factors. We chose not to adjust in this case because the risk factor categories included the potential confounders. Cox proportional hazard models were also used to estimate adjusted HRs for death, diabetes, cardiovascular disease, ESRD, and development of eGFR <60, <45, and <30 mL/min/1.73 m² in those with and without hypertension. HRs were adjusted for the same variables as described earlier and for the development of postdonation CVD, diabetes, and proteinuria as time-varying covariates. Cox proportional hazard models were also used to estimate, in those with postdonation hypertension, HRs for all clinical outcomes with death as a competing risk factor between those on ACEI or ARB (ACE/ARB) vs those on other agents. HRs were adjusted for sex, race, current age, relationship to recipient time to diagnosis of hypertension and covariates present at time of last follow-up: BMI, fasting glucose, SBP, DBP, hyperlipidemia, presence of CVD, diabetes, smoking history, and eGFR. Because diagnosis of hypertension and time of initiation of antihypertensive treatment occurred at varying times during follow-up, hypertension and antihypertensive treatment were modeled as time-varying covariates. Hypertension, diabetes, and proteinuria were also modeled as time-varying covariates. A Cox proportional hazard model was also used to estimate HRs for clinical outcomes in those with hypertension at time of donation and those without baseline hypertension after adjustment for the same variables as described for the multivariate stepwise procedure using death as a competing risk factor. Statistical significance was set at a P-value of 0.05. SAS version 9.3, SAS Institute Inc., Cary, NC, was used for all statistical analysis.
3 RESULTS
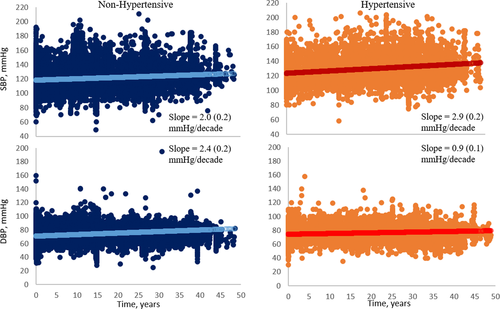
Of the 4296 individuals who donated a kidney between 1963 and 2014, 96 were excluded from the incidence analysis for having predonation hypertension and 489 donors with unknown postdonation hypertension status (Figure 1). Donors with unknown hypertensive status were more likely to be women (54.8 vs 31.5%), more likely to be smokers (45.7 vs 29.2%), and had a lower eGFR at donation (99.7 vs 103.4 mL/min/1.73 m²), but were otherwise comparable to those with known hypertension status (data not shown). Of the remaining 3700, 1126 (30%) donors reported hypertension and 894/1126 (79.4%) reported receiving treatment and provided the name of antihypertensive agent(s) they were receiving (Figure 1). Donors who developed hypertension were on average 2 years older, were more likely to have donated to a first-degree relative, have smoked, and had a higher BMI, higher SBP, higher DBP, higher fasting glucose, and higher total cholesterol (Table 1). eGFR at donation was lower in those who later developed hypertension: mean (SD), 99.4 (33.8) vs 105.1 (33.2), P < .001. In those with postdonation hypertension, SBP rose by 2.9 (0.2) mm Hg/decade, progressing at a greater rate than in those without postdonation hypertension 2.0 (0.2) mm Hg/decade, P < .0001 (Figure 2). DBP rose by 0.9 (0.1) mm Hg/decade in those who developed postdonation hypertension compared to 2.4 (0.2) mm Hg/decade, P < .0001, in those without postdonation hypertension.
| Postdonation hypertension | |||
|---|---|---|---|
| No2574 (69.6) | Yes1126 (30.4) | P-value | |
| Male, % | 41.0 | 42.7 | .3 |
| Age, y | 38.3 (11.4) | 40.4 (11.9) | <.001 |
| White | 95.1 | 94.4 | .4 |
| First-degree relative, % | 67.8 | 85.1 | <.001 |
| Smoker, % | 26.5 | 36.0 | <.001 |
| Family history of HTN, % | 32.0 | 33.0 | .6 |
| BMI, kg/m2 | 25.6 (4.3) | 26.3 (4.5) | <.001 |
| eGFR, mL/min/1.73 m2 | 105.1 (33.2) | 99.4 (33.8) | <.001 |
| SBP, mm Hg | 118.8 (12.7) | 121.4 (13.2) | <.001 |
| DBP, mm Hg | 72.1 (9.7) | 75.5 (9.8) | <.001 |
| Creatinine, mg/dL | 0.89 (0.16) | 0.92 (0.17) | <.001 |
| Glucose, mg/dL | 92.4 (12.8) | 95.7 (15.9) | <.001 |
| Total cholesterol, mg/dL | 190.7 (37.7) | 197 (41.5) | .002 |
- HTN, hypertension; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; DBP, diastolic blood pressure.
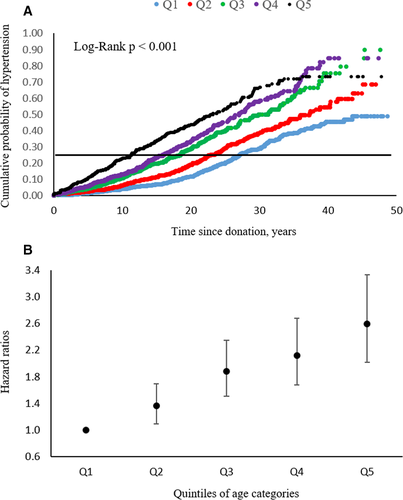
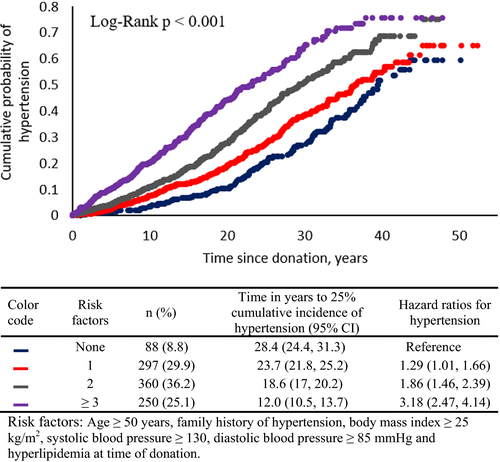
The median (interquartile range [IQR]) time to diagnosis of hypertension was 15.3 (range 7.9-23.7) years after donation and mean (SD) age at diagnosis was 56.7 (12.6) years. Figure 3A shows cumulative probability of hypertension by quintiles of age at time of donation. The number of years from donation to reach a 25% cumulative probability of hypertension for the group of individuals who at the time of donation were in the lowest quintile of age was 29.8 years compared to 13.2 years for those who were in the highest quintile of age at time of donation (log-rank test P < .001). Figure 3B shows the adjusted HRs for incident hypertension in kidney donors by quintiles of age at donation. Risk of hypertension development was 2.6-fold greater in those who donated in the highest quintile of age compared to those who donated in the lowest quintile of age. Cumulative probability of hypertension was also higher in donors who had a greater number of any of the following risk factors at time of donation: age ≥49.6 years, family history of hypertension, BMI ≥25 kg/m2, SBP ≥130 mm Hg, DBP ≥85 mm Hg, and hyperlipidemia. For those with no risk factors, the mean time to hypertension was 31.0 years (95% CI 28.1-34.1) years compared to 13.2 (95% CI 11.4-15.0) years for those with 3 or more risk factors (log-rank, P < .001). Accordingly, the HR for developing hypertension progressively increased with more risk factors, and in those with 3 or more risk factors the HR was 3-fold higher than for those with no risk factors at time of donation (Figure 4).
3.1 Predictors of hypertension development
Older age, family history of hypertension, higher BMI, higher fasting serum glucose, higher SBP, higher DBP, hyperlipidemia, and being a smoker were associated with a higher risk of incident hypertension (Table 2). The strongest covariates associated with this risk were family history of hypertension, HR 1.25 (95% CI 1.08-1.46) and hyperlipidemia 3.1 (95% CI 2.65-3.63). Being white was associated with a 30% lower risk of developing hypertension (P = .03). Donating to a first-degree family member was, however, not associated with incident hypertension (Table 2).
| At donation | HRs (95% CI) | P-value |
|---|---|---|
| Age, y | 1.03 (1.03, 1.04) | <.001 |
| White | 0.7 (0.51, 0.97) | .03 |
| Family history of HTN | 1.25 (1.08, 1.46) | .004 |
| BMI, kg/m2 | 1.05 (1.04, 1.07) | <.001 |
| Glucose, mg/dL | 1.00 (1.00, 1.01) | .03 |
| SBP, mm Hg | 1.02 (1.01, 1.02) | <.001 |
| DBP, mm Hg | 1.01 (1.00, 1.02) | .005 |
| Hyperlipidemia | 3.1 (2.65, 3.63) | <.001 |
| Smoker | 1.12 (0.97, 1.31) | .1 |
| eGFR, mL/min/1.73 m2 | 1.00 (1.00, 1.01) | .07 |
- N = 3445.
- For continuous variables HR is per unit value. Variables entered in the model included: sex, age, race, relationship to recipient, family history of HTN, and the following variables at time of donation (BMI, serum glucose, eGFR, systolic and diastolic blood pressure, hyperlipidemia, and smoking status). A total of 3700 individuals were entered into the mode, but due to missing values only 3445 were used in the multiple regression procedure.
- HRs, hazard ratios; HTN, hypertension; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; DBP, diastolic blood pressure.
3.2 Antihypertensive use and adequacy of blood pressure control
Most (61.2%) hypertensive donors are treated with 1 antihypertensive agent, 25.3% are treated with 2, and 13% required ≥3 agents (data not shown). The most commonly prescribed agents were ACE/ARB alone or combined with other agents (38%). In 19.1%, ACEIs were used as the only treatment, and 6% were treated with an ARB alone. The combination of a diuretic or a beta-blocker with ACE/ARB represented 10.8%, and ACE/ARB with a calcium channel blocker or a vasodilator was used in 2.3% of hypertensive donors. At last follow-up, donors on ACE/ARB were highly comparable to donors treated with other agents, except for having a lower pulse pressure and being 4 years older (Table 3).
| Categories of antihypertensive agents | P-value | ||
|---|---|---|---|
| ACEI or ARB | Other antihypertensive agents | ||
| n (%) | 340 (38) | 554 (62) | |
| Male, % | 45.0 | 40.4 | .2 |
| Age at HTN diagnosis | 56 (11.8) | 57.2 (12.6) | .2 |
| Current age | 71.2 (12.6) | 67.6 (11.9) | <.001 |
| Time to HTN, y | 16.2 (10.2) | 16.1 (10.3) | .9 |
| eGFR, mL/min/1.73 m2 | 61.3 (21.1) | 58 (20.5) | .5 |
| SBP, mm Hg | 128.4 (14.7) | 129.8 (16.6) | .2 |
| DBP, mm Hg | 76.7 (9) | 75.3 (11.3) | .06 |
| Pulse pressure, mm Hg | 51.7 (13) | 54.5 (15.6) | .006 |
| BMI, kg/m2 | 29.8 (5.3) | 29.6 (5.9) | .6 |
- Values are means (SD).
- HTN, hypertension; eGFR, estimated glomerular filtration rate; SBP and DBP, systolic and diastolic blood pressure; ESRD, end-stage renal disease; CVD, cardiovascular disease.
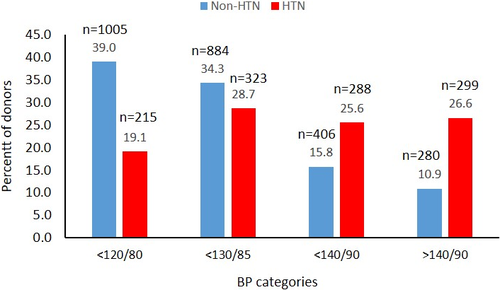
At last follow-up, 73.4% of hypertensive donors had BP <140/90 mm Hg and 19% had systolic, and diastolic blood pressure values in the optimal range <120/80 mm Hg (Figure 5). In those without a diagnosis of hypertension, 280 (10.9%) reported blood pressure values in the hypertensive range and 15.8% in the prehypertensive range.
3.3 Hypertension and risk of major events
After accounting for covariates present at the time of donation and postdonation, conditions including diabetes, hyperlipidemia, and new cardiovascular disease, we found that hypertensive donors were more likely to have diabetes, HR 1.77 (95% CI 1.2-2.6), P = .004, and more likely to have proteinuria, HR 1.55 (95% CI 1.03-2.32), P = .03 (Table 4). A sensitivity analysis excluding donors who developed postdonation diabetes continued to show an increase in HR for proteinuria for those who developed postdonation hypertension, HR 1.84 (95% CI 1.15-2.90), P = .01. Hypertensive donors were more likely to have eGFR <60, <45, and <30 mL/min/1.73 m² (Table 4). The risk of developing ESRD, however, was not higher in those with hypertension: HR 0.96 (95% CI 0.15-8.23), P = 1.0. Similarly, the risk of death was not different between those with and without hypertension (Table 4).
| Outcomes | Non-HTN | HTN | Hazard ratios (95% CI) | P-values |
|---|---|---|---|---|
| Death | 3.8 (99/2579) | 11.7 (131/1121) | 1.03 (0.46-2.40) | .9 |
| Diabetes | 2.1 (53/2579) | 15.8 (177/1121) | 1.77 (1.20-2.61) | .004 |
| Proteinuria | 3.2 (83/2576) | 14.6 (163/1118) | 1.55 (1.03-2.32) | .03 |
| eGFR <60 | 32.2 (831/2579) | 56.6 (634/1121) | 1.44 (1.21-1.72) | <.0001 |
| eGFR <45 | 6.6 (170/2579) | 24.9 (279/1121) | 1.89 (1.42-2.52) | <.0001 |
| eGFR <30 | 0.89 (23/2579) | 7.1 (80/1121) | 2.26 (1.24-4.25) | .009 |
| ESRD | 0.16 (4/2579) | 2.1 (23/1118) | 0.96 (0.15-8.23) | .97 |
| CVD | 4.4 (113/2572) | 25.8 (288/1117) | 1.42 (1.05-1.92) | .02 |
- HRs adjusted for age, race, relationship category, and at time of donation (fasting glucose, body mass index, systolic and diastolic blood pressures, smoking, estimated glomerular filtration rate, diabetes postdonation (except when diabetes was the dependent variable), hyperlipidemia, and cardiovascular disease (except when CVD was the dependent variable). Hypertension, diabetes, and proteinuria were modeled as a time-varying covariate for death, proteinuria, ESRD, and eGFR <60, <45, and <30. In the case of CVD only hypertension and diabetes were modeled as time-varying covariates. For diabetes, only hypertension was modeled as a time-varying covariate.
- All events occurred after diagnosis of hypertension.
- ESRD, end-stage renal disease; CVD, cardiovascular disease.
3.4 Antihypertensive agents and outcomes
Hypertensive donors on ACE/ARB when compared to use of other agents, had a lower risk of eGFR <45; HR 0.64 (95% CI 0.45, 0.90) P = .01, and lower risk of ESRD: 0.03 (95% CI 0.001, 0.21), P = .004 (Table 5). ACEI or ARB use was not associated with proteinuria development: 1.04 (95% CI 0.63, 1.68), P = .9, or death from any cause 1.25 (95% CI 0.67, 2.27), P = .5 (Table 5). An additional analysis comparing nonhypertensive donors, hypertensive donors treated with ACEI/ARB, and hypertensive donors treated with other agents, showed that postdonation hypertension was associated with greater HRs for all clinical outcomes, except for eGFR<60 mL/min/1.73 m², regardless of the type of treatment received. The risk of eGFR<30 mL/min/1.73 m² or ESRD in those treated with ACE/ARB was not different from those observed in donors who did not develop postdonation hypertension (Table 6).
| Antihypertensive category | ||||
|---|---|---|---|---|
| ACEI/ARB, n (%) | Other, n (%) | |||
| Clinical outcome | 340 (38) | 554 (62) | HRs (95% CI) | P-value |
| Death | 23 (6.8) | 64 (11.5) | 1.25 (0.67, 2.27) | .5 |
| CVD | 41 (12.3) | 94 (17.3) | 0.8 (0.52, 1.22) | .3 |
| Diabetes | 27 (8.1) | 56 (10.2) | 0.96 (0.57, 1.58) | .4 |
| Proteinuria | 32 (9.61) | 58 (10.6) | 1.04 (0.63, 1.68) | .9 |
| eGFR <60 | 123 (36.3) | 237 (42.6) | 0.88 (0.69, 1.11) | .3 |
| eGFR <45 | 53 (15.6) | 136 (24.3) | 0.64 (0.45, 0.9) | .01 |
| ESRD | 1 (0.3) | 15 (2.7) | 0.03 (0.001, 0.21) | .004 |
- HRs adjusted for sex, race, and the following variables at time of last follow-up: age, fasting glucose, body mass index, systolic and diastolic blood pressures, smoking, estimated glomerular filtration rate, diabetes post-donation (except when diabetes was the dependent variable), hyperlipidemia, and cardiovascular disease (except when CVD was the dependent variable).
- Hypertension, diabetes, and proteinuria were modeled as a time-varying covariate.
- All events occurred after diagnosis of hypertension. Use of ACE inhibitors (ACEIs) or ARB and diabetes were included as a time-varying covariate.
- ESRD, end-stage renal disease; CVD, cardiovascular disease.
| Clinical outcome | HRs (95% CI) | P-value, 2 vs 1 | P-value, 3 vs 1 | ||
|---|---|---|---|---|---|
| Non-HTN (1) | HTN other meds (2) | HTN ACE/ARB (3) | |||
| Diabetes | 1 | 2.75 (1.90, 3.98) | 2.70 (1.77, 4.08) | <.0001 | <.0001 |
| CVD | 1 | 2.05 (1.60, 2.62) | 1.74 (1.27, 2.35) | <.0001 | .0004 |
| Proteinuria | 1 | 2.40 (1.74, 3.29) | 2.59 (1.80, 3.70) | <.0001 | <.0001 |
| eGFR 60 | 1 | 1.12 (0.97, 1.28) | 1.14 (0.96, 1.35) | .12 | .13 |
| eGFR 45 | 1 | 1.96 (1.57, 2.45) | 1.61 (1.20, 2.13) | <.0001 | .001 |
| eGFR 30 | 1 | 3.73 (2.33, 6.08) | 1.83 (0.92, 3.47) | <.0001 | .07 |
| ESRD | 1 | 4.99 (1.82, 15.1) | 0.81 (0.11, 3.72) | .002 | .07 |
| Death | 1 | 2.71 (1.82, 3.98) | 2.30 (1.29, 3.86) | .0005 | .0005 |
- HRs adjusted for sex, age, race, relationship to recipient, family history of hypertension (HTN), and the following variables at time of donation: serum glucose, eGFR, systolic and diastolic blood pressure, presence of hyperlipidemia, and smoking status. Time of initiation of ACEI/ARB or other medications were treated as a time-varying covariates.
3.5 Outcomes in donors who were hypertensive at donation
Donors with hypertension prior to donation (n = 96) were more likely to have a family history of hypertension and hyperlipidemia. They were about 10 years older, had greater BMI, SBP, DBP, and higher serum glucose values than those without hypertension at the time of donation (Table 7). Donors with predonation hypertension were diagnosed with hypertension 4.3 (1.9) years before donation. Risks for the different clinical outcomes between those with and without hypertension at time of donation were not different (Table 8).
| HTN at baseline | |||
|---|---|---|---|
| No | Yes | ||
| n | 4200 | 96 | P value |
| Female, % | 56.8 | 56.3 | .9 |
| Age, y | 38.9 (11.6) | 49.9 (10.7) | <.0001 |
| White | 94.2 | 96.9 | .3 |
| First-degree relative | 74.1 | 68.8 | .2 |
| Smoker | 31.2 | 22.9 | .08 |
| Family history of HTN | 31.8 | 47.7 | .002 |
| Hyperlipidemia | 4.9 | 25.0 | <.0001 |
| BMI, kg/m2 | 25.8 (4.3) | 27.4 (3.8) | .0006 |
| eGFR, mL/min/1.73 m2 | 103.0 (33.9) | 98.0 (35.3) | .2 |
| SBP, mm Hg | 119.6 (13) | 130.2 (12.8) | <.0001 |
| DBP, mm Hg | 73.3 (9.9) | 78.7 (10) | <.0001 |
| Creatinine, mg/dL | 0.90 (0.16) | 0.89 (0.18) | .4 |
| Glucose, mg/dL | 93.2 (14.5) | 99.6 (16.1) | <.0001 |
| Total cholesterol, mg/dL | 192.0 (39.1) | 200.6 (37.6) | .1 |
- Data are mean (SD) or %.
- HTN, hypertension; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; DBP, diastolic blood pressure.
| Outcomes | Non-HTN, % (n/N) | HTN, % (n/N) | HRs (95% CI) | P-value |
|---|---|---|---|---|
| Diabetes | 6.2 (230/3700) | 8.3 (8/96) | 0.83 (0.20, 2.31) | .8 |
| Proteinuria | 6.7 (246/3694) | 11.5 (11/96) | 1.42 (0.42, 3.49) | .5 |
| eGFR <60 | 39.59 (1465/3700) | 58.33 (56/96) | 1.12 (0.73, 1.63) | .6 |
| eGFR <45 | 12.14 (449/3700) | 20.83 (20/96) | 0.98 (0.46, 1.84) | 1.0 |
| eGFR <30 | 2.78 (103/3700) | 6.25 (6/96) | 1.89 (0.53, 5.22) | .3 |
| ESRD | 0.73 (27/3697) | 1.05 (1/95) | — | |
| CVD | 10.87 (401/3689) | 19.15 (18/94) | 0.89 (0.39, 1.75) | .8 |
| Death | 6.2 (230/3700) | 8.3 (8/96) | 0.76 (0.18, 2.18) | .7 |
- HRs adjusted for: age, sex, race, relationship, family history, BMI, glucose, SBP, DBP, eGFR, smoking history, and hyperlipidemia. HRs for ESRD could not be calculated. eGFR <60, 45, 30 = estimated glomerular filtration rate <60, 45, 30.
4 DISCUSSION
These results demonstrate that roughly one-third of kidney donors develop hypertension after donation, and risk factors for its development are similar to what is seen in the general population. We found that one-fourth of donors receiving antihypertensive medications are poorly controlled (>140/90 mm Hg) and that one-tenth of donors without a diagnosis of hypertension had blood pressure readings in the hypertensive range.
We have previously shown that the prevalence of hypertension is similar to general population controls drawn from the 2003-2004 and 2005-2006 waves of the National Health and Nutrition Examination Survey (NHANES) after matching for age, gender, race, and BMI.11 The prevalence of hypertension in our current cohort compared to US adults from a more recent wave of NHANES 2011-2014 is shown in Table 9. The prevalence in US adults is 2-fold higher in those ≤59 years of age and 1.7-fold higher in those >59 years of age when compared to our cohort of mostly white kidney donors. Prevalence of hypertension, however, in nonwhite kidney donors does appear to be higher. Lentine et al, using medical claims and drug-treated hypertension definitions, demonstrated a 30%-50% higher prevalence of hypertension in non-Hispanic black donors compared to non-Hispanic white donors, but no difference between non-Hispanic black donors and NHANES controls of the same ethnicity.13 Hispanic donors, however, had a higher prevalence of hypertension than the general population Hispanic controls. Collectively, these studies do not suggest that the prevalence of hypertension is higher in donors, with the exception of Hispanic donors. Our data cannot shed light on hypertension in minorities as most of our donors are white.
| Age categories, y | Donors | NHANES 2011-2014 |
|---|---|---|
| 18-39 | 4.2% | 7.3% |
| 40-59 | 15.6% | 32.4% |
| >59 | 47.7% | 65.0% |
Comparing incidence of hypertension in donors and appropriate controls has been difficult because most donors are not followed prospectively. In addition, most data regarding incident hypertension in the general population come from cohorts, like the Framingham Study,14 Atherosclerosis Risk in Communities (ARIC) Study,15 and others in which the ascertainment of incident hypertension has been carried out for the near term only. For example, the incidence of hypertension in 5554 ARIC participants followed for a median of 11.9 years was 21.6%. The mean age of these participants was 61.9 years. The older age (compared to kidney donors) and the observation that minimal hypertension is seen in the first 10 years after donation limit the ability to make any meaningful comparisons regarding incident hypertension in kidney donors. Perhaps the most comprehensive and careful attempt to answer whether the incidence of hypertension is higher in kidney donors comes from the meta-analysis by Boudville et al.4 In 6 studies involving 249 donors and 161 controls, only 1 study reported a higher incidence in donors.16 Of note, a recent meta-analysis of 52 studies comparing 118 426 kidney donors to 117 656 controls suggests no evidence of higher all-cause mortality, CVD, or hypertension in donors.17 Standardized mean difference of DBP (mean difference in DBP between donors and controls divided by pooled standard deviation) was 0.17 mm Hg higher in donors. We noted a greater rise in DBP in donors without postdonation hypertension (compared to hypertensive donors). Nevertheless, a higher risk of incident cardiovascular disease was mainly in those who developed postdonation hypertension. A plausible explanation for this apparently paradoxical association is that SBP and pulse pressure are better predictors of cardiovascular diseases than DBP or mean blood pressure.18-21 In addition, those with postdonation hypertension were more likely to be diabetics, which is an independent risk factor for cardiovascular disease.22, 23 The rates of CVD we observed in nonhypertensive donors of 4.5% and in hypertensive donors of 15.3% are considerably lower than the rate of 36% reported in non-Hispanic whites.24
The covariates that we found to be associated with incident hypertension (age, gender, family history of hypertension, SBP, DBP, and BMI) carried weights almost similar to what they do in the general population. For example, family history of hypertension was associated with a 25% higher risk in our cohort and in the Framingham cohort it was associated with a 20% higher risk. BMI, SBP, and DBP at the time of donation conveyed almost identical risks in donors and Framingham participants.14 This may suggest indirectly that there might be no effect modification between uninephrectomy and other risk factors for the development of hypertension.
Most kidney donors had blood pressure value <140/90 mm Hg while receiving treatment. Data from the 2009-2010 NHANES wave indicate that only 45.5% of the general population have adequately controlled blood pressure.25 However, 25% have poorly controlled blood pressure and 1 of 10 donors with repeated readings >140/90 mm Hg was not receiving treatment. Donors deserve to have a long-term plan for medical care so conditions that are readily treatable such as hypertension and diabetes do not go unaddressed.
These results suggest that hypertension is associated with reduced eGFR and proteinuria. This association is far from causal, as the link between non-malignant hypertension and CKD is weak. In fact, a meta-analysis of 10 randomized trials of 26 521 patients assigned to antihypertensive therapy or a lower blood pressure target failed to show benefit in terms of reducing renal endpoints that spanned rises in creatinine, blood urea nitrogen (BUN), or ESRD.8 Therefore, in the general population and also in kidney donors, it remains unclear whether preexistent renal disease is sufficient to explain the association of hypertension and future loss of renal function.
A third of donors received ACE/ARB. We expected to see more frequent use of these agents, considering their ability to abrogate intraglomerular hypertension and reducing the likelihood of native proteinuric kidney disease progression.26, 27 Although currently unknown, it is conceivable that the general practitioner would be reluctant to use these agents in someone with a single kidney. The use of these agents in our cohort was associated with fewer donors reaching an eGFR <45 mL/min/1.73 m² or ESRD as compared to those treated with other agents, and the risk was similar to nonhypertensive donors. Although these data, by no means, provide conclusive evidence of the superiority of ACEI/ARB in this population, the observed associations provide a rationale for performing further research to determine the utility of ACEI/ARB use to decrease the risk of low GFR and the development of ESRD in individuals with postdonation hypertension. It is important to note that the mechanism of hyperfiltration after donation is not driven by a rise in intraglomerular pressure, but rather by an increase in the glomerular surface area9; therefore, such an observed benefit cannot be readily explained by the ability of these agents to alleviate intraglomerular hypertension. In reality, only a large-sized, randomized clinical trial can provide evidence supporting the associations observed in this retrospective analysis. One must also consider that the demonstrated benefit of these ACEI/ARB are largely seen in patients with proteinuria and that extrapolating that information to kidney donors who are generally nonproteinuric is not without limitations. Nevertheless, we feel that ACEI and ARB should be considered among the preferred agents in kidney donors who are hypertensive.
These analyses have limitations. Most of our donors are Caucasian (97% vs 75% in US kidney donors), which limits extrapolating the results from this analysis to other ethnic groups. The issue of self-report is also important. However, previous studies have shown that the concordance between hypertension diagnoses was extremely high when it was defined by need for treatment.28, 29 Moreover, the majority of diagnosis was abstracted from medical records, as well. The associations we observed between ACEI/ARB and eGFR <45 mL/min/1.73 m2 or and ESRD is greatly limited by the retrospective design of the study and possible selection bias.
In all, this analysis shows that kidney donors have risk factors for developing hypertension that are similar to those of the general population. ACEI or an ARB are the most commonly used antihypertensive medications, and their use appears to be associated with lower risks of eGFR<45 mL/min/1.73 m2 ESRD. The latter can be confirmed only in a prospectively designed study involving a much larger number of donors. Opportunities exist to optimize level of blood pressure control in hypertensive donors and to actively follow donors so that hypertension does not go untreated.
ACKNOWLEDGMENTS
This study was supported by the National Institutes of Health (5P01 DK013083).
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.




