Innate Immune Cell Collaborations Instigate Transplant Tolerance
Abstract
This editorial provides commentary on Hongo et al's (page 2467) demonstration that transplant tolerance induction after recipient conditioning with total lymphoid irradiation, antithymocyte serum, and donor bone marrow transplantation requires arginase-1–expressing myeloid-derived suppressor cells that are generated in response to invariant natural killer T cell interleukin-4 production.
Total lymphoid irradiation (TLI), T cell depletion and donor bone marrow transplantation (BMT) consistently induces mixed chimerism and a state of donor-specific immunological tolerance to allografts in laboratory animals 1. The induction of a similar robust tolerant state clinically would remove the recipient's need for toxic immunosuppressants and provide freedom from acute and chronic rejection. Indeed, when comparable regimens are tried clinically, they have shown promise by inducing transplant tolerance in a subset of patients and often enable recipient tapering off immunosuppression 1. Yet there has been a failure to fully recapitulate the consistent and robust immunological tolerance observed experimentally. Recipient chimerism tends to be transient and cellular and antibody-mediated rejection often thwarts successful immunosuppression withdrawal 1. Thus, a better understanding of the precise immunological mechanisms that consistently mediate tolerance in animals will hopefully lead to uniform and robust tolerance induction in the clinic.
Previous rodent studies have identified two innate immune cell populations, invariant natural killer T (iNKT) cells and myeloid-derived suppressor cells (MDSC), which are both required for experimental transplantation tolerance. Initial studies from Seino et al 2 reported that interleukin IL-4 secreting iNKT cells play an important role in the establishment of costimulatory blockade-mediated transplantation tolerance. MDSC, a heterogenous population of myeloid-lineage cells that act as potent negative regulators of the immune response, are also required for costimulatory blockade induction of tolerance following heart transplantation 3. Yet how or if iNKT cells and MDSC interact with each other during the induction of tolerance has not been clearly defined. It also has not previously been shown if there is a similar need for either population in other models of tolerance induction, particularly the clinically relevant combination of TLI, T cell depletion and BMT.
Cytokines are well-appreciated mediators of MDSC development and activators of their suppressive mechanisms (reviewed in 4, 5). Seminal findings from Bronte et al reported IL-4 induced arginase 1 (Arg1) in MDSC-mediated suppression of alloreactive T cell responses in tumor-bearing mice 6. L-arginine is the substrate of Arg1 and Arg1+ MDSC-mediated deprivation of this essential amino acid, which is required for T cell growth and differentiation, is a critical MDSC inhibitory mechanism 5. Other recent studies suggest that IL-4 7 and IL-13 8 drive myeloid cell regulatory functions, including induction of Arg1 expression 8. The cytokines also support an MDSC protective role against graft-versus-host disease following BMT 7, 8. Thus, a network involving T helper type 2 (Th2) cytokine-driven MDSC regulatory activity is emerging as a crucial tolerogenic mechanisms in both transplantation and cancer.
In this issue of the American Journal of Transplantation, Hongo et al provide novel mechanistic insights that link essential IL-4-producing iNKT cells and IL-4 receptor (R)-expressing MDSC for the induction of heart transplant tolerance following a conditioning regimen of TLI, anti-thymocyte serum (ATS) and donor BMT 9. Specifically, the authors establish that this conditioning regimen generates a tolerogenic environment with augmented numbers of MDSC and iNKT cells that are of critical importance for regulation of alloimmunity. Using a model of C57BL/6 heart transplantation into BALB/c recipients followed by ATS and TLI, the authors demonstrate that a narrow time window exists when increased numbers of MDSC set the stage for transplant tolerance. Specifically, tolerance is fully ablated if MDSC are depleted with anti-Gr-1 antibodies on postoperative day (POD) 13. Importantly, delivery on POD 15 of purified CD11b+Gr-1+ cells from nontransplanted, but TLI/ATS-conditioned mice, restores tolerance in 40% of Gr-1+ cell-depleted allograft recipients. Interestingly, the current data also support that it is TLI, and not ATS or BMT, that initiates MDSC increases in lymphoid tissues. Finally, the authors perform compelling experiments utilizing CD1d−/− and Ja18−/− mice, both lacking iNKT, as well as IL-4-deficient mice, to establish that IL-4 produced by iNKT cells is paramount to MDSC-mediated transplant tolerance following TLI/ATS/BMT.
While robust in their nature, the current set of studies leave several aspects of iNKT and MDSC immunobiology unanswered. Unfortunately, while the general suppressive capacity of CD11b+ Gr-1+ cells was established, limited cell numbers precluded definitive assessment of the suppressive contribution of Arg1 to the suppressive function of the various subsets of CD11b+ Gr-1+ cells modulated by TLI and iNKT cells in this model. Likewise, MDSC administration only partially restored heart transplant tolerance. These data suggest either that the presence of MDSC at POD 13-14 is critical or that mechanisms other than MDSC also contribute to tolerance. An additional question that was not addressed is the identity of the stimuli promoting IL-4 production by iNKT cells.
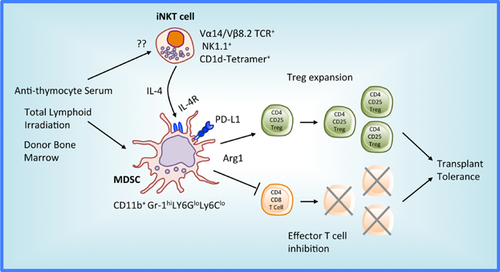
In conclusion, the authors report that a clinically relevant conditioning regimen of TLI/ATS/BMT promotes mouse heart transplant tolerance associated with mixed chimerism and augmentation of immunosuppressive cells, including Arg1+ MDSC. Significantly, the suppressive function of the MDSC requires the influence of IL-4-secreting host iNKT cells. The findings by Hongo et al 9 support an important and powerful tolerogenic mechanism where Th2 cytokines, including IL-4, up-regulate Arg1 in MDSC that block T effector proliferation and promote Treg (Figure 1). Accordingly, these current insights 9 and recent related publications 7, 8 suggest delivery of IL-4 or IL-13, as part of a TLI/ATS/BMT regimen, could promote Arg1+ MDSC generation and improve obtainment of clinical tolerance following transplantation. Likewise, monitoring Arg1 in MDSC for patients enrolled in tolerance protocols may be a valuable surrogate to assess induced tolerant states.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.