Overview of the Landscape of HIV Prevention
Abstract
In this introductory essay on the landscape of HIV prevention, my intent is to provide context for the subsequent topics discussed at the Symposium on Hormone Regulation of the Mucosal Environment in the female reproductive tract (FRT) and the Prevention of HIV infection: FRT immunity, mucosal microenvironment and HIV prevention, and the risk and impact of hormonal contraceptives on HIV transmission.
Introduction
In this introductory essay on the landscape of HIV prevention, my intent is to provide context for the subsequent topics discussed at the Symposium on Hormone Regulation of the Mucosal Environment in the female reproductive tract (FRT) and the Prevention of HIV infection: FRT immunity, mucosal microenvironment and HIV prevention, and the risk and impact of hormonal contraceptives on HIV transmission.
High-risk populations of women and the pandemic's progress
Let us begin with the magnitude of the problem and the focus on transmission to women. In 2012, there were just over 35 million adults and children living with HIV infection, 2.3 million new infections, and 1.6 million people who succumbed to infection.1 Sub-Saharan Africa is still home for more than two-thirds of those living with and dying from infection and the center of newly acquired infections that particularly afflict young women in the region.2, 3 Indeed, not only can we not treat our way out of the pandemic, we must also prevent transmission to those most at risk if we are to stem the pandemic's progress.
Pathogenesis of transmission in the SIV-monkey model and Interventions
Preventing transmission to women was of course the overarching goal of the research and topic that participants in the Symposium presented and discussed. As a general introduction and context for those presentations and discussions, I provide first a framework on the intersections between pathogenesis studies and prevention interventions that inform the design of those interventions, based on the understanding of the ‘when, where, and how’ of acquisition.
This is a monkey ‘tale’, so-to-speak, because the monkey models of HIV-1 transmission to women provide a window through which we can view the earliest and critical events in transmission4 that would otherwise be in a black box (Fig. 1a). In this black box, following exposure to virus, HIV or SIV must cross the mucosal barrier, establish a founder population of infected cells at the portal of entry, and subsequently seed the draining lymph nodes (LNs) with sufficient virus and infected cells to establish and amplify infection there. Subsequent hematogenous dissemination then establishes systemic infection, mainly throughout the lymphoid tissues (Fig. 1b). This is the first time when HIV-1 infection might be manifest in an infected woman, and hence the need to investigate particularly the antecedent events at the portal of entry in the highly relevant nonhuman primate (NHP) model.
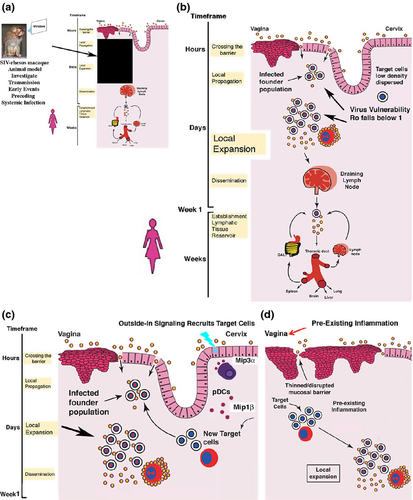
Studies in the NHP animal model have indeed revealed some early opportunities for prevention interventions to be successful. First, the infected founder populations are small, at least in part because of the difficulty in gaining access to a low density and dispersed population of susceptible target cells (mainly CD4+ T cells) in the submucosa, and thereafter maintaining infection at a basic reproductive rate (Ro) ≥1 (Fig. 1c). Microbicides, antibodies, and resident or rapid responder virus-specific CD8 T cells thus will all be working with favorable odds altogether to prevent establishment of these founder populations or constrain local expansion to bring Ro below 1.
Mucosal microenvironment and transmission
The mucosal environment will clearly influence the success of interventions based on access to and availability of the target cells. In the cervix, for example, successive vaginal exposures to a high-dose SIV inoculum elicit outside-in signaling that recruits target cells to expand infection locally and thereby facilitate transmission (Fig. 1c). The protective effects of glycerol monolaurate (GML) may have been mediated by modulating this MIP3-α/β-chemokine recruitment system.5 The mucosal epithelium under hormonal influences produces chemokines6 that recruit target cells as well, and in the vagina, pre-existing inflammation will thin or denude the normally multilayered squamous epithelial barrier as well as increase the supply of target cells in the submucosa to facilitate transmission via increased access to and availability of target cells (Fig. 1d).
This framework clearly predicts that inflammation and hormonal changes in the mucosal microenvironment will influence transmission through access, target cell availability, and the effectiveness of innate and adaptive immune defenses. Genital tract inflammation and associated changes in the cytokine milieu will predictably not only increase susceptibility to HIV infection through increased access and target cell availability,8-11 but, moreover, correlate with diminished effectiveness of a tenofovir microbicide gel in preventing HIV transmission.12
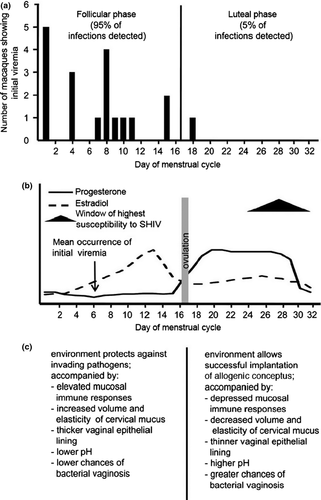
Progesterone profoundly alters transmission by several mechanisms. These include thinning of the vaginal epithelium, which enhanced transmission more than sevenfold in the SIV-pigtail macaque NHP model, and, moreover, accelerated progression to simian AIDS in infected animals with progesterone implants.13 The late luteal phase of the menstrual cycle is also the time of maximum susceptibility to vaginal transmission of SHIV, which again correlated with progesterone effects on the thickness of the vaginal mucosal barrier but also other changes in the mucosal microenvironment.14-16
These include (Fig. 2) decreased volume and elasticity of cervical mucus, higher pH, and the depressed mucosal immune responses in preparation for implantation of the conceptus. Given these effects of high progesterone levels on transmission, it is thus perhaps not too surprising that injectable hormonal contraceptives particularly have been reported to increase HIV acquisition by twofold.17
Translating understanding into design principles for preventive interventions
- Prevent, eliminate, or constrain the establishment and expansion of infected founder populations at the portal of entry. Thus, microbicides that provide sustained levels of antiretrovirals (ARVs) at the portal of entry and vaccines that provide high concentrations of antibodies and virus-specific CTLs with favorable ratios over the initially small numbers of infected target cells will satisfy this principle.
- Modulate the counter-productive effects of inflammation and innate immunity on access to and availability of target cells, for example, microbicide that combines ARVs with an agent such as GML.
- Reduce transmission-promoting hormonal effects such as those described for progesterone, for example, developing non hormonal or low concentration hormonal contraceptives.




