Kidney Transplant Recipients Treated With Belatacept Exhibit Increased Naïve and Transitional B Cells
Abstract
Phase III clinical studies have shown that kidney transplant (KT) recipients treated with the costimulation blocker belatacept exhibited a better renal allograft function and lower donor-specific anti-HLA immunization when compared to recipients treated with calcineurin inhibitors (CNI). We analyzed B cell phenotype in KT recipients treated with belatacept and stable renal function (N = 13). Results were compared to those observed in stable patients treated with CNI (N = 12), or with chronic antibody-mediated rejection (N = 5). Both transcriptional profile and phenotypic characterization of peripheral B cells were performed by real-time polymerase chain reaction and flow cytometry, respectively. In belatacept group, the frequency and absolute number of transitional B cells as defined by both phenotypes: CD19+CD24hiCD38hi and CD19+IgDhiCD38hiCD27−, as well as naïve B cells were significantly higher compared with CNI group. B cell activating factor (BAFF) and BAFF receptor mRNA levels were significantly lower in belatacept group than in CNI group. These results show for the first time that belatacept influences B cell compartment by favoring the occurrence of transitional B cells with potential regulatory properties, as described in operational tolerant patients. This role may explain the lower alloimmunization rate observed in belatacept-treated patients.
Abbreviations
-
- APRIL
-
- A proliferating inducing ligand
-
- BAFF
-
- B cell activating factor
-
- BAFF-R
-
- BAFF receptor
-
- BANK1
-
- B cell scaffold protein ankyrin repeats 1
-
- BCMA
-
- B cell maturation protein
-
- CNI
-
- calcineurin inhibitors
-
- CR
-
- chronic antibody-mediated rejection
-
- DC
-
- dendritic cells
-
- DSA
-
- donor-specific alloantibodies
-
- KT
-
- kidney transplant
-
- PBMC
-
- peripheral blood mononuclear cells
-
- PCR
-
- polymerase chain reaction
-
- qPCR
-
- quantitative PCR
-
- sHLA-G
-
- soluble HLA-G
-
- TACI
-
- transmembrane activator and CAML interactor
Introduction
Extensive studies carried out in the recent years have clearly highlighted the importance of humoral immunity as a major component of the alloimmune response in the field of solid organ transplantation. Actually, the production of donor-specific alloantibodies (DSA) after transplantation is clearly involved in both acute and chronic graft injury, and graft lost 1-3. By contrast, recent data have also suggested that B cells could play a role in operational tolerance. Indeed, a B cell signature has been identified in two distinct cohorts of tolerant kidney transplant (KT) recipients 4, 5. Such signature includes an increase in naïve and transitional B cells with potential regulatory properties, when compared to KT recipients with stable graft function or with biopsy-proven chronic rejection. Thus and as previously established in the field of cellular alloimmune response, the belief is currently emerging that the outcome of allograft response–rejection or tolerance after transplantation could be in part determined by the balance between DSA-producing to tolerogenic B cells. In this context, monitoring B cell phenotype could be of importance to both analyze and better predict the characteristics of the humoral alloimmune response. Moreover, the influence of immunosuppressive drugs on the B cell compartment should now be taken into account in order to define the most protective strategy against B cell–mediated injury.
Belatacept, a first-in-class selective costimulation blocker, inhibits the interaction between CD80/CD86 and CD28 preventing T cell activation. This drug is currently designed in solid organ transplantation to provide effective immunosuppression while avoiding both the renal and nonrenal toxicities associated with calcineurin inhibitors (CNI). Belatacept was approved by the US Food and Drug Administration in June 2011 based on the 3-year results of two open-label, randomized, multicenter Phase III trials. These studies compared a more or less intensive regimen of belatacept to cyclosporine in patients receiving a KT from either living or standard criteria deceased donors (BENEFIT) or extended-criteria deceased donors (BENEFIT-EXT) 6, 7. The 3-year results demonstrated comparable patient and graft survival between belatacept and cyclosporine with better renal function despite higher rates and grades of early (within 6 months) T cell–mediated acute rejection 8, 9. However, acute rejection in patients receiving belatacept was not typically associated with DSA 9, 10. In contrast, cyclosporine was more frequently associated with the development of DSA 7.
Taking also into account that the engagement of CD28 on plasma cells 11 with CD80/CD86 induces antibody secretion 12, 13, we therefore hypothesized that belatacept influences B cell compartment. We report here the first B cell phenotype analysis in KT recipients who had stable renal function treated with belatacept as maintained immunosuppression.
Materials and Methods
Patients and study design
- First kidney recipients with stable graft function treated with belatacept (Bristol-Myers Squibb [BMS] Company, New York, NY), mycophenolate mofetil and steroids (n = 13; belatacept). We picked out patients from Phases II (n = 1) and III (n = 10) clinical studies cohorts (BMS) and local cohort treated after full market approval (n = 2). These patients have had stable renal function (GFR) as estimated by MDRD formula, nonsignificant proteinuria and no acute rejection within the year before inclusion. Four patients have presented an acute T cell–mediated rejection episode, all were treated with high doses of steroids alone and none was classified as resistant (resistant if the estimated GFR level did not return to within 15% of the baseline within 2 weeks after initiation of antirejection treatment). Time between acute rejection and blood collection was 45 (23–110) months. Nineteen samples were analyzed from the 13 patients. Time between transplantation and sample analysis ranged between 12 and 113 months
- First kidney recipients with stable graft function treated with CNI, mycophenolate mofetil and/or steroids (CNI; n = 12). Patients were selected from our database and closely matched to belatacept patients on the basis of date of transplantation, age, number of HLA mismatches, sensitization status at the time of renal transplant, donor type and age, induction and maintenance immunosuppressive treatment. All patients have had stable renal function, nonsignificant proteinuria and no acute rejection within the year before inclusion. Two patients have an acute T cell–mediated rejection episode both 1 month after transplantation. Both episodes were sensitive to high dose of steroids. Blood samples were collected 67 and 84 months after acute T cell–mediated rejection episode. Fourteen samples were analyzed from the 12 patients.
- First KT recipients with chronic antibody-mediated rejection (CR; n = 5). All CR were biopsy proven and presented histological evidences of chronic tissue injury (i.e. allograft glomerulopathy (cg) and/or interstitial fibrosis/tubular atrophy (ci + ct) and/or fibrous intimal thickening in arteries (cv) associated with the presence of C4d and DSA) 14. DSA were not detected in two patients. We chose patients closest to the stables treated with belatacept or CNI on the basis of date of kidney transplantation, age, donor type and age and immunosuppressive regimen. Patients treated with rituximab within 1 year before blood collections were excluded. Time between diagnosis of CR and sampling for the studies ranged from 1 to 8 months. Three patients from the five included were treated for at least one episode of acute antibody-mediated rejection, respectively, 4, 3 and 2 years before the diagnosis of CR. Treatment included in all patients plasmapheresis, intravenous immunoglobulins and high doses of steroids, and rituximab in two.
| Variables | Stable graft function | p-Value1 | Chronic antibody-mediated rejection (CR) | |
|---|---|---|---|---|
| Belatacept | CNI | |||
| Patients, N | 13 | 12 | 5 | |
| Samples, | ||||
| Early analysis, N | 13 | 12 | — | 5 |
| Delay from renal transplant, months, mean (SD2) | 64 ± 25 | 71 ± 27 | 0.49 | 149 ± 126 |
| Late analysis, N | 7 | 2 | — | 0 |
| Delay from renal transplant, months, mean (SD) | 87 ± 4 | 90 ± 1 | 0.30 | — |
| At the time of transplantation | ||||
| Sex (male/female), N | 10/3 | 11/1 | 0.59 | 2/3 |
| Recipient age, years, mean (SD) | 50 ± 15 | 51 ± 17 | 0.89 | 46 ± 20 |
| Donor age, years, mean (SD) | 55 ± 14 | 56 ± 13 | 0.84 | 56 ± 24 |
| Living/deceased donor, N | 0/13 | 2/10 | 0.48 | 0/5 |
| Donor-specific alloantibodies, N | 0 (0) | 0 (0) | — | 0 (0) |
| HLA mismatches, mean (SD) | 3.4 ± 1 | 2.9 ± 1 | 0.27 | 2.4 ± 1.6 |
| Cold ischemia time, min, mean (SD) | 1291 ± 543 | 1138 ± 339 | 0.45 | 1149 ± 141 |
| Cause of end-stage kidney disease, N (%) | ||||
| Glomerulonephritis | 6 (46) | 4 (33) | 0.23 | 0 |
| Chronic interstitial nephropathy | 3 (23) | 2 (17) | 1 (20) | |
| Unknown nephropathy | 2 (15) | 0 (0) | 2 (40) | |
| Other | 2(15) | 6 (50) | 2 (40) | |
| Viral infectious disease, N (%) | ||||
| CMV viremia | 3 (25) | 2 (17) | 1.00 | 1 (20) |
| BK viruria | 6 (50) | 2 (17) | 0.19 | 1 (20) |
| Immunosuppression, N (%) | ||||
| Induction | 13 (100) | 11 (92) | 1.00 | 2 (40) |
| Thymoglobulin | 1 (8) | 0 (0) | — | 1 (50) |
| Belatacept | 12 (100) | 0 (0) | — | 0 |
| Calcineurin inhibitors | 0 (0) | 12 (100) | — | 4 (80) |
| Mycophenolate mofetil | 13 (100) | 11 (92) | 1.00 | 3 (60) |
| Steroids | 13 (100) | 9 (75) | 0.22 | 4 (80) |
| Rejection, | ||||
| Acute T cell–mediated rejection, N (%) | 4 (33) | 2 (17) | 0.64 | 0 |
| Delay from transplant, months, median (min-max) | 2.5 (2–47) | 1 (1–1) | — | — |
| Acute antibody-mediated rejection, N (%) | 0 (0) | 0 (0) | — | 2 (40) |
| Delay from transplant, months, median (min-max) | — | — | — | 80 (62–98) |
| Chronic antibody-mediated rejection | 0 (0) | 0 (0) | — | 5 (100) |
| Delay from transplant, months, median (min-max) | — | — | — | 104 (58–368) |
| End of follow-up | ||||
| Time from transplant, months, median (min-max) | 86 (9–127) | 84 (27–136) | 0.95 | 105 (89–384) |
| eGFR mL/min/1.73 m2, mean (SD) | 57.18 ± 16.31 | 63.93 ± 17.43 | 0.34 | 32.05 ± 7.8 |
| Proteinuria, g/day, median (min-max) | 0.13 (0.03–0.51) | 0.12 (0.07–0.80) | 0.86 | 0.75 (0.25–1.61) |
| Donor-specific alloantibodies, N (%) | 1 (8) | 2 (17) | 1.00 | 4 (80) |
| Number, median (min-max) | 0 (0–1) | 0 (0–5) | — | 1 (0–2) |
| MFI, median (min-max) | 2459 | 2159 (1437–6136) | — | 3934 (743–19 734) |
- CMV, cytomegalovirus; CNI, calcineurin inhibitors; eGFR, estimated GFR; MFI, mean fluorescence intensity.
- 1 p-Value between the two stable groups.
- 2 Standard deviation.
Cell isolation and flow cytometry
Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Paque (GE Healthcare, Marolles-en-Hurepoix, France) gradient centrifugation. Total B cells from freshly isolated PBMC were purified by negative selection using the B cell isolation kit II for magnetic cell separation (MACS system; Miltenyi Biotec SAS, Paris, France). PBMC and purified B cells were stained according to standard protocols using the following mAbs purchased from Beckman-Coulter (Villepinte, France), unless otherwise specified: CD3-ECD, CD19-ECD or CD19-PECy5, CD138-PE, CD24-FITC (BD Pharmingen [Becton, Dickinson and Company, Le Pont de Claix, France]), CD38-ECD, CD27-PE, IgD-FITC, CD5-FITC (EXBIO Praha, a.s. Vestec, Czech Republic), CD1d-PE (BD Pharmingen) alongside istotype-matched controls. CD45-FITC, -PE, -ECD and -PECy5 were used to perform fluorescence signal compensation. Cells (2 × 105 to 4 × 105) were analyzed using EPICS™ XL4 flow cytometer (Beckman-Coulter). CD4-FITC, CD127-PE and CD25-PECy5 were used to stain T cells.
Messenger RNA isolation and real-time quantitative polymerase chain reaction
Messenger RNA was extracted from 5 × 106 PBMC using the RNeasy mini kit (Qiagen, Valencia, CA) according to the manufacturer's instructions for nine patients from the belatacept group and nine from the CNI group. Genomic DNA was removed by DNase treatment (Qiagen). Total RNA was reverse transcribed to complementary DNA using reverse transcription reagents (Thermo Scientific, Courtaboeuf, France). Real-time quantitative polymerase chain reaction (qPCR) was performed using commercially available primer and probe sets (Applied Biosystems, Foster City, CA): HPRT: Hs99999909_m1, CD19: Hs00174333_m1, CD32a: Hs00234969_m1, CD32b: Hs00269610_m1, BANK1: Hs00215678_m1, BAFF-R: Hs00606874_g1, BAFF: Hs00198106_m1, APRIL: Hs00601664_g1, TACI: Hs00963364_m1, BCMA: Hs03045080_m1. We used 2−ΔΔct method to figure out relative expression of RNAs between a sample and a reference. All samples were tested in duplicates in 96-well plates with the 7900HT fast real-time PCR system (Applied Biosystems). HPRT and CD19 were used as endogenous controls to normalize RNA amounts.
Statistics
Quantitative variables are presented as means (SD) and medians with range (min-max) values, according to the distribution of the variable concerned. Qualitative values are expressed as percentages. Stable KT groups were compared at the time of inclusion. Quantitative variables were compared with unpaired t-test or Mann–Whitney, depending on the distribution of the variable concerned. Qualitative variables were compared with Fisher test or chi-square test.
Biological data were analyzed with the nonparametric Mann–Whitney test (flow cytometry and qPCR data). To compare early and late B cell phenotype, we used paired t-test. A p-value < 0.05 was considered as significant. All analyses were performed using GraphPad Prism version 5.01 for Windows (GraphPad Software, Inc., San Diego, CA).
Results
Belatacept patients display higher proportion of transitional B cells than CNI patients
The total lymphocyte, CD3+ T and CD19+ B cells counts were assessed in the peripheral blood of patients from the three groups: belatacept or CNI-treated patients with stable graft function and CR patients. The total lymphocyte count was not significantly different between the three groups (Figure 1A). The absolute number of CD3+ T cells in the PBMC of the three groups was also similar (Figure 1B). While the frequency of CD19+ B cells did not differ between the belatacept and CNI groups (2.9 [0.2–12] % vs. 2.4 [1.3–6] %), it was significantly higher when both were compared to the CR group (0.5 [0.1–2.0] %, p = 0.011 and p = 0.011, respectively) (Figure 1C). Accordingly, the absolute number of B cells was higher in the belatacept and CNI groups compared with the CR group (3.38 [2.00–24.000] and 3.59 [1.26–7.7] vs. 0.360 [0.08–4.00]; p = 0.036 and p = 0.012, respectively) (Figure 1D).

To identify peripheral B cell developmental stages among the CD19+ B cells by flow cytometry, we used the Bm1–Bm5 classification based on the expression of CD27, CD38 and IgD (Figure 2), and thresholds of expression of CD24/CD38 (Figure 3). Analysis of B cell subsets was performed on purified B cells from stable belatacept- and CNI-treated patients. CR patients were not analyzed. Actually, these patients exhibited a small amount of B-lymphocytes (0.5% of PBMC, even after B-lymphocyte enrichment number of the different subclasses of B cells remains under the threshold required for analysis. Using the CD27/IgD classification 15, we did not observe any significant difference in the percentage of CD19+ B cells displaying a memory phenotype (CD27+) or a naïve phenotype (CD27−) between the belatacept and CNI groups (Figure 2A). However, mean frequencies of CD27+ and of unswitched CD27+IgD+ subset memory B cells were slightly lower in the belatacept group compared with the CNI group (22.5 [6.92–78] % and 7.5 [2.7–34] % vs. 33.35 [11–71.5] % and 11 [3–41.5] %, respectively) (Figure 2A). Consequently, the proportion of CD27− (IgD+) naïve B cells was increased in the belatacept group (Figure 2A).

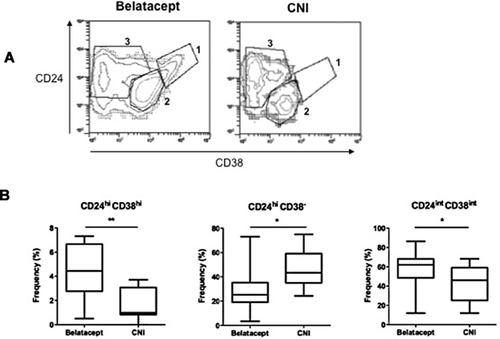
The analysis of memory B cell populations according to the Bm classification 16 showed that the belatacept group had a significantly lower number of late Bm5 cells, which are CD27+ switched memory cells, than the CNI group (7.3 [2.9–21.4] % vs.15.9 [6.7–45.5] %; p = 0.02) (Figure 2C). Both belatacept and CNI groups displayed similar numbers of early Bm5 cells (also switched memory B cells). As previously described for peripheral blood 16, there were virtually no Bm3+4 cells. The Bm1 virgin naïve IgD+ cells were significantly decreased in the belatacept group compared with the CNI group (7 [2.4–24.5] % vs. 25.3 [14.3–52] %; p = 0.0015). By contrast, activated naïve Bm2 cells were significantly increased in the belatacept group compared with the CNI group (66.5 [34.7–82.2] % vs. 40 [12.6–66.2] %; p = 0.004). This result is consistent with the increasing trend of CD27− cells and particularly of CD27−IgD+ naïve B cells, in the belatacept group. Altogether belatacept patients display a lower number of memory B cells and, consequently, a higher proportion of B cells having a naïve phenotype, than CNI patients.
The proportion of Bm2′ cells, which are mainly CD27− transitional B cells 17, 18, was significantly higher in the belatacept group than in the CNI group (4.5 [1–7] % vs. 0.98 [0.2–4.4] %; p = 0.0009) (Figure 2C). To confirm that this B cell subset was enriched in the belatacept group, we analyzed expression levels of CD24 and CD38 since transitional B cells can also be identified by their high expression levels of both these markers 17, 18. We indeed observed higher numbers of CD24hiCD38hi immature B cells in the belatacept group than in the CNI group (4.45 [0.5–7.3] % vs. 1 [0–3.7] %; p = 0.003) (Figure 3). In accordance with above results, CD24hiCD38− cells, which are primarily memory B cells, were less frequent in the belatacept group (25.3 [3.37–73] % vs. 43.4 [24.2–75] %; p = 0.01). The proportion of CD24intCD38int, comprising primarily mature B cells, was increased in the belatacept group when compared to the CNI group (62 [12–86.3] % vs. 46 [12–68.1] %; p = 0.049), although the difference reached the limit of statistical significance. This is likely to be related to the increase in transitional B cells and decrease of the memory B cells.
Belatacept patients maintain higher proportion of transitional B cells along KT
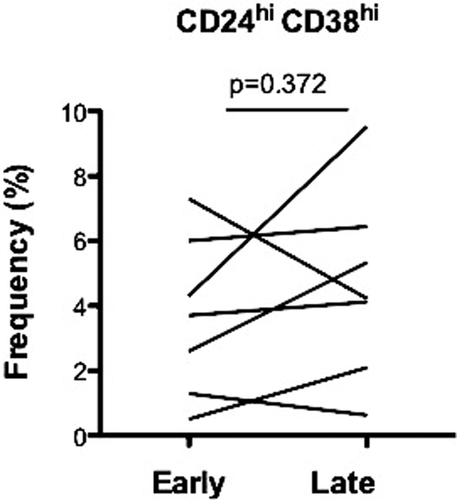
We performed a late B cell phenotype in seven patients treated with belatacept and in two patients treated with CNI. Late blood samples were collected 20.80 ± 0.79 months after the initial one. The proportion of naïve transitional B cells (CD24hiCD38hi) was similar in both time points (N = 7; p = 0.37) (Figure 4). In CNI patients, lower proportion of naïve transitional B cells was stable (N = 2; data not shown). B cell phenotype observed in stable KT recipients remained stable within almost 6 years posttransplant.
Belatacept patients show lower levels of BAFF and BAFF-R transcripts than CNI patients
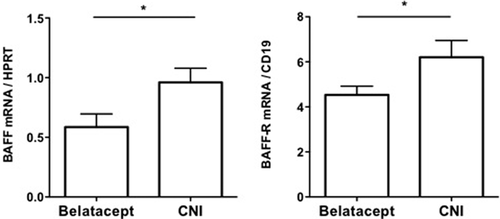
We next analyzed the transcript levels of molecules involved in inhibitory B cell profiles (CD32 isoforms and the B cell scaffold protein ankyrin repeats 1 [BANK1]) and in B cell survival and differentiation (the A proliferating inducing ligand [APRIL], the B cell activating factor [BAFF] and its receptors: BAFF receptor [BAFF-R], the transmembrane activator and CAML interactor [TACI] and the B cell maturation protein [BCMA]) in the PBMC from belatacept and CNI patients. The transcript levels of both CD32a and CD32b isoforms and BANK1 were comparable in both patient groups. APRIL, TACI and BCMA transcripts were also expressed at similar levels in both groups. By contrast, we observed a significant difference in the quantity of BAFF and BAFF-R transcripts. The belatacept group displayed significantly less BAFF and BAFF-R transcripts than the CNI group (0.59 [0.11] vs. 0.96 [0.12] and 4.53 [0.39] vs. 6.21 [0.74], respectively) (Figure 5).
Discussion
Here we report for the first time the analysis of peripheral B cell compartment in renal transplant patients treated with belatacept. The present study was prompted by the observation that KT patients enrolled in Phase III clinical studies have lower incidence of DSA upon belatacept (CTLA-4-Ig) treatment compared with CNI. Since a B cell signature has been described in operationally tolerant renal transplant recipients 4, 5, we analyzed whether B cell compartment was modified by belatacept. We showed that belatacept patients have a higher number of naïve and transitional B cells. Moreover, we showed that such B cell signature is maintained over time with same proportion of naïve transitional B cells.
In our study, patients from the belatacept, CNI and CR groups had similar count of both total lymphocytes and T cells. While both the frequency and absolute number of B cells were comparable between the belatacept and CNI groups, they were significantly lower by comparing these two groups with the CR group. These results contrast with previous findings showing a similar number and frequency of B cells in patients with stable renal function when compared to patients with chronic rejection. The only significant difference was the higher number of B cells in drug-free tolerant recipients 5, 19. Our results may be related to the limited number of analyzed patients, rather than to the immunosuppression strategy, which was not modified within the previous 12 months. However, our results reinforce the recent concept of the association between graft acceptance and the predominance of B cells with tolerogenic phenotype.
Our observation of increased number of naïve B cells in belatacept patients was described by other groups in long-term immunosuppressive drug-free tolerant KT patients 4 and in alemtuzumab-treated KT patients 20. In line with this result, immature B cells, namely the CD19+CD24hiCD38hi transitional B cells, was increased in belatacept patients compared to CNI patients. This B cell subset was shown as exhibiting regulatory capability through IL-10 release 21 and interestingly was also found in operational tolerant KT patients 4. The development of naïve and/or transitional B cells with regulatory functions has been hypothesized as being involved in graft tolerance induction and/or maintenance in such KT patients 4.
Since CD24hiCD38hi transitional B cells have regulatory capacity 21, we analyzed the transcript levels of molecules involved in regulatory B cell profiles and in B cell maturation and activation 19. Unlike operational tolerant KT patients, expression of BANK1 transcripts, which negatively regulates CD40-mediated AKT activation and which has been described as a leader gene in these patients, was not significantly different in both belatacept and CNI groups. Two isoforms of the low-affinity Fcγ receptor CD32 are expressed on B cell surface: while CD32b mediates an inhibitory signal upon co-ligation with the B cell receptor, CD32a, transduces an activating signal. Although decreased CD32a/CD32b ratio was described for tolerant patients, it was similar between both our groups. Interestingly, we observed a decrease in BAFF and BAFF-R transcripts in patients from the belatacept group when compared to patients treated with CNI. BAFF has emerged as a critical factor for B cell survival and differentiation. BAFF exerts these actions by binding to BAFF-R with a high affinity, and to TACI and BCMA with a low affinity. While BAFF is predominantly produced by innate immune cells, BAFF-R expression begins at the late transitional stage and is present on all mature B cells. BAFF-R signaling was demonstrated to determine transitional B cell maturation and the subsequent survival of mature B cells 22, 23. Our observation of reduced BAFF and BAFF-R transcripts are in accordance with our B cell phenotypic analysis and other studies reporting an association of high levels of BAFF with abnormal renal function and HLA immunization or a local humoral alloimmune response in kidney transplantation 24-26. Levels of BAFF transcripts were also increased in rejected allografts 27. In line with our results, elevated BAFF-R transcripts were also associated with long-term graft dysfunction, as described in a previous study measuring BAFF-R levels in a cohort of 143 kidney recipients with stable graft function at enrollment for more than 6 years 28.
The rationale for the use of belatacept in organ transplantation is primarily focused on its ability to block CD80/86–CD28 mediated T cell activation and its potential activity on other immune cells such as B cells was initially thought to be mainly mediated by an alteration of the T cell/B cell cooperation. Our results suggest that belatacept could also act directly on B cells. Mechanisms by which belatacept could influence B cell compartment remain however to be determined. First, belatacept could act directly on B cells through CD28–CD80/CD86 costimulation blockade. CD28 expression in B cells is repressed by the transcription factor Pax5 and de-repressed during differentiation to plasma cells. The role of CD28 in humoral immune responses has been predominantly attributed to helper T cell costimulation and germinal center formation in lymphoid organs 29, 30. An intrinsic B cell role for CD28 has been identified in controlling the differentiation or function of plasma cells beyond germinal center formation 11. Recently, it has been shown that sustained antibody responses depend on the function of CD28, which selectively supports the survival of bone marrow–resident nonproliferating plasma cells, the long-lived plasma cells 13. Second, belatacept could act indirectly through the induction of the tolerogenic HLA-G molecule. Indeed, it has been demonstrated that dendritic cells (DC) generated in vitro in presence of belatacept released soluble HLA-G (sHLA-G) in response to allostimulation; these belatacept-treated DC acting as tolerogenic antigen-presenting cells. In addition, renal transplant patients receiving belatacept exhibited increased levels of plasma HLA-G than those receiving CNI. This sHLA-G secretion by DC during an allogeneic challenge was thus suggested as an explanation to the better graft acceptance observed in belatacept treated renal transplant patients 31. Another study on a large cohort of renal transplant patients showed an inverse association between plasma sHLA-G and the presence of anti-HLA alloantibodies 32. Finally, we demonstrated that sHLA-G inhibits the proliferation and differentiation of B cells through binding to the ILT2 receptor expressed to their cell surface 33. Through these direct/indirect mechanisms, we hypothesize that the belatacept may affect the B cell maturation process and promote the maintenance of transitional B cells, which could help explain the weak anti-HLA alloimmunization.
In conclusion, present data showed that KT recipients treated with belatacept exhibited a B cell phenotype that shares similarity to that observed in operational tolerant patients, suggesting that costimulation blocker may, at least partially, create an environment that limits anti-donor-specific B cell alloimmune response. Whether the B cell pattern observed predicts further occurrence or not of DSA and antibody-mediated events remains to be established.
Acknowledgments
This study was supported by grants from the Bristol-Myers Squibb France and the Commissariat à l'Energie Atomique et aux Energies Alternatives.
Disclosure
The authors of this manuscript have no conflicts of interest as described by the American Journal of Transplantation.