Single Donor-Derived Strongyloidiasis in Three Solid Organ Transplant Recipients: Case Series and Review of the Literature
Abstract
Donor-derived Strongyloides stercoralis infections in transplant recipients are a rare but recognized complication. In this case series, we report donor-derived allograft transmission of Strongyloides in three solid organ transplant recipients. Following detection of infection in heart and kidney–pancreas recipients at two different transplant centers, a third recipient from the same donor was identified and diagnosed. S. stercoralis larvae were detected in duodenal aspirates, bronchial washings, cerebrospinal fluid, urine and stool specimens. Treatment with ivermectin and albendazole was successful in two of the three patients identified. The Centers for Disease Control and Prevention was contacted and performed an epidemiologic investigation. Donor serology was strongly positive for S. stercoralis antibodies on retrospective testing while all pretransplant recipient serum was negative. There should be a high index of suspicion for parasitic infection in transplant recipients and donors from endemic regions of the world. This case series underscores the need for expanded transplant screening protocols for Strongyloides. Positive serologic or stool tests should prompt early treatment or prophylaxis in donors and recipients as well as timely notification of organ procurement organizations and transplant centers.
Abbreviations
-
- AST
-
- American Society of Transplantation
-
- CDC
-
- Centers for Disease Control and Prevention
-
- CSF
-
- cerebrospinal fluid
-
- CVVH
-
- continuous veno-venous hemodialysis
-
- EGD
-
- esophagogastroduodenoscopy
-
- ELISA
-
- enzyme-linked immunosorbent assay
-
- HTLV-I
-
- human T cell lymphotrophic virus-1
-
- MMF
-
- mycophenolate mofetil
-
- O&P
-
- ova and parasite
-
- OPO
-
- organ procurement organization
-
- OPTN
-
- Organ Procurement and Transplantation Network
Introduction
An estimated 100 million cases of strongyloidiasis are reported worldwide; however, the true burden is unknown due to the parasite's ability to cause chronic asymptomatic infections and autoinfection 1. Immunocompromised patients are at high risk for hyperinfection and disseminated disease 2. Strongyloides infections have been previously reported in transplant patients. In most cases, dissemination of latent infection in the recipient following immunosuppression has been suspected. Although rare, donor-derived disease has been reported 3.
In July 2012, a 24-year-old Puerto Rico-born Hispanic man was hospitalized for multiple gunshot wounds. He required intubation, multiple vasopressors and packed red blood cell transfusion. He developed cerebral edema. Certification of brain death was performed. On hospital Day 10, his organs were procured and transplanted. He received 2 g of methylprednisolone and 1 g of methylprednisolone with cefazolin at 12 and 3 h prior to cross-clamp, respectively. He had no history of symptoms suggestive of Strongyloides infection but had traveled to Puerto Rico often.
Case 1
A 60-year-old Hispanic man with end-stage ischemic cardiomyopathy underwent orthotopic heart transplantation. He was born in the United States and lived in Puerto Rico as a teenager. Induction therapy consisted of methylprednisolone followed by maintenance immunosuppression with tacrolimus, mycophenolate mofetil (MMF) and prednisone. Posttransplant course was complicated by recurrent episodes of grade 3A(2R) rejection in the first month requiring two courses of methylprednisolone 1 g daily for 3 days and one course of prednisone 270 mg daily for 3 days.
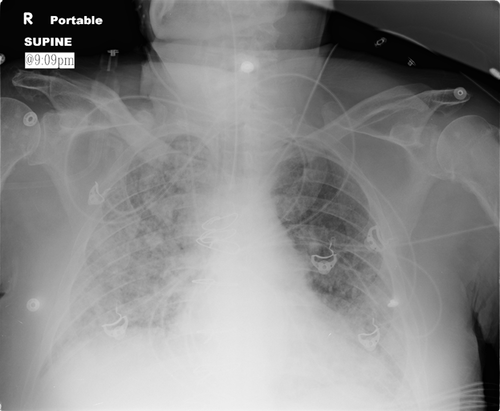
On posttransplant Day 48, he presented with fatigue, sore throat and hemoptysis. An endomyocardial biopsy demonstrated grade 1A rejection and multifocal myocyte necrosis. Echocardiography and right heart catheterization revealed normal allograft function and hemodynamics. Respiratory distress and hypotension developed shortly after catheterization requiring intubation and vasopressor support. Empiric vancomycin, piperacillin–tazobactam, fluconazole and oseltamivir were initiated. Chest radiograph revealed diffuse bilateral interstitial infiltrates (Figure 1). The organ procurement organization (OPO) was contacted about the clinical course of the other transplant recipients; however, no problems were reported at this time. Over the next 72 h, septic shock developed secondary to Enterobacter cloacae. Continuous veno-venous hemodialysis (CVVH) was initiated due to deteriorating renal function and persistent metabolic acidosis. On posttransplant Day 55, Strongyloides stercoralis larvae were found on bronchoalveolar lavage. Ivermectin and albendazole were initiated. Immunosuppression was maintained with a lower dose of MMF and a continuous infusion of tacrolimus. Three vasopressors, mechanical ventilation and CVVH were continued. Meropenem and daptomycin were started for bacteremia due to E. cloacae, Klebsiella pneumoniae and vancomycin-resistant enterococci. Lumbar puncture revealed Strongyloides in the cerebrospinal fluid (CSF). CSF cultures became positive for vancomycin-resistant enterococcus. Linezolid was added. On posttransplant Day 70, the transplant center was contacted by the OPO with the diagnosis of Strongyloides in the kidney–pancreas recipient. Care was withdrawn 7 days later. On autopsy, adult Strongyloides and rhabditiform and filariform larvae were found within a perirectal peritoneal parasitoma and intestinal mucosa; larval forms were found in the lung parenchyma, lymph nodes and cardiac allograft.
Case 2
Two months after travel to Louisiana and Florida, a 64-year-old US-born Caucasian man with end-stage renal disease secondary to type I diabetes mellitus underwent simultaneous pancreas and kidney transplant. Induction therapy consisted of antithymocyte globulin and 500 mg methylprednisolone followed by maintenance immunosuppression with tacrolimus, MMF and prednisone. Posttransplant course was complicated by small bowel obstruction requiring multiple surgeries and multidrug-resistant E. cloacae sepsis. Pseudo-aneurysm hemorrhage necessitated allograft pancreatectomy on posttransplant Day 33. Pathology showed fat necrosis and acute hemorrhage without evidence of Strongyloides larvae.
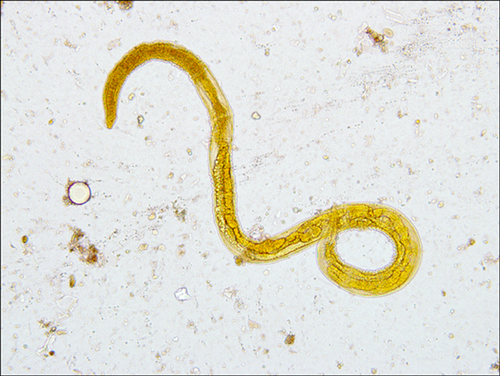
On posttransplant Day 66, a 2-week history of nausea, anorexia and abdominal fullness precipitated an esophagogastroduodenoscopy (EGD), which demonstrated diffusely ulcerated duodenal mucosa and adult Strongyloides on biopsy. The patient also had a maculopapular, nonpuritic rash on his trunk, which showed nonspecific porokeratosis on biopsy without evidence of parasitic infection. The patient was subsequently admitted and treated with ivermectin and albendazole. Immunosuppression was transitioned to cyclosporine; MMF and prednisone were discontinued. Empiric therapy with vancomycin, cefepime and metronidazole was started. Stool and urine ova and parasite (O&P) exams were positive for rhabditiform larvae (Figure 2) and cleared after 3 days of treatment. The patient was discharged on posttransplant Day 85 with good renal function. Albendazole and ivermectin were continued for 1 and 3 weeks, respectively.
Case 3
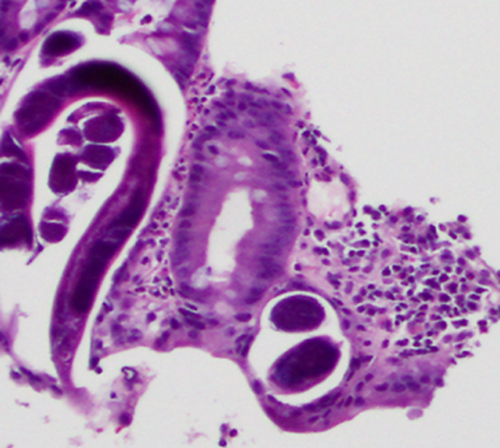
A 14-year-old US-born Caucasian boy with end-stage renal disease secondary to single dysplastic kidney underwent a preemptive renal transplant. He was a life-long resident of central West Virginia with no travel history. Induction therapy consisted of basiliximab and 360 mg methylprednisolone followed by maintenance immunosuppression with tacrolimus, MMF and prednisone. On posttransplant Day 72, the patient was admitted to another hospital because of fever, vomiting and diarrhea. He was started on cefepime. Knowledge of Strongyloides infection in the heart and kidney–pancreas recipients prompted patient transfer to the transplant center. His examination was notable for a diffuse petechial rash over the lower abdomen, buttocks and thighs. Renal function was stable. Duodenal aspirates and biopsies obtained by EGD revealed Strongyloides rhabditiform larvae (Figure 3). On posttransplant Day 74, he was started on ivermectin and albendazole; cefepime was continued. Immunosuppression was transitioned to cyclosporine; MMF and prednisone were discontinued. Stool O&P exam was positive for Strongyloides while urine was negative. The gastrointestinal symptoms and rash resolved. Stool O&P became negative after 3 days of treatment. On posttransplant Day 79, he was discharged to home with good renal function. Albendazole and ivermectin were continued for 2 and 4 weeks, respectively.
On posttransplant Day 114, the patient was diagnosed with acute T cell–mediated rejection. He was treated with three doses of methylprednisolone at 10 mg/kg followed by a prednisone taper. Empiric ivermectin treatment was restarted and continued for 2 weeks following completion of the prednisone taper.
The fourth organ recipient underwent orthotopic liver transplant and expired posttransplant Day 4 of undetermined causes. No evidence of Strongyloides was found on autopsy.
Because reactivation was suspected in the heart and kidney–pancreas recipients due to travel history, strongyloidiasis was not immediately reported to the OPO. No additional testing was completed to differentiate between reactivation and donor transmission. Prompted by the kidney recipient's symptoms, the transplant coordinator contacted the OPO 5 days after the kidney–pancreas recipient's Strongyloides diagnosis to report infection and inquire about the other recipients. Two days later, the Organ Procurement and Transplantation Network (OPTN) notified the Centers for Disease Control and Prevention (CDC) of strongyloidiasis in the three transplant recipients. Serology testing was performed on stored recipient and donor serum. All recipients were negative; however, the donor tested positive at 13.68 U/µL (positive >1.7 U/µL) (Table 1). In addition, the donor had no eosinophilia and tested negative for human T cell lymphotrophic virus-1 (HTLV-1).
| Patient | Specimen | Date of collection | From | Sent | Testing completed | Strongyloides IgG ELISA1 |
|---|---|---|---|---|---|---|
| Donor | Serum | July 21, 2012 | OPO | October 9, 2012 | October 17, 2012 | Positive (13.68) |
| Heart recipient | Serum (pretransplant) | June 23, 2012 | TC 1 | October 11, 2012 | October 17, 2012 | Negative (0.26) |
| Serum (posttransplant) | September 15, 2012 | TC 1 | October 11, 2012 | October 17, 2012 | Negative (0.00) | |
| Liver recipient | Serum (pretransplant) | July 9, 2012 | TC 1 | October 11, 2012 | October 17, 2012 | Negative (0.52) |
| Kidney–pancreas recipient | Serum (pretransplant) | July 22, 2012 | TC 2 | October 10, 2012 | October 17, 2012 | Negative (0.21) |
| Kidney recipient | Serum (pretransplant) | July 21, 2012 | TC 2 | October 10, 2012 | October 17, 2012 | Negative (0.06) |
- ELISA, enzyme-linked immunosorbent assay; OPO, organ procurement organization; TC, transplant center.
- 1 Reactions >1.7 U/µL should be considered positive.
Discussion
These cases represent probable transmission of Strongyloides infection from a common donor. Pretransplant Strongyloides serology results imply that the donor had chronic Strongyloides infection, which reactivated and became disseminated prior to transplantation of his organs. Guidelines from the American Society of Transplantation (AST) recommend screening for Strongyloides in at-risk recipients, living donors and, where feasible, deceased donors 4. No recipients in this series were screened or received prophylaxis pretransplant despite history suggesting potential exposure.
OPTN policy 4.5 requires reporting of potential disease transmissions within 24 h of any substantial concern for donor derivation. Early diagnosis and treatment may have mitigated morbidity and mortality in these cases. Our series demonstrates the need for timely communication between transplant centers and OPOs as there was a significant delay in diagnosis between cases 5, 6. Furthermore, a high index of suspicion of potential donor-transmitted disease is essential.
Donor-derived strongyloidiasis in transplant recipients
Donor-derived Strongyloides infection is rare, but has been reported (Table 2). In the case reports reviewed, seven patients were successfully treated while five succumbed to Strongyloides or related complications. Donor-derived infection was confirmed by serologic testing in only six reported cases 7-14. Symptoms generally present within 6 weeks, but have been reported up to 9 months posttransplantation.
| Case | Patient | Allograft | Time of onset | Demographic risk factor | Presenting symptoms | Transplant immunosuppression | Treatment |
|---|---|---|---|---|---|---|---|
| Le et al (2014) | 59-year-old male | Heart | 51 days | Donor from Puerto Rico | Respiratory distress | Induction: methylprednisolone | Ivermectin 200 mcg/kg per day and albendazole 400 mg BID |
| Maintenance: tacrolimus, MMF, prednisone | |||||||
| 64-year-old male | Kidney/pancreas | 64 days | Donor from Puerto Rico | Rash, nausea, vomiting, anorexia | Induction: antithymocyte globulin methylprednisolone | Ivermectin 200 mcg/kg per day and albendazole 400 mg BID for a month | |
| Maintenance: tacrolimus, MMF, prednisone | PPX with biweekly pulse ivermectin | ||||||
| 14-year-old male | Kidney | 72 days | Donor from Puerto Rico | Rash, fever, nausea, vomiting | Induction: basiliximab and methylprednisolone | Ivermectin 200 mcg/kg per day and albendazole 400 mg BID for a month | |
| Maintenance: tacrolimus, MMF, prednisone | PPX with biweekly pulse ivermectin | ||||||
| Hamilton et al 7 | 39-year-old female | Kidney | 10 weeks | Donor from Dominican Republic | Rash, nausea, vomiting, diarrhea, abdominal pain | Induction: antithymocyte globulin | Ivermectin 200 mcg/kg per day SQ q 48 h for 11 days |
| Maintenance: tacrolimus, MMF, prednisone | Ivermectin 200 mcg/kg per day for 14 days | ||||||
| 53-year-old female | Kidney | # | Donor from Dominican Republic | Epigastric pain, gastric ulcers, hematemesis, right lobe lung infiltrate | Induction: antithymocyte globulin | Ivermectin 200 mcg/kg per day for 2 days then weekly for five doses | |
| Maintenance: tacrolimus, MMF, prednisone | Ivermectin 200 mcg/kg per day for 7 days given 26 days later | ||||||
| 58-year-old male | Liver | # | # | # | Induction: basiliximab | Ivermectin 200 mcg/kg per day for 2 days and albendazole 400 mg daily for 7 days | |
| Maintenance: tacrolimus, MMF, prednisone | |||||||
| Weiser et al 3 | 68-year-old male | Kidney | 3 months | Donor from Honduras | Periumbilical purpura, fever, nausea, vomiting, lower lobe consolidations | # | Albendazole 400 mg BID PR for 5 days |
| Day 6: albendazole 400 mg BID PR and PO as well as ivermectin 18 mg daily | |||||||
| Day 9: ivermectin 18 mg SQ q 48 h | |||||||
| Brügemann et al 2 | 36-year-old male | Heart | 43 days | Donor from Suriname | Abdominal pain, nausea, vomiting, purpuric skin rash | No induction | Albendazole 400 mg BID for 10 days |
| Maintenance: tacrolimus, MMF, prednisone taper | At 16 weeks: ivermectin 15 mg for one dose | ||||||
| At 22 weeks: 5-day prednisone taper | |||||||
| At 23 weeks: methylprednisolone 1 g/day for 3 days | |||||||
| # | Liver | 4 months | Donor from Suriname | Eosinophilia | # | Ivermectin for 4 days | |
| Second course of ivermectin 6 months later | |||||||
| # | Kidney | # | Donor from Suriname | Died 6 months postop of E. coli sepsis | # | # | |
| Rodriguez-Hernandez et al 10 | 67-year-old male | Liver | 2.5 months | Donor from Ecuador | Asthenia, anorexia, diarrhea, purpuric rash, dyspnea, cough, fever, right lobe lung consolidation | Maintenance: tacrolimus, MMF, prednisone | Albendazole 400 mg BID and ivermectin 200 mcg/kg per day for 1 week then albendazole 400 mg BID and ivermectin 200 mcg/kg per day three times a week for 2 weeks |
| PPX with biweekly pulse ivermectin | |||||||
| # | Kidney/pancreas | # | Donor from Ecuador | Present in GI tract | # | # | |
| # | Kidney | # | Donor from Ecuador | No disease | # | # | |
| # | Lungs | # | Donor from Ecuador | No disease | # | # | |
| Huston et al 9 | 61-year-old female | Kidney | 90 days | Donor from Puerto Rico | Fever, acute kidney injury, headache, dizziness, tremors, acute respiratory distress syndrome | # | Ivermectin PO and PR with albendazole |
| Trial of veterinary ivermectin for three doses | |||||||
| Patel et al 10 | 62-year-old female | Intestine | 9 months | Donor from Honduras | Nausea, vomiting, abdominal pain, constipation, fevers, headaches, photophobia, bilateral interstitial infiltrates | Induction: basiliximab | Ivermectin 200 mcg/kg per day and thiabendazole 25 mg/kg BID with ivermectin 15 mg enemas daily |
| Maintenance: tacrolimus, MMF, corticosteroids | |||||||
| Said et al 11 | 52-year-old male | Kidney | 48 days | Donor from South Asia | # | Induction: antithymocyte globulin | Albendazole and ivermectin orally and rectally |
| Maintenance: tacrolimus, MMF, prednisone | |||||||
| 43-year-old female | Kidney | 90 days | Donor from South Asia | # | Induction: antithymocyte globulin | Albendazole and ivermectin orally and rectally | |
| Maintenance: tacrolimus, MMF, prednisone | |||||||
| 43-year-old female | Kidney | 92 days | Donor from South Asia | # | Induction: antithymocyte globulin | Albendazole and ivermectin orally and rectally | |
| Maintenance: tacrolimus, MMF, prednisone | |||||||
| Ben-Youssef et al 12 | 41-year-old male | Pancreas | 5 weeks | Donor immigrant to US | Fever, chills, dyspnea, vomiting, diarrhea, epigastric pain, hematuria | Induction: antithymocyte globulin | Thiobendazole 50 mg/kg per day and ivermectin 200 mcg/kg per day for 7 days |
| Maintenance: tacrolimus, MMF, prednisone | |||||||
| Hoy et al 14 | 52-year-old female | Kidney | 33 days | # | # | # | # |
| 10-year-old female | Kidney | 64 days | # | Cough, hematuria, fever | # | # |
- #, data not reported in original publication; BID, bis in die (twice a day); GI, gastrointestinal; kg, kilograms; mcg, micrograms; mg, milligrams; MMF, mycophenolate mofetil; PO, per os (by mouth); PPX, prophylaxis; PR, per rectum; q, every; SQ, subcutaneous.
S. stercoralis has a life cycle consisting of both parasitic and free-living phases. After infecting the host, filariform larvae enter the circulatory system, are transported to the lungs and infiltrate the alveoli 1, 15.They ascend the tracheobronchial tree, are swallowed and migrate to the small intestine where they become adult female worms and invade the mucosa 1, 15. Via parthenogenesis they produce eggs, which yield rhabditiform larvae 16, 17. These larvae migrate to the lumen of the intestine and are excreted in feces. They can also travel to the large intestine and become filariform larvae 1, 15. This process, called autoinfection, almost invariably leads to chronic infection 17. An amplified cycle of autoinfection, termed hyperinfection, occurs in immunocompromised hosts. This can lead to disseminated infection in which the parasite is found in remote sites including the skin, cardiovascular and central nervous systems 18, 19. Hyperinfection and dissemination carry significant morbidity and mortality with fatality rates reaching 85% 5, 6, 19. Hyperinfection and dissemination have been reported in patients with diabetes, hematologic malignancies, malnutrition, hypogammaglobulinemia and with the use of immunosuppressive drugs in autoimmune disease and organ transplantation 1, 7, 19. Patients with HTLV-1 are also at increased risk of Strongyloides infections because the virus increases interferon-gamma production and decreases IL-1, IL-3, IL-5 and IgE, which play an important role in parasitic defense 19.
Donor and recipient screening
The AST guideline recommends screening by serum IgG enzyme-linked immunosorbent assay (ELISA) 4, 6, 19. The ELISA has a sensitivity of 90%, specificity of 99% and positive and negative predictive values of 97% and 95%, respectively 17. Immunocompromised patients, such as chronic renal failure patients on dialysis, may be among the 5–10% with false-negative or indeterminate serology tests. False-positives can occur in the presence of other parasitic infections 20.The diagnostic gold standard, stool exam for O&P, is only reliable during larval shedding; however, sensitivity approaches 100% when it is performed in triplicate 18. Our case series exemplifies the importance of more rigorous Strongyloides screening in recipients and donors who have traveled to endemic areas including the Caribbean, Mexico, South and Central America, Africa, Southeast Asia and temperate regions such as southern and eastern Europe, the United Kingdom and southeastern United States 4. Autoinfection produces long-term chronic infections that can be reactivated at any time even with remote and limited exposure. Positive serology should prompt treatment of but not exclude potential donors and recipients.
Treatment and prophylaxis
Treatment options for Strongyloides infection include ivermectin, thiabendazole and albendazole. Ivermectin is preferred in transplant patients as it is well tolerated and does not interact with immunosuppressant drugs 19. Table 2 lists the treatments used in previously reported cases of donor-derived strongyloidiasis 7-14. While most reports describe parasite clearance within 21 days, clearance may take 30 days or longer with large parasite burden. Our surviving patients were maintained on a regimen of pulsed dosed ivermectin consisting of two doses every 2 weeks for 3 months. Stool exams for O&P have remained negative.
Implications for management
This case series raises many questions concerning our understanding of the parasitology of Strongyloides and the management of strongyloidiasis in transplant patients. Currently, there is no consensus on treatment regimen. Treatment decisions should be made on a case-by-case basis tailoring the length of therapy to clearance of parasites from endoscopic or stool specimens. When allograft rejection is diagnosed in a transplant patient previously treated for strongyloidiasis, the degree of increased immunosuppression must be balanced with the risk of reactivation. In our patient, starting empiric ivermectin with standard rejection therapy did not result in reactivation. Further studies are needed to understand the appropriate strategy. We recognize that retransplantation is a significant possibility for our surviving patients. The choice of induction and maintenance immunosuppression therapy as well as the timing and length of Strongyloides prophylaxis will require careful study.
Conclusions
This case series of suspected donor-derived strongyloidiasis, once thought to be a rare phenomenon, draws attention to diligent screening in all at-risk recipients and donors. Further research on treatment regimens for immunocompromised hosts is also needed to maintain the safety of transplant recipients. Timely notification of OPOs and transplant centers as well as high index of suspicion of possible donor-derived infections is paramount.
Acknowledgments
The findings and conclusions are those of the authors and do not necessarily represent the official position of the CDC. We would like to thank the following individuals: Harold Harrison, MD, from Microbiology Division of Geisinger Medical Center; Zong Ming Chen, MD, PhD, from Pathology Division of Geisinger Medical Center; Ashokkumar Jain, MD, and Elaine Lander, NP, from the Temple University Transplant Center; Christine McGarry and Richard Hasz from Gift of Life; Michael Foltzer, MD, Robert Gotoff, MD and Carlos Jaramillo, MD, from the Infectious Disease Division at Geisinger Medical Center; Jeremy Scott, Pharm D, from the Pharmacy Department at Geisinger Medical Center and Isabel McAuliffe and Elizabeth Bosserman, MPH, from CDC Parasitic Diseases Branch.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.