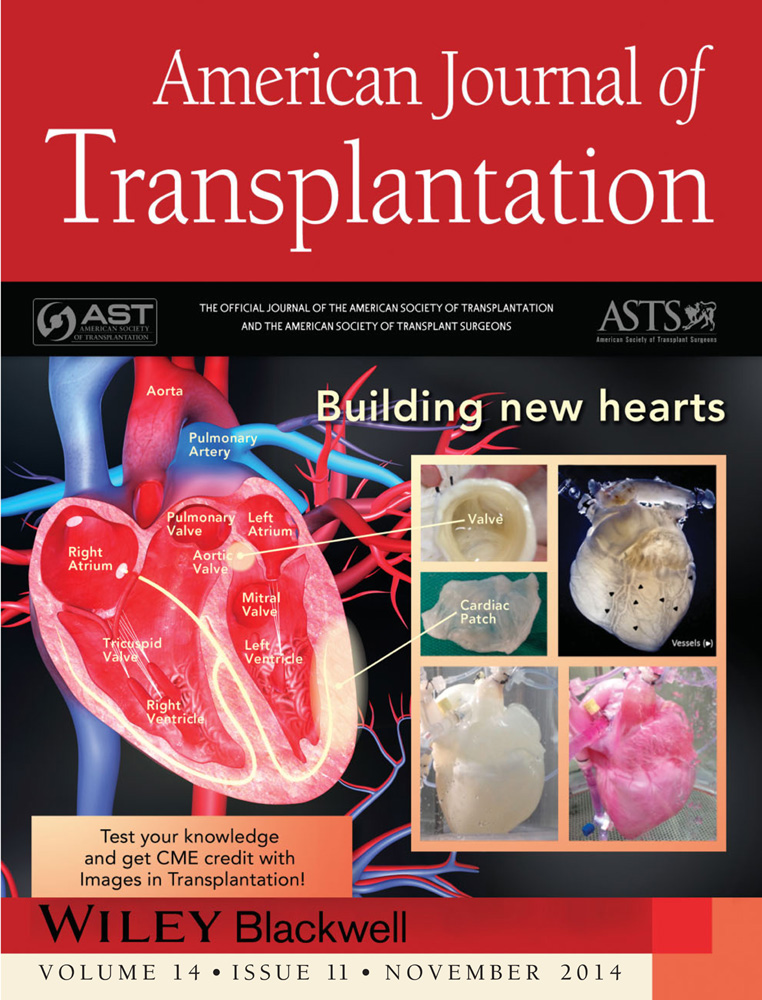
Building New Hearts: A Review of Trends in Cardiac Tissue Engineering
Abstract
Cardiovascular disease (CVD) is the number one cause of death in the United States. However, few treatments for CVD provide a means to regain full cardiac function with no long-term side effects. Novel tissue-engineered products may provide a way to overcome the limitations of current CVD therapies by replacing injured myocardium with functioning tissue or by inducing more constructive forms of endogenous repair. In this review, we discuss some of the factors that should be considered in the development of tissue-engineered products, and we review the methods currently being investigated to generate more effective heart valves, cardiac patches and whole hearts.
Abbreviations
-
- 3D
-
- three-dimensional
-
- ATDPCs
-
- adipose tissue-derived progenitor cells
-
- CNT
-
- carbon nanotube
-
- CVD
-
- cardiovascular disease
-
- ECM
-
- extracellular matrix
-
- EPC
-
- endothelial progenitor cell
-
- ESC
-
- embryonic stem cell
-
- FDA
-
- Food and Drug Administration
-
- hiPS-CMs
-
- human induced pluripotent stem cell–derived cardiomyocytes
-
- HUVEC
-
- human umbilical vein endothelial cell
-
- LVAD
-
- left ventricular assist device
-
- MI
-
- myocardial infarction
-
- MSCs
-
- mesenchymal stem cells
-
- NYHA
-
- New York Heart Association
-
- OCP
-
- Office for Combination Products
-
- RV
-
- right ventricular
-
- SIS
-
- small intestinal submucosa
-
- SMC
-
- smooth muscle cell
-
- TAH
-
- total artificial heart
-
- VEGF
-
- vascular endothelial growth factor
-
- VIC
-
- valve interstitial cell
Introduction
Cardiovascular disease (CVD), the number one cause of death worldwide, accounts for one of every three deaths in the United States 1. This disease category includes a wide range of pathological conditions of the heart and vasculature, including ischemic heart disease, valve disease, cardiac and vascular structural malformations, cardiomyopathies, microvascular diseases and congestive heart failure. The treatments for these conditions vary according to the underlying diagnosis but may include a combination of medications, surgical interventions, mechanical or bioprosthetic implants, cell or gene therapies or whole-tissue/organ transplants to address the underlying injury or disease, to intervene in its progression or to treat end-stage organ failure.
Despite the benefits of these therapeutic options, most patients fail to regain full cardiac function with no long-term side effects. Instead, cardiovascular damage often leads to the loss of functional myocardium, which is replaced with scar tissue, and a loss of cardiomyocytes, which weakens contractility, ultimately resulting in compensatory pathology that increases the risk of organ failure. By intervening in this cascade and directly replacing injured tissue with functioning muscle or by inducing more constructive forms of endogenous cardiac repair, tissue-engineered products could enable recovery of lost cardiovascular function.
Herein, cardiac engineered tissues are defined as scaffolds (either biological or synthetic) implanted into a cardiac or vascular environment—with or without genes, cells or small molecules—for the purpose of either replacing injured tissue or augmenting endogenous repair. The goal of tissue engineering is to generate tissues that are biocompatible, fully functional, able to grow with the recipient over time and durable enough to last for the lifetime of the patient. In designing a cardiovascular construct, one of the first requirements is to understand the functional demands of the de novo tissue. Based on the tissue and its associated functional requirements, specific elements must be considered, such as what type of scaffold to use for each application; whether to seed the scaffold with cells, genes or other molecules before implantation; what cells/genes/molecules would be appropriate for this purpose; and what in vitro environment is necessary to promote the proper growth and function of the construct (Table 1). In addition, once a product has been designed, many steps are necessary to make it ready for clinical studies.
| Considerations | Options | Purpose |
|---|---|---|
| Scaffold | Synthetic or biologically derived | Provide mechanical support, physical and biological cues. Be degradable and capable of integration with host tissue |
| Cells | Autologous or allogeneic stem cells or progenitor cells | Differentiate into cardiomyocytes, fibroblasts and neural, pacemaker and endothelial cells |
| Performance | At the cellular or tissue level | Enhance cellular viability, proliferation and functionality; strengthen mechanical and cardiac function |
| Regulation | Preclinical and clinical | Determine safety and potential for compassionate use; provision of both positive and negative data to community |
This review will discuss the types of scaffolds and cells that can be used for tissue engineering and the process of translating research findings into clinical methods for use in patients with CVD. Current research in the development of heart valves, cardiac patches and whole hearts will be highlighted (Figure 1).

Scaffolds
When developing engineered tissues, one of the first choices to be made is whether to use a scaffold alone or a scaffold combined with cells. The role of the scaffold is to provide not only mechanical support in the face of injury but also the physical and biological cues necessary to direct engraftment, differentiation and maturation of cells and to guide proper alignment to produce a functional tissue. In addition, the implanted scaffold should be biodegradable and should be nontoxic, resorbable and able to be replaced with the cardiac extracellular matrix (ECM) secreted by stromal cells/fibroblasts. Scaffolds can be generated from either synthetic matrices, such as polylactic acid and polyglycolic acid, which are often used for simpler constructs, or from biologically derived matrices, such as fibrin, collagen and tissue ECM. For synthetic matrices, properties such as composition, porosity, biodegradation rate and architecture can be precisely controlled, but these materials often fail to recapitulate the properties of native tissue and can be poor substrates for cell attachment and growth 2. In contrast, biologically derived ECM matrices, if left in a native state, can provide natural cues for cell migration, proliferation and differentiation, but scaffolds made from single ECM components often lack the sophistication to develop into a functional tissue.
One of the most significant developments in this field within the past 5 years has been the expansion and refinement of the decellularization process to produce decellularized organ or tissue matrices from deceased donor tissues. Originally, decellularization was an immersion-based technique that was routinely applied to valves and partial organ pieces 3, but with the introduction of perfusion decellularization, whole organs and tissues could be decellularized 4. Since then, incremental improvements have been made in decellularization protocols, which have helped preserve native ECM characteristics and, thereby, have enhanced the recellularization and function of the resulting decellularized matrices 3, 5, 6. Decellularized matrices are the first scaffolds to provide the natural tissue ultra-architecture, mechanical strength and biological components (such as sugars and growth factors) needed to help modulate and direct cell differentiation, growth organization and development 5, 7, 8. Furthermore, decellularized matrices can be used to recruit endogenous cells to accelerate the repair process 9-13. Another advantage of decellularized matrices is that the vascular structure is maintained, allowing for the anastomosis and perfusion of generated tissues. Some drawbacks of decellularized matrices include the potential loss of physical properties, such as mechanical strength and stiffness, and the introduction of cytotoxic residues during the decellularization process 5, 14, 15. In addition, the in vivo use of these acellular matrices in the vascular flow has been associated with complications such as thrombogenicity; thus, for these constructs to be a viable therapeutic option, they will most likely need to be reseeded with cells before implantation. However, it is not currently known to what degree these scaffolds will need to be reseeded and what cell types should be used.
Cell Types for Seeding Constructs
Cells can be either recruited endogenously to a tissue-engineered product or transplanted onto a scaffold before implantation. If the goal is to seed the scaffold with endogenous cells, growth factors and small molecules can be added to the matrix before implantation to enhance cellular recruitment. Another approach would be to capitalize on the acute inflammatory response to mobilize bone marrow stem cells into the nascent construct. This can be accomplished in a preparatory fashion by implanting the construct in a heterotopic location before final orthotopic placement 16, or the construct can be implanted in situ. However, if the goal is to seed the scaffold before implantation, a large number of cells (millions to billions, depending on tissue volume) will need to be generated and maintained in vitro. Terminally differentiated cardiomyocytes have a limited ability to proliferate; therefore, these cells cannot be propagated in culture for use in most tissue-engineering applications. Furthermore, these cells are extremely sensitive to hypoxia and are unlikely to survive in a poorly vascularized environment, such as injured myocardium. Thus, many studies have assessed the efficacy of cellularizing scaffolds with various stem and progenitor cell populations, including mesenchymal stem cells, resident cardiac stem cells, embryonic stem cells or induced pluripotent stem cells, instead of mature cardiovascular cells.
There are many benefits to using stem and progenitor cells for tissue engineering. They can be propagated to large numbers and differentiate into diverse cell types, such as cardiomyocytes, fibroblasts, neural cells, pacemaker cells, smooth muscle cells (SMCs) and endothelial cells 5, 17-19. Furthermore, stem and progenitor cells are more resistant to hypoxia than are mature cells. In addition, stem and progenitor cells can be isolated from either autologous or allogeneic sources, each of which has its own advantages and limitations. The use of autologous cells limits the likelihood of rejection or transmission of infectious agents, but time is required to expand these cells in vitro and to derive the necessary cell types in high numbers. On the other hand, allogeneic cell populations can be grown at any time and used to produce off-the-shelf products for emergent situations, but both in vitro and in vivo tests are still required to ensure that the cells will not be immunogenic.
Despite the many advantages of using stem cells, there are still numerous issues to be addressed: If and how stem cells are correctly differentiated into the diversity of cells needed for cardiovascular tissues? What are the correct (stem) cell types for complex tissues? How is sterility maintained for extended periods in a complex in vitro tissue environment? What is the optimum process for efficiently generating the numbers and types of cells needed? Should cells be delivered in an immature or mature state? How should cellular viability and function be measured once cells are implanted in a construct?
Culture Requirements
To guide the cellularization process and the development of cardiac tissues in vitro, bioreactors are being designed to simulate natural physiological conditions 20, 21.The maturation of cells and the functionality of a final product are affected by biochemical cues, physical and electrical stimulation, scaffold ultrastructure and other parameters. These bioreactors can be used not only to control traditional culture conditions, such as temperature and gas concentration, but also to distribute oxygen and nutrients throughout three-dimensional (3D) matrices via circulation/perfusion systems and to provide preload, afterload, mechanical strain, electrical stimulation and pulsatile fluid-flow characteristics that mimic human physiologic conditions at the local (valve) or tissue level. The use of automated bioreactors may also improve the reproducibility of in vitro conditions and reduce the risk of construct contamination. Finally, as we learn more about the environmental cues and physiological conditioning necessary to promote cellular differentiation and tissue growth, bioreactors can be developed that more accurately replicate these conditions to improve in vitro generation of cardiac tissues and whole hearts.
Heart Valve Replacement
Currently, failing heart valves are usually replaced with either mechanical or bioprosthetic valves. Neither of these valve types is able to grow with the patient, so their use in pediatric patients is limited. Although mechanical valves are very durable, they can become obstructed and can malfunction. Because they are not able to grow, they can result in a size mismatch. Moreover, these valves also increase the risk of infection or thromboembolic complications 22, so recipients must remain on life-long anticoagulation therapy, which increases the chance of hemorrhage. On the other hand, bioprosthetic valves are either scaffolds of cryopreserved human allografts or valves derived from glutaraldehyde-fixed porcine or bovine tissues, essentially first-generation tissue-engineered constructs. These valves deteriorate quickly, can rupture or calcify and often must be replaced. In addition, complications such as immune reactions and infective endocarditis can cause these valves to fail 23. Clearly, alternative replacement valves are needed that have the potential to remodel, regenerate when damaged and grow with patients. Tissue-engineered valves are being designed to meet these demands. Table 2 summarizes some of the recent research findings for tissue-engineered heart valves.
| Structure | Material | Cell type(s) | Inflammation | Model | Failure | Reference | Summary |
|---|---|---|---|---|---|---|---|
| Leaflet | Metal mesh core covered by multilayered tissue | SMCs, adventitial fibroblasts and HUVECs | Decreased inflammation | In vitro | N/A | Alavi et al 9 | Coating a stainless steel or nitinol mesh with tissue layers reduced activation of monocytes, thus improving the biocompatibility of the metal leaflet while maintaining the strength to withstand pressure |
| Pulmonary valve | Decellularized porcine pulmonary valve | Acellular | Severe inflammation | Clinical use | 52% of cases | Voges et al 25 | Matrix P and Matrix P plus tissue-engineered pulmonary valves showed high rates of failure due to inflammation and fibrosis |
| Decellularized, commercially available, cryopreserved pulmonary allograft composed of collagen and elastin | Autologous vascular endothelial cells | Decreased inflammation | Clinical use | No | Dohmen et al 26 | This prospective study evaluated patients who received a cryopreserved pulmonary allograft that was decellularized and then recellularized with autologous endothelial cells. At 10 years, all patients were in NYHA class I and no calcification was found. No mortality or device-specific complications occurred during follow-up observation | |
| Collagen produced from autologous fibroblasts | Acellular | N/A | In vitro | N/A | Syedain et al 27 | Valves were made by molding fibrin gel with fibroblasts into a tubular shape, culturing the tube within a bioreactor for 5 weeks and then decellularizing the tissue. The engineered tissue tube displayed tensile mechanical properties and anisotropy similar to that of native pulmonary valve tissue | |
| Aortic valve | Alginate/gelatin hydrogel | SMCs and VICs | N/A | In vitro | N/A | Duan et al 10 | Aortic valve conduits were 3D bioprinted with SMCs encapsulated in the valve root and VICs in the leaflets. The cells remained viable for over a week and retained their respective phenotypes |
| Decellularized human heart valve | HUVECs | N/A | In vitro | N/A | Weymann et al 13 | Pulmonary and aortic human heart valves were decellularized and then recellularized with HUVECs. A confluent monolayer formed, and markers of endothelial cells were detected | |
| Decellularized porcine aortic heart valve | EPCs | N/A | In vitro | N/A | Ye et al 19 | Coating decellularized matrices with heparin-VEGF multilayer film reduced platelet adhesion and increased human EPC adhesion, proliferation and migration | |
| Decellularized aortic root from Sprague–Dawley rats | Bone marrow-derived MSCs | No sign of inflammation | In vitro and Sprague–Dawley rats | N/A | Friedrich et al 5 | A detergent-enzymatic perfusion method to decellularize aortic root tissue was developed. The method did not alter the morphological or functional characteristics or the biocompatibility of the scaffold but increased rigidity. Subcutaneous implantation of the scaffold did not cause inflammation | |
| Decellularized ovine aortic valve conduit | Autologous endothelial cells | No sign of inflammation | Sheep | No | Tudorache et al 12 | Decellularized valve allografts were reseeded with endothelial cells and implanted in sheep. No signs of dilation, stenosis, cusp mobility reduction or transvalvular gradient were seen at 3 months postimplant |
- 3D, three-dimensional; EPC, endothelial progenitor cell; HUVEC, human umbilical vein endothelial cell; MSC, mesenchymal stem cell; NYHA, New York Heart Association; SMC, smooth muscle cell; VEGF, vascular endothelial growth factor; VIC, valve interstitial cell.
Tissue-engineered, low-pressure, pulmonary heart valve conduits have been used in the clinical setting for more than a decade. They have typically been used during reconstruction of the right ventricular outflow tract or to replace the pulmonary valve as part of a Ross procedure. Cryopreserved valve allografts, the valves commonly used for these procedures, can induce an immunological response, resulting in early valve deterioration. One method used to reduce the immunogenicity of these valves has been to decellularize them, with the hope that they will be repopulated with host cells. Initial clinical studies of decellularized allografts have shown mixed results. Brown et al 24 found that decellularized valves (CryoValve®; CryoLife, Inc., Kennesaw, GA) had failure rates similar to those of nondecellularized valves 5 years postimplantation, but patients who received decellularized valves had significantly lower valve insufficiency grades, most likely because of the decreased antigenicity of these valves. In contrast, Voges et al 25 reported that patients who received decellularized valve implants (Matrix P®, autotissue; TÜV Rheinland LGA Products GmbH, Cologne, Germany) had high rates of adverse outcomes, and histologic analysis of explanted conduits showed severe inflammation and fibrosis. Another approach involves reseeding decellularized valves with autologous endothelial cells before implantation. Early clinical results in a small patient population indicated good hemodynamic performance for these reseeded valves, but late valve degeneration still needs to be assessed 26. Thus far, tissue-engineered pulmonary valves have shown some promise, but further development is required. Design of tissue-engineered aortic valves will be considerably more difficult due to the higher pressure these valves face; however, many researchers are now investigating methods to address this issue.
One aspect that has been assessed is the benefit of mechanically conditioning the tissue-engineered constructs. Syedain et al 27 fabricated valves by molding fibrin gel with encapsulated fibroblasts into a tubular shape, culturing the tube within a bioreactor to mimic physiological strain, and then decellularizing the generated tissue. The resulting valves had mechanical properties and anisotropy similar to those of native pulmonary valves. To develop valves that can withstand the strenuous dynamic pressure the aortic valve is subjected to, researchers have investigated the use of hybrid valves composed of biologically derived and synthetic materials. For example, Alavi et al 9 created heart valve leaflets by coating a metal mesh core with multilayered tissue. The inflammatory response induced by these hybrid valves was less than that induced by bare metal implants, so this strategy could improve mechanical stability while maintaining good biocompatibility.
Another research goal is to develop valves that are capable of growing with the patients, and this will require the incorporation of cells to promote remodeling of the matrix. Duan et al 10 bioprinted aortic valve conduits with encapsulated smooth muscle and interstitial cells. They found that the cells remained viable for more than a week, suggesting that complete fabrication of the aortic valve with viable cells may be feasible. Studies of decellularized aortic tissue have shown that it is possible to maintain the morphological and functional characteristics of the scaffold during the decellularization process 5, 12, 13, 19. These studies have also evaluated the potential for recellularizing these scaffolds with various cell types. Early in vitro studies have indicated good cell attachment and proliferation with human umbilical vein endothelial cells and human blood-derived endothelial progenitor cells 13, 19. Moreover, in vivo studies have shown that the inflammatory response and the occurrence of calcification are decreased with decellularized scaffold implants versus cryopreserved implants 5, 12.
Repair Using Cardiac Patches
Engineering of a biocompatible, nonimmunogenic cardiac patch with morphological and functional properties similar to those of native tissue could provide a clinical alternative for almost half a million patients annually who have an acute myocardial infarction (MI) and subsequent heart failure. Development of replacement tissues or patches that can prevent acute MI-induced progression to heart failure or that can restore function to the failing heart is a major aim of tissue engineers. The goals are to design replacement tissues that promote cell viability and cell differentiation and, where necessary, offer mechanical support, conduct electrical signals and provide the mechanical force required for contractions. Table 3 provides an overview of the research currently being conducted to develop tissue-engineered cardiac patches. Investigators are currently developing such patches by using biopolymers such as alginate, fibrin, collagen, self-assembling peptides and a range of synthetic polymers, such as poly(glycerol sebacate) and polyurethanes 13.
| Scaffold material | Cell type(s) | Maximum thickness | Vasculature1 | Model | Reference | Summary |
|---|---|---|---|---|---|---|
| Acellular | ||||||
| Porous polyurethane (poly[ester urethane]urea) | Acellular | 800 µm | None | Pig (MI, implantation 2 weeks after induction) | Hashizume et al 28 | Eight weeks after the patch was implanted, the treatment group had a lower end-diastolic area, a higher fractional area change and a thicker infarcted ventricular wall than the control group |
| Type I collagen | Acellular | Not provided | None | Mouse (acute transmural MI, immediate implantation) | Serpooshan et al 30 | Four weeks after the patch was implanted, the treatment group showed reduced left ventricular remodeling and improved cardiac function, as compared to controls |
| Porcine cardiac extracellular matrix | Acellular | Average thickness 2.5 mm | Native architecture present | Rat (right ventricular outflow tract repair) | Wainwright et al 31 | At 16 weeks after repair, the patch had remodeled into dense, cellular connective tissue that was the same thickness as the native tissue and small islands of cardiomyocytes were present within the patch. Ventricular function and size were similar to baseline levels |
| Porcine urinary bladder matrix | Acellular | 0.25 mm | Native architecture present | Chimeric rat (right ventricular outflow-tract repair) | Remlinger et al 29 | At 4 weeks after repair, the LV ejection fraction was decreased and RV shortening fraction was increased. By 16 weeks, both had nearly returned to native values, and the patch had been remodeled and replaced with new host tissue. Also, an endothelial lining had developed with no fibrotic tissue detected |
| Injectable extracellular matrix from decellularized porcine myocardial tissue | Acellular | N/A | None | Pig (MI, injections given 2 weeks after MI) | Seif-Naraghi et al 53 | At 3 months postinjection, the global wall-motion index was reduced, ejection fraction was enhanced and foci of neovascularization were present at the injection sites |
| Porcine small intestinal submucosa (CorMatrix) | Acellular | ?? | Native architecture present | Human (repair of false aneurysm) | Yanagawa et al 32 | At 1 year, the patient returned to baseline function without limitations, with full restoration of left ventricular function, increased wall thickness and synchronous contractile activity |
| Cellular | ||||||
| Fibrin | Neonatal rat heart cells | 824 ± 22 µm | Channels | In vitro | Vollert et al 34 | Using an alginate-dissociation technique, lumina were generated that enabled perfusion of engineered heart tissue |
| Poly(glycerol sebacate) | C2C12 mouse myoblasts, neonatal rat heart cells | 250 µm | None | In vitro | Neal et al 37 | Three different scaffold designs revealed properties of cardiac mimetics and promoted the functional assembly of rat heart cells |
| CNTs in gelatin methacrylate hydrogels | Neonatal rat cardiomyocytes | 100 µm | None | In vitro | Shin et al 18 | The compression modulus and electrical conductivity of the gels, the percentage of cell retention and viability and the cell alignment index were CNT-dose dependence. Tissue cultured on the CNT gels showed strong spontaneous synchronous beating and were resistant to the effects of doxorubicin and heptanol |
| Scaffold-free cell implantation | Sheet of hiPS-CMs | N/A | None | Mini-pig (noninjury model) | Kawamura et al 36 | Transplanting cell sheets of magnetically labeled hiPS-CMs with an omental flap covering promoted angiogenesis, resulting in greater cell survival |
| Scaffold-free cell implantation | Bi-level sheet of EPCs and SMCs | N/A | None | Rat (ischemic cardiomyopathy) | Shudo et al 39 | Co-culturing EPCs and SMCs induced higher cytokine production in vitro than culturing either alone. In vivo, the bi-level sheet increased capillary density and improved blood perfusion in the infarct border zone, and the cells were shown to migrate into the myocardium |
| Scaffold-free cell implantation | Three layers of cells sheets comprising mouse ESC-derived cardiomyocytes, endothelial cells and mural cells | Unknown | None | Rat (MI, implantation 1 week after induction) | Masumoto et al 38 | Four weeks after implantation of the cell sheets, significant and sustained improvement in cardiac function was seen, which was attributed primarily to increased endogenous neovascularization |
| Poly(glycerol sebacate) | Neonatal mouse skeletal myoblasts releasing controlled levels of VEGF combined with neonatal rat cardiomyocytes | 1 mm | Channels | Mouse (MI, implantation 1 week after induction) | Marsano et al 35 | At 4 weeks after implantation, the patch had induced the formation of vascular networks and improved cardiac function |
| Fibrin | Neonatal rat heart cells | 657 ± 41.3 µm | None | Rat (acute myocardial injury, immediate implantation) | Wendel et al 33 | At 4 weeks after implantation, the patch had reduced LV remodeling and improved LV contractile function |
| Fibrin | Human subcutaneous or cardiac ATDPCs | Not provided | None | Mouse (MI, immediate implantation) | Bago et al 17 | When implanted as part of a fibrin patch, both cardiac and subcutaneous ATDPCs underwent endothelial differentiation, but cardiac ATDPCs were more effective at inducing vascularization and produced greater levels of cTnI. Mice that received patches with cardiac ATDPCs showed improvement in LV ejection fraction (21.5% recovery at 3 weeks postimplant) |
- 1 Vasculature refers to vascularization of the patch before implantation. ATDPCs, adipose tissue-derived progenitor cells; CNT, carbon nanotube; ECM, extracellular matrix; ESC, embryonic stem cell; hiPS-CMs, human induced pluripotent stem cell–derived cardiomyocytes; LV, left ventricular; MI, myocardial infarction; RV, right ventricular; SMC, smooth muscle cell; VEGF, vascular endothelial growth factor.
Studies have focused on patches that vary with regard to materials, thicknesses and cellularity. In animal models of ischemic injury or an anatomical congenital anomaly, acellular patches have been shown to improve cardiac function and tissue remodeling within 8–16 weeks after implantation 28-31. Eight weeks after implanting a porous polyurethane patch in a porcine MI model, Hashizume et al 28 observed improved cardiac function. Decellularized matrices of varying thicknesses have been used in right ventricular outflow tract repairs. In rats, endogenous endothelial cells and cardiomyocytes were recruited into decellularized porcine ECM derived from either cardiac 31 or bladder tissue 29. Most recently, decellularized porcine small intestinal submucosa (SIS) (CorMatrix ECM; CorMatrix Cardiovascular, Inc., Roswell, GA) was used in a patient with a false aneurysm 32. One year later, the patient's ventricular contraction had improved, although the authors did not show cellular regeneration. To improve the healing time, cells can be added to the constructs before implantation to actively promote healing. Scaffolds implanted with neonatal rat cells have been shown to improve left ventricular function in as little as 3–4 weeks 17, 33. However, in these studies, clinical timing of surgical placement was not taken into consideration; therefore, it is difficult to determine how these patches will translate to a clinical setting.
One of the challenges researchers have faced when developing cellularized patches is the need for prevascularization to support the oxygen and nutritional needs of the cells on the patch. To address this issue, several investigators have assessed materials engineered with predefined channels. These channels have been shown to enhance perfusion in vitro 34 and to aid in the formation of vascular networks in an in vivo model 35. Another method that has been explored to improve vascularization is the use of a pedicled omental-flap covering. For example, Kawamura et al 36 implanted cell sheets of human induced pluripotent stem cell-derived cardiomyocytes with an omental flap, which enhanced survival as compared to no flap; however, these researchers did not assess whether this method improved function.
Other aspects being investigated include the modulation of material architecture 37 and the incorporation of conductive materials, such as nanotubes 18, to improve myocyte morphology and function. In addition, investigators are trying to determine which cell types should be delivered to the injured area to achieve the greatest improvement in cardiac function (i.e. cardiomyocytes alone vs. cardiomyocytes in combination with endothelial cells, SMCs or other support cells). In a study by Masumoto et al 38, transplanting cell sheets consisting of cardiomyocytes, endothelial cells and mural cells significantly improved systolic function and increased neovascularization after MI in a rat model. In addition, the functional improvements were shown to derive from proangiogenic factors expressed by the transplanted cardiomyocytes and paracrine signaling that occurred between the various cell types, rather than from direct cardiomyocyte incorporation. Likewise, in a rat model of ischemic cardiomyopathy, Shudo et al 39 showed that implantation of a “bi-level” cell patch containing endothelial and smooth muscle cells increased ventricular function; this bi-layer strategy appeared to produce a positive synergistic effect on cytokine production, leading to increased endogenous neovascularization and the observed improvements in cardiac function.
Whole Heart Replacement
For patients with end-stage heart failure, heart transplantation is currently the best treatment option. However, the number of patients on the waitlist for heart transplantation far exceeds the number of available donor hearts. In addition, patients who do receive a heart transplant must remain on long-term immunosuppressive therapy to prevent rejection of the transplanted organ; thus, they are more prone to infections. Another treatment option for patients with heart failure is mechanical circulatory support. The two main choices are the total artificial heart (TAH) and the left ventricular assist device (LVAD), each of which has its own set of complications and limitations. For example, use of a TAH for support in biventricular heart failure has been associated with bleeding, neurologic events, infections, persistent anemia and other complications 11, 40. Furthermore, because of the large size of TAHs, implantation of this device is restricted primarily to men. Likewise, although the design enhancements of second-generation, continuous-flow LVADs have led to improvements in clinical outcomes, these devices are still associated with hemorrhagic events, stroke, pump thrombosis and driveline infections 7, 41.
One promising alternative for treating heart failure is the use of tissue-engineered whole hearts. Thus far, the most successful approach has been to decellularize whole hearts to generate an extracellular matrix scaffold, which can then be recellularized with autologous cells. Because decellularized scaffolds retain their composition (such as glycoproteins, proteoglycans and growth factors) and ultrastructure (such as topology, fiber orientation and ligand presentation), they are able to provide biomechanical support and important microenvironmental cues for the transplanted cells 4. In two studies, this method has shown some initial success in small-animal models (Table 4). Ott et al 4 were the first to demonstrate the feasibility of this approach using rat heart constructs recellularized with neonatal cardiac cells. After being cultured for 8 days under physiological conditions, the engineered whole heart was contracting and drug-responsive, generating about 2% of adult pump function. Similarly, Lu et al 42 recently described a mouse model in which a decellularized scaffold was reseeded with progenitor cells derived from inducible pluripotent stem cells. After 20 days in culture, that heart was contracting and drug-responsive.
| Scaffold material | Cell type(s) | Model | Reference | Summary |
|---|---|---|---|---|
| Decellularized deceased donor rat hearts | Neonatal cardiac cells or aortic endothelial cells | In vitro and rat heterotopic | Ott et al 4 | Whole hearts were decellularized by coronary perfusion with detergents, leaving an acellular scaffold with competent valves, intact chambers and perfusable architecture. Hearts were reseeded by either intramural injection of cardiac cells or perfusion of endothelial cells into vascular conduits. Resulting constructs were contracting and drug-responsive after 8 days in culture |
| Decellularized mouse hearts | Human induced pluripotent stem cell-derived multipotent cardiovascular progenitor cells | In vitro | Lu et al 42 | Decellularized hearts were seeded with multipotent progenitor cells, which migrated, proliferated and differentiated in situ. The resulting heart tissue showed spontaneous contractions, could generate mechanical force, and was drug-responsive after 20 days in culture |
Some of the numerous challenges in building a total bioartificial heart include optimizing the recellularization techniques, defining the most appropriate cell types to be used, obtaining the amount of cells needed to fully populate the scaffold and keeping the organ safe and sterile during this process. Additionally, the electrical stimuli and mechanical drive, which are important for cell differentiation, must not be forgotten. Once the bioartificial heart starts to present the characteristics of a working complex organ, a combined transplant with mechanical devices should be considered. Mechanical circulatory support is currently an important therapy in advanced heart failure 43. The possibility of temporally combining the new heart with a mechanical device could lead to the ability to transplant a partially recellularized organ, leaving the completion of recellularization to the potential recruitment of endogenous stem cells 8. Electrical stimuli could be provided by concomitantly implanting a multichamber pacemaker 44.
Translational Process and Clinical Trials
As discussed in this article, many tissue-engineered products are beginning to transition to clinical studies. In the United States, the Food and Drug Administration (FDA) regulation of new drugs, biologics and devices falls under the purview of the Center for Drug Evaluation and Research, the Center for Biologicals Evaluation and Research and the Center for Devices and Radiologic Health, respectively, each of which has its own set of regulations regarding premarket approval. For example, all new drugs and biologics must undergo clinical trial testing to demonstrate safety and efficacy before being approved, whereas the majority of devices are cleared by the FDA through the 510[K] process, which only requires evidence that the new device is substantially equivalent to a predicate device. In fact, only about 2% of new devices are required to undergo clinical testing for safety and efficacy through the Premarket Application process 45. Because bioengineered products may include new devices, biologics, pharmaceuticals and/or surgical techniques alone or in combination, they could potentially be reviewed by any one of the three centers mentioned above. Thus, not all bioengineered products are subject to the same regulatory guidelines, and the exact pathway for market approval of these combination products is often unclear. To address this issue, the Office for Combination Products (OCP) was created in 2002. The OPC is responsible for classifying each combination product as a device, biologic or pharmaceutical based on the product's primary mode of action and then assigns it to the appropriate center for review. This decision, therefore, dictates the pathway for market approval, although the manufacturer can submit an appeal and ask for the product to be reclassified if it disagrees with the decision. It should also be noted that some bioengineered products are initially approved for first-in-human use as treatments in “compassionate-use” cases, although no bioengineered cardiovascular products have been introduced into clinical study by this means thus far.
Despite the current uncertainties regarding the exact pathway to market approval for each bioengineered product, we provide some suggestions here regarding testing that should accompany the translational process. Because each type of tissue-engineered product (e.g. valve or cardiac patch) has its own set of structural and functional demands when used as a clinical treatment, a specific set of performance criteria should be developed for each type to determine how well the engineered product will translate to the intended clinical use. Criteria to be considered include function, durability, connectivity and—if cells are present—cell viability, proliferation, differentiation, transformation (oncogenicity) and function. These tests should include both in vitro and in vivo investigations. For example, the function of tissue-engineered heart valves would probably first be assessed in vitro by determining the tensile and flexural mechanical properties, mechanical anisotropy and fatigue resistance, whereas in vivo testing could be used to assess valve patency and performance (e.g. transvalvular pressure gradient and grade of insufficiency); the occurrence of calcification, rupture and thrombosis; and the ability of the valve to grow with the host. This testing should include rigorous large animal studies developed to mimic the biochemical and physiological interactions likely to occur in humans. When designing these tests, researchers should aim to simulate the actual clinical circumstance in which these end products will be used to increase the accuracy with which these tests predict patients' responses. This includes using appropriate models and performing implantation procedures in a manner that adheres to current clinical guidelines, as set by medical societies such as the American Heart Association, the American College of Cardiology and the Society of Thoracic Surgeons. For example, in vivo studies designed to determine the effectiveness of a tissue-engineered valve could include models that promote either stenosis or insufficiency to simulate the health status of patients receiving these implants. Alternatively, valves could be implanted in models of rapidly growing animals (e.g. piglets) to determine growth compatibility. With endovascular implantation gaining ground, testing could also include intra-aortic peripheral implantation of valves in large animal models, such as sheep. Similarly, in vivo testing of cardiac patches should simulate actual clinical scenarios. For example, when testing the effectiveness of a patch for treating MI, implantation should occur in the postacute phase (i.e. 7–14 days post-MI), as dictated by medical guidelines 46. Efficacy should also be assessed at different anatomical sites when relevant, as was done for the in vivo evaluation of pig SIS, which was tested in the left ventricle after occlusion of the left anterior descending coronary artery to simulate post-MI treatment and in the right outflow tract to evaluate use for transmural vascular grafting 47, 48. To expedite progress and increase confidence in the products tested, both negative and positive results from all preclinical and early clinical studies should be published.
As these products move into clinical trials, additional factors will need to be considered, including the release criteria used to confirm the safety, efficacy and durability of the product. The exact criteria used will depend on the composition of the specific product. For example, products with cellular components will probably require testing for contamination (e.g. endotoxins and mycoplasma) and potency. Bioengineered products could also be assessed using the guidelines provided by the International Standards Organization and the FDA for determining the biocompatibility of medical devices. These tests may include cytotoxicity, systemic toxicity, sensitization, hemocompatibility, pyrogenicity, genotoxicity, carcinogenicity and the biodegradation rate. Furthermore, testing should be completed to ascertain product attributes such as shelf life and stability before implantation. Finally, as the scale of production increases, quality control measures must be instated to verify the uniformity of the products being produced.
Once trials are initiated, cost, scientific rigor and means to avoid bias in scope and results will have to be addressed, especially since these trials are likely to be conducted in a single-blind or unblinded manner. Another consideration is that many tissue-engineered constructs will be integrated into the body after implantation and, thus, will be extremely difficult—if not impossible—to remove. Therefore, patient education, posttreatment care and follow-up evaluation will be especially important. It is critical that patient safety be a top priority during all research studies.
One example of an engineered cardiovascular product that has undergone the translational process to first-in-human use is porcine SIS, which has been used for pericardial reconstruction, repair of heart valves and the treatment of some congenital heart diseases 49-51. The 5-year follow-up results have been reported for the use of porcine SIS (CorMatrix ECM) in pericardial reconstruction. The pathological analysis of the explanted tissue showed remodeling of the bioscaffold into a viable, fully cellularized, vascularized and nonfibrotic connective tissue similar to native pericardium 51. Currently, four different clinical cardiovascular trials (ClinicalTrials.gov Identifier: NCT01535807/NCT02073331/NCT01569594/NCT01247974) are being conducted to further study CorMatrix ECM and determine its potential use for carotid artery repair, its direct implications on postoperative atrial fibrillation and its induction of inflammatory markers after coronary artery bypass graft surgery.
Ethics
For the field of tissue engineering and regenerative medicine to progress, ethical and organ brokering regulatory issues need to be addressed. In designing trials, several issues are significant: determining which evidence is sufficient for safety, defining control groups and their potential alternatives, establishing the inclusion criteria for the patient selection process, obtaining full patient consent for the trial, determining which outcomes should be assessed and how product failure will be handled, and identifying and registering other uses for the product. Patient safety should be a foremost concern. All parties—researchers, clinicians, regulatory agencies, industry representatives, ethics committees and patient coalitions—must be involved in the complex steps of the translation process, including clinical trials. Furthermore, as these bioengineered products reach the clinical trial stage, it is incumbent upon the tissue engineering community to educate the public, the medical community and the regulatory agencies about the additional risks and rewards of engineered products (for a review of ethics in tissue engineering, see Taylor et al 52).
Conclusions
Tissue engineering promises to provide many new therapeutic options for individuals with CVD. The studies summarized herein represent the advancements being made in this field and highlight areas in which further development is needed. Future research endeavors should be aimed at designing more advanced 3D culturing systems (i.e. bioreactors) and biomimetic materials, establishing means to functionally integrate tissue-engineered constructs with host tissue, determining ways to increase vascularization in engineered constructs and defining the best cell sources and appropriate methods for guiding cell growth and differentiation. Educating the public, patients and regulatory agencies concerning regenerative-medicine strategies is another important step in promoting the development of optimal ethical guidelines and regulations.
Acknowledgments
Dr. Doris A. Taylor's work was funded in part by the American Heart Association's Jon Holden DeHaan Cardiac Myogenesis Research Center #AHA09070499N, the Texas Emerging Technology Fund and the Houston Endowment. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. We would like to thank Heather Leibrecht, MS, of the Texas Heart Institute for editorial assistance.
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Dr. Doris A. Taylor, PhD, FAHA, FACC, holds a financial interest in Miromatrix, Inc. and is entitled to sales royalty through the University of Minnesota for products related to the research described in this paper. This relationship has been reviewed and managed by the University of Minnesota in accordance with its conflict of interest policies. This does not alter the authors' adherence to the American Journal of Transplantation's policies on sharing data and materials.