Comparative ampelographic and genetic analysis of grapevine cultivars from Algeria and Morocco
Abstract
Background and Aims
North Africa has a long history of viticulture and a wide diversity of grape cultivars. Ampelographic studies have been made of grapevine cultivars grown all over the world, but only a few describe those of Algeria and Morocco. Many Maghrebi cultivars held in germplasm banks or found growing wild in this region have recently been subjected to microsatellite profiling by different researchers, though little comparative analysis has been undertaken. The aim of the present work was to clarify the identity of the grapevine cultivars growing in the Maghreb via ampelographic and single-nucleotide polymorphism analysis.
Methods and Results
Seventy-one accessions were studied through the ampelographic construction of their mean leaves, via genotypic analysis using single-nucleotide polymorphism markers, and the comparison of these results with previously reported single sequence repeat marker profiles and ampelographic data for other grapevine material from the Maghreb.
Conclusion
New synonyms and homonyms were detected between Maghrebi cultivars. Some misinterpretations and errors of identification made during the making of the studied germplasm collections were identified.
Significance of the Study
This study helps clarify the confusion over the identity of Algerian and Moroccan grapevine cultivars and provides a general picture of grapevine diversity in the Maghreb.
Introduction
North Africa has a long history of viticulture and a great diversity of grape cultivars. In fact, the north of Algeria, Morocco and Tunisia are within the area of distribution of the original wild species Vitis vinifera L. ssp. sylvestris (Gmelin) Hegi from which present day cultivated grapes (Vitis vinifera L. spp. sativa) were domesticated (This et al. 2006). Cultivated vines were brought into the region by Phoenicians and Carthaginians (Isnard 1951, McGovern 2004) and these likely hybridised among themselves and with wild forms over centuries of viticultural history. Viticulture became consolidated in Northern Africa under Roman influence (McGovern 2004, This et al. 2006). Centuries later, Islam enhanced the expansion of table rather than winemaking cultivars (Isnard 1951, Aldebert and Orsat 1959), bringing new cultivars from Eastern regions (Fregoni 1991). More recently, European influence in the region promoted grapevine cultivation in Algeria, with colonists making their own wine (Föex 1891, Johnson 1990). The arrival of phylloxera in Europe at the end of the 19th century, however, led to a great increase in the area cultivated to the grapevine in Algeria, with French vine growers planting large vineyards. Many of the cultivars planted were those grown at that time in France (Föex 1891), while others were brought from Spain (Isnard 1951, Levadoux et al. 1971). Today, information held by the Organisation Internationale de la Vigne et du Vin (OIV) (http://www.oiv.int/) Internationale de la Vigne et du Vin 2009) for the period 1986–2007 shows that Algeria and Morocco were responsible for a mean 0.42% and 0.41%, respectively, of the world's entire grape production. In Algeria, 59.41% of this production was destined for table consumption, 40.47% for winemaking and 0.10% for raisin production. In Morocco, these figures were 76.40, 23.24 and 0.36%, respectively.
Many ampelographic studies have been made of grapevine cultivars from all over the world, but only a few have described those of Algeria and Morocco (Föex 1891, Isnard 1951, Vidal 1951, Levadoux et al. 1971), the most recent being a collection of the descriptions made by Galet since 1952 under the title Dictionaire Encyclopédique des Cépages (Galet 2000). In recent years, however, several molecular studies on North African cultivars have been made, identifying the accessions held in germplasm banks through the study of DNA polymorphisms. As a consequence, many of the Maghrebi cultivars have now been profiled by nuclear and chloroplast microsatellite [simple sequence repeats (SSR)] analysis (El Oualkadi et al. 2009, 2011, Laiadi et al. 2009, Riahi et al. 2010, Zinelabidine et al. 2010). Unfortunately, these studies were published over a short period of time that made it difficult for their authors to compare their results. Thus, much confusion regarding the true number of cultivars continues to exist, the consequence of the existence of synonyms, homonyms, errors of identification and the difficulty in transcribing Arab words into the Latin alphabet.
Recently, single-nucleotide polymorphisms (SNPs – another type of molecular marker) have been used to identify grapevine cultivars, to construct genetic maps and to perform parentage analysis (Cabezas et al. 2011, Ibanez et al. 2012, Zinelabidine et al. 2012). In the genome, SNPs are more abundant than SSRs, and further, they have the advantage of being bi-allelic, co-dominant, robust and of allowing for high-throughput genotyping and automation. Importantly, their allele binning is easy (bi-allelic and based on nucleotide sequence instead of amplicon length), which makes these markers particularly suitable for comparing the data obtained by different laboratories.
The aim of the present work was to clarify the identity of the grapevine cultivars growing in the Maghreb via ampelographic and SNP analysis, and to compare the results obtained with previously published SSR marker profiles and ampelographic data. This information was used to detect synonyms, homonyms and possible errors of identification in previous reports.
Materials and methods
Plant material
The Algerian material used in this study included 34 accessions (two plants per accession) (codes A1–A34; Table 1) of the germplasm collection belonging to the M'zej Edchiche Institut Téchnique d'Arboriculture Fruitière (ITAF), (Ministère de l'Agriculture), Skikda (northeastern Algeria). The Moroccan material included 37 accessions (two plants per accession) and wild plants (codes M1–M37; Table 1); these were either maintained as part of the grapevine collection of the Société du Développement Agricole (SODEA) de Meknés (n = 19), found growing in vineyards in the Demnate region (n = 11), or found growing spontaneously in non-cultivated areas in Oum-er-Rbia, Ouaoumana and El Ksiba (n = 7). Different leaf samples were taken from the same plants for the ampelographic and molecular analysis.
| Accession name | Code | Berry colour | Origin‡ | Country | SNP analysis |
|---|---|---|---|---|---|
| Aberkane | A1 | B† | ITAF | Algeria | Yes |
| Adadi | A2 | G | ITAF | Algeria | Yes |
| Adari des Bibans | A3 | G | ITAF | Algeria | Yes |
| Ahchichene | A4 | G | ITAF | Algeria | Yes |
| Ahmar de Mascara | A5 | R | ITAF | Algeria | Yes |
| Ahmar Mechtras2 | A6 | P | ITAF | Algeria | Yes |
| Ahmar Mechtras3 | A7 | P | ITAF | Algeria | Yes |
| Ahmed dra el Mizen | A8 | G | ITAF | Algeria | Yes |
| Aïn el Couma | A9 | G | ITAF | Algeria | Yes |
| Aïn el Kelb | A10 | G | ITAF | Algeria | Yes |
| Amellal | A11 | G | ITAF | Algeria | Yes |
| Amokrane | A12 | G | ITAF | Algeria | Yes |
| Aneb el Cadi | A13 | G | ITAF | Algeria | Yes |
| Baladi | A14 | G | ITAF | Algeria | Yes |
| Bezzoul el Khadem | A15 | B | ITAF | Algeria | Yes |
| Boghni | A16 | G | ITAF | Algeria | Yes |
| Bouaber des Aures | A17 | B | ITAF | Algeria | Yes |
| Bouni | A18 | G | ITAF | Algeria | Yes |
| Cherchelli | A19 | G | ITAF | Algeria | Yes |
| Farana Blanc | A20 | G | ITAF | Algeria | Yes |
| Farana de Mascara | A21 | G | ITAF | Algeria | Yes |
| Farana Noir | A22 | B | ITAF | Algeria | Yes |
| Ghanez | A23 | G | ITAF | Algeria | Yes |
| Kabyl Aldebert | A24 | B | ITAF | Algeria | Yes |
| Lakhzine | A25 | G | ITAF | Algeria | Yes |
| Lekhdari | A26 | G | ITAF | Algeria | Yes |
| Louali | A27 | G | ITAF | Algeria | Yes |
| Muscat de Berkain | A28 | G | ITAF | Algeria | Yes |
| Muscat de Fandouk1 | A29 | G | ITAF | Algeria | Yes |
| Muscat el Adda | A30 | B | ITAF | Algeria | Yes |
| Sbaa Tolba | A31 | G | ITAF | Algeria | Yes |
| Sultanine Fandouk | A32 | G | ITAF | Algeria | Yes |
| Tadelith | A33 | B | ITAF | Algeria | Yes |
| Tizi Ouinine | A34 | G | ITAF | Algeria | Yes |
| Abbou | M1 | B | SODEA | Morocco | Yes |
| Abbouhou | M2 | B | Demnate | Morocco | Yes |
| Aguyar | M3 | B | Demnate | Morocco | Yes |
| Arbia | M4 | B | SODEA | Morocco | Yes |
| Azizi El Hor | M5 | G | SODEA | Morocco | No |
| Bezoul el Aouda | M6 | B | SODEA | Morocco | Yes |
| Bouchouka blanc | M7 | G | Demnate | Morocco | Yes |
| Boujlida | M8 | G | SODEA | Morocco | Yes |
| Boukhanzir | M9 | G | SODEA | Morocco | No |
| Bouqseb | M10 | G | SODEA | Morocco | Yes |
| Bouzouga | M11 | B | SODEA | Morocco | Yes |
| Carignan | M12 | B | SODEA | Morocco | Yes |
| Djinani | M13 | G | SODEA | Morocco | Yes |
| El Biod | M14 | G | SODEA | Morocco | Yes |
| El Farryali | M15 | B | Demnate | Morocco | No |
| El Karim | M16 | G | SODEA | Morocco | Yes |
| El-Ksiba1 | M17 | Nd | Wild (El-Ksiba) | Morocco | No |
| El-Ksiba2 | M18 | Nd | Wild (El-Ksiba) | Morocco | No |
| Farana | M19 | G | SODEA | Morocco | Yes |
| Laadari | M20 | G | Demnate | Morocco | No |
| Laaderi | M21 | B | SODEA | Morocco | Yes |
| Maccabeo | M22 | G | SODEA | Morocco | Yes |
| Muscat Doukkala | M23 | G | SODEA | Morocco | Yes |
| Muscat Sefrou | M24 | G | SODEA | Morocco | Yes |
| Oualgdid | M25 | B | Demnate | Morocco | Yes |
| Ouaoumana1 | M26 | Nd | Wild (Ouaoumana) | Morocco | No |
| Ouaoumana2 | M27 | Nd | Wild (Ouaoumana) | Morocco | No |
| Oum-er-Rbia1 | M28 | Nd | Wild (Oum-er-Rbia) | Morocco | No |
| Oum-er-Rbia3 | M29 | Nd | Wild (Oum-er-Rbia) | Morocco | No |
| Oum-er-Rbia4 | M30 | Nd | Wild (Oum-er-Rbia) | Morocco | No |
| Rarjij | M31 | B | Demnate | Morocco | No |
| Sbaa Lebnat | M32 | G | SODEA | Morocco | Yes |
| Sidi Taybi | M33 | B | SODEA | Morocco | Yes |
| Tagulayt | M34 | G | Demnate | Morocco | Yes |
| Tanakert | M35 | B | Demnate | Morocco | Yes |
| Tinouine | M36 | B | Demnate | Morocco | Yes |
| Tyliote | M37 | G | Demnate | Morocco | No |
- †Berry colour: B, black, P, pink; R, red; G, green; Nd, not determined. ‡Origin: ITAF, Germplasm collection of the M'zej Edchiche Institut Téchnique d'Arboriculture Fruitière (Skikda, Algeria); SODEA, Germplasm collection of the Société du Développement Agricole (Meknés, Morocco).
Ampelographic characterisation
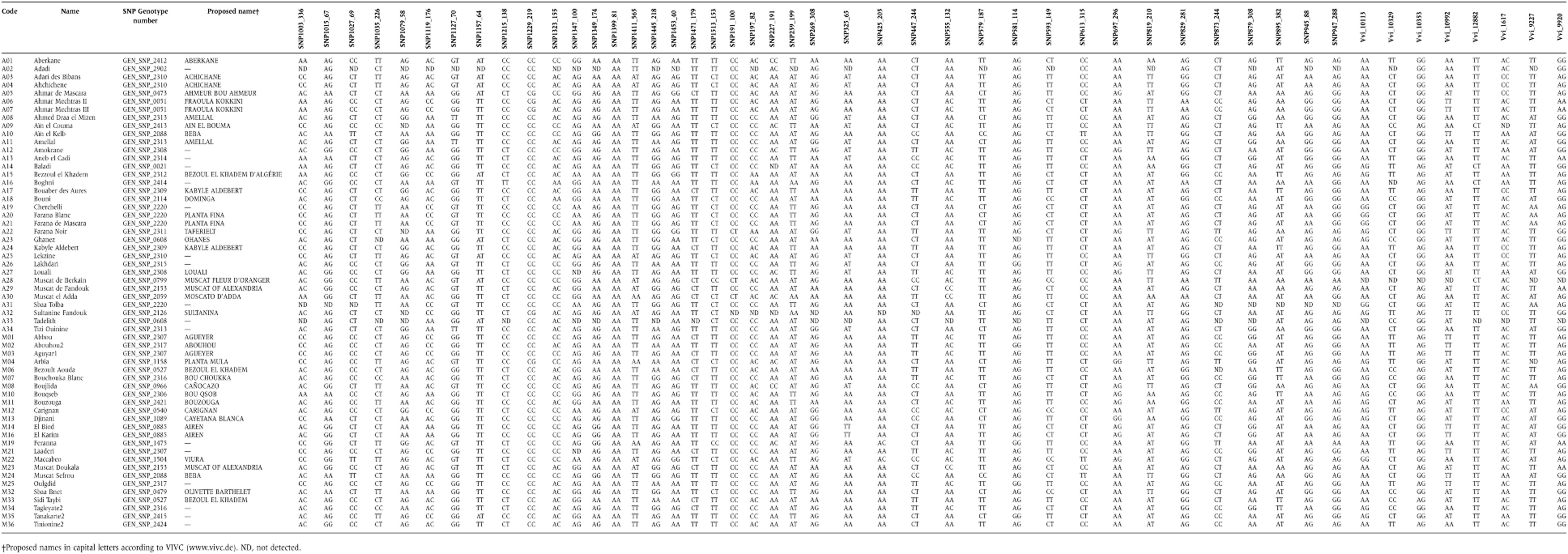
After blooming, 10–11 fully expanded leaves from nodes eight to nine of green shoots were collected from each accession. Measurement of the variables required for reconstructing an average leaf for each plant was taken following the method of Martinez and Grenan (1999) (Figure 1a). Image analysis of digital photographs was performed with analySIS 3.0 software (Soft Imaging System GmbH, Münster, Germany). The number of teeth between the major veins was also recorded following the proposal of the same authors (Figure 1b).
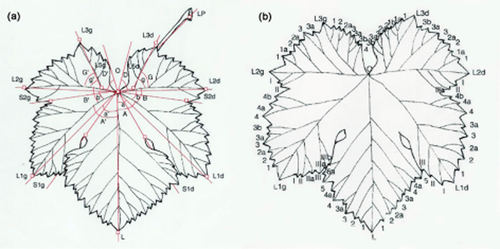
Construction of mean leaves (based on the method of Martinez and Grenan 1999): (a) lengths and angles measured in real leaves; (b) number of teeth in a real leaf.
The variables measured (lengths and angles) were used to calculate the following relationships: Rel.1:L1d/L; Rel.2:L1g/L; Rel.3:A + B + G; Rel.4:A' + B' + G'; Rel.5:a + b + g; Rel.6:a' + b' + g'; Rel.7:(S1d + S2d)/(L1d + L2d); Rel.8:(S1g + S2g)/(L1g + L2g); Rel.9:S1d/L1d; Rel.10:S1g/L1g; Rel.11:S2d/L2d; Rel.12:S2g/L2g (Martinez and Grenan 1999).
DNA analysis
DNA was isolated from young frozen leaves using the DNeasyTM Plant Mini Kit (Qiagen, Valencia, CA, USA). The genotype of most of the grapevine cultivars examined was determined with a set of 48 selected SNPs (Table 1). Genotyping was performed at CEGEN (http://www.cegen.es), using the SNPlex high-throughput genotyping platform (Applied Biosystems, Foster City, CA, USA), according to Cabezas et al. (2011). The results obtained were compared with the Instituto de Ciencias de la Vid y el Vino database, which includes the genotypes of more than 6000 grapevine samples.
Data analysis
Principal component analysis (PCA) of the raw data from the above-defined leaf relationships was performed using SAS statistical software v.9.1 (SAS Institute, Cary, NC, USA). This analysis avoids problems caused by differences in leaf size because of growing conditions. SNP data were analysed using GenAlEx (Peakall and Smouse 2006, 2012).
Results
The average leaf of every cultivar studied was constructed. Figures 2 and 3 show the mean leaves constructed for the Algerian and Moroccan cultivars, respectively. The first three axes determined in PCA explained 88% of the variation between cultivars in terms of leaf morphology. Axis 1 (Prin 1) explained 49.5% of the variation, with variables related to the depth of the lateral sinuses (Rel. 7, Rel. 8, Rel. 9, Rel. 10, Rel. 11 and Rel. 12) having the greatest weight. Axis 2 (Prin 2) explained 25.28% of the variation, with variables related to the angles formed by the main veins (Rel. 3, Rel. 4, Rel. 5 and Rel. 6) showing the greatest weight. Finally, the third axis (Prin 3) explained 13.55% of the variation, with variables indicating the orbicular or cuneiform shape of the leaf (Rel. 1 and Rel. 2) having the greatest weight.
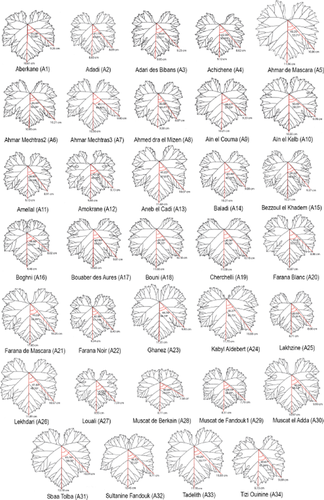
Mean leaves of the Algerian cultivars (A1 to A34).
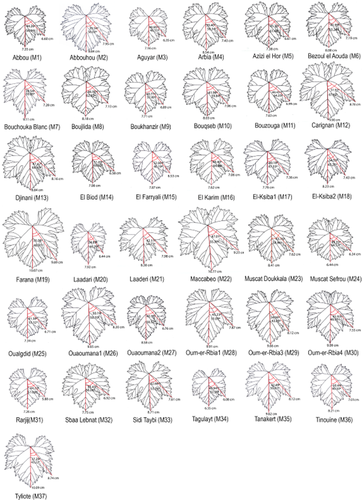
Mean leaves of the Moroccan cultivars and wild plants (M1 to M37).
Figure 4 shows the PCA distribution plot for the studied plants with respect to the variables analysed. The accessions to the right of the graph on Prin 1 are those with shallow lateral sinuses, including accession Oum-er-Rbia 1 (M28), and the cultivars Bouchouka (M7), El Farryali (M15), Laadari (M20), Bouzouga (M11), Tagulayt (M34) and Oualgdid (M25), all from Morocco. On the left on Prin 1 lie the cultivars with deeper lateral sinuses, such as Amokrane (A12), Carignan (M12), Louali (A27), Muscat el Adda (A30), Bezzoul el Khadem (A15), Farana (M19), El Karim (M16) and Sidi Taybi (M33). Muscat de Berkaim (A28), with the most open vein angles, is located at the back of the graph on Prin 2, while El-Ksiba 2 (M18), Farana (M19) and Bezzoul el Khadem (A15), with the most closed angles, are located in the foreground. The bottom of the graph on Prin 3 is occupied by Ouaoumana 2 (M27), Farana Blanc (A20), Oum-er-Rbia 1 (M28), Bouchouka Blanc (M7) and Rarjij (M31), all plants with longer leaves, i.e. those in which the relationship between L1g or L1d with respect to L is small. The upper part of the graph is occupied by Muscat Sefrou (M24), Sultanina Fandouk (A32), El-Ksiba 1 (M17), Bouzouga (M11), Muscat de Fandouk1 (A29) and Muscat de Berkaim (A28), the leaves of which are orbicular in shape. The remaining cultivars are distributed in intermediate positions.
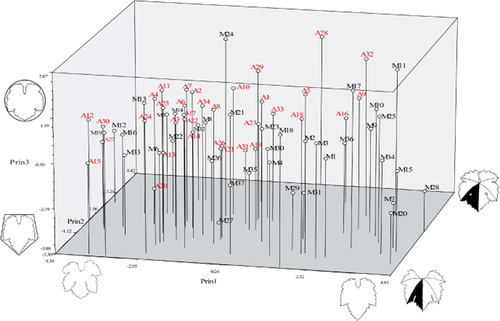
Principal component analysis showing the projection of the 34 Algerian (A1 to A34) and 37 Moroccan (M1 to M37) grape cultivars on the axes defined by the first three principal coordinates.
Given the above PCA distribution of the accessions and the mean leaf data (Figures 2 and 3), the Moroccan cultivars were found to show (at least in grosso modo) more variation in terms of leaf morphology than those of Algeria, which generally were characterised by deep lateral sinuses.
In the samples studied, SNP1399_81 was found to be monomorphic and SNP analysis of 58 cultivar samples (34 Algerian and 24 Moroccan) revealed a total of 39 non-redundant genotypes (Table 2). Most redundancy was found among accessions of the same country, but two matching pairs were found between accessions from different countries: Aïn el Kelb (A10) with Muscat Sefrou (M24), and Muscat de Fandouk 1 (A29) with Muscat Doukkala (M23). A total of 23 different genotypes were observed among the Algerian samples, and 18 among the Moroccan samples. The total probability of identity for multi-locus genotypes for the 48 SNPs, calculated from the 39 non-redundant genotypes, was 3.2·10−16.
Discussion
Viticulture in the Maghreb has experienced great changes over its history, a consequence of the different political and cultural influences to which the region has been subjected. At the end of the protectorate in Morocco, the country's vineyards covered more than 52 000 ha (37 000 ha planted with European cultivars and 15 000 ha with indigenous cultivars) (Vidal 1951). Economically important plantations existed in several regions, including Doukkala, Haouz, Benslimane, Essaouira and Moulouya Khémisset. Presently, local cultivars occupy 59.5% of the total Moroccan vineyard area given over to table grape production. The cultivar Doukkala occupies 41.1%, followed by Abbou with an area of 6%. The most common of the introduced cultivars is Muscat d'Italie, which occupies 9.9% of the total area under vineyards, followed by Valency which occupies 8.6%, and the cultivar Muscat of Alexandria with 6.9% (Ezzahouani 2002). The most important winemaking grape cultivars grown in Morocco are Cinsaut, Carignan, Alicante Bouschet, Grenache Noir and Cabernet Sauvignon. These cultivars occupy 72% of the total area of winegrape vineyards, which have enjoyed some recovery through partnership agreements between national and foreign (mainly French) wine companies (Ezzahouani 2002).
Less information is available for Algeria. The only information on the extension of land under vineyards, and of the cultivars grown, is provided by the Organisation Internationale de la Vigne et du Vin (2009) (originally provided by the ITAF), by Isnard (1951), and by Levadoux et al. (1971). According to the ITAF, some 94 025 ha were given over to vineyards in 2013. The only information available on the cultivars grown (and their relative proportion) is now many years old (Isnard 1951, Levadoux et al. 1971). Although grapevine cultivation is thought to be in recession in Algeria, the conservation of the country's grapevine genetic resources is both scientifically and economically important.
Several authors have recently published microsatellite profiles for many of the cultivars examined in the present work, insome cases using the same accessions examined here [those of the ITAF (Algeria), and the SODEA and Demnate (Morocco) collections]. The present work compiles and compares all the microsatellite and ampelographic data published for North African grape cultivars, and along with the ampelographic and SNP results obtained, provides a greater understanding of the true identity behind the names given to cultivars in different areas. Supporting Information Table S1 provides a comprehensive summary of the comparisons made and conclusions reached. Samples are grouped according to their SNP genotypes and ordered according to their codes (Table 1), except in those cases in which homonyms or similar names appear; these are grouped together for better understanding. Where synonyms, homonyms, similar names and possible sampling and/or collection errors come together, proper identification can be complicated. For example, the cultivars Ahmed dra el Mizen (A8), Amellal (A11) and Tizi Ouinine (A34) (GEN_SNP_2313, Supporting Information Table S1), all turned out to be the same cultivar. The same happened for Amokrane (A12) and Louali (A27), (GEN_SNP_2308, Supporting Information Table S1). Another example is the complex use of ‘Farana’, which includes several Algerian and Moroccan cultivars (GEN_SNP_2220, GEN_SNP_1475 and GEN_SNP_2311, Supporting Information Table S1). The present work shows that several old cultivars grown both in Spain and in the Maghreb countries, especially in Morocco (where they still grow but under different names), include Ahmeur bou Ahmeur, Airen, Cañocazo, Beba, Cayetana Blanca, Dominga, Ohanes, Planta Fina and Planta Mula (Supporting Information Table 1). This is probably the result of cultural and material exchange between North Africa and the Iberian Peninsula over many centuries. There are also cultivars of wide distribution outside the Maghreb, such as several Muscats, and Sultanina, that were also found in the Moroccan and Algerian collections. Finally, there are several cultivars (Aberkane, Achichene, Adadi, Amellal, Aneb el Cadi, Bezzoul el Khadem, Taferielt, Agueyer, Abbouhou, Bouqseb, Bouchouka and Bouzouga) for which no synonyms were found outside of the region; these cultivars are probably native to the Maghreb.
The molecular profiles for Ouaoumana 1 and Ouaoumana 2 (wild grapevine samples from Morocco) reported by Zinelabidine et al. (2010) were identical, but different to any other wild plants from different locations in Morocco. No matches were seen with the profiles of other North African materials reported by other authors (El Oualkadi et al. 2009, Laiadi et al. 2009, Riahi et al. 2010). Figure 3 shows the mean leaves for Ouaoumana 1 (M26) and Ouaoumana 2 (M27) to be similar in terms of lateral sinus depth and vein angles, even though Ouaoumana 2 (M27) has more cuneiform leaves. This great similarity in their leaves, and the above-mentioned molecular results, show that they are the same genotype.
The three wild plants found at Oum-er-Rbia (M28, M29 and M30) had clearly different leaves to one another and to plants from the other locations. The wild plant M28 had more cuneiform leaves, M29 more closed lateral sinuses and superimposed lobules, and M30 a closed petiolar sinus and U-shaped lateral sinuses.
In terms of their vein angles, M17 and M18 from El-Ksiba also showed leaves that differed from one another, and from those of the other cultivars studied. This suggests that they represent different cultivars; however, it would appear that they are similar at the molecular level because 8 of the 11 microsatellites analysed by Zinelabidine et al. (2010) coincide.
The present results highlight the need for collaboration between countries if problems of homonyms and synonyms are to be resolved. They also show the need to combine ampelographic and molecular results. Cultivars with similar leaf or bunch morphology might be difficult to distinguish by ampelographic means, but be clearly distinguishable by molecular techniques. For example, Bouchouka Blanc (M7) and Laadari (M20), which have leaves of similar morphology, are clearly shown by molecular methods to be different cultivars. Combining these techniques can also help confirm identity. For example, Amokrane (A12) and Louali (A27) have similar leaf morphologies, but leaves may vary greatly in size (because of the properties of the soil and water availability, or even because their parent plants belong to different clones). Molecular analysis shows them to be the same cultivar.
Conversely, some cultivars show identical molecular profiles for the markers analysed, but have different ampelographic characteristics, even to the extent that the grapes of one might be red and those of the other green. This was seen for the Pinot cultivars (Pinot Noir, Pinot Blanc and Pinot Gris) and for Muscat de Frontignan Rouge and Muscat de Frontignan Blanc.
The accessions Ghanez (A23) and Tadelith (A33) had identical SNP profiles (SNP genotype number: GEN_SNP_0608; proposed name: Ohanes), and their mean leaves were similar (Figure 2) – even clustering close to one another in PCA (Figure 4). The fact that they have different coloured grapes, however, is enough to consider them different cultivars. The reporting of contradictory results in previous studies (Supporting Information Table S1) suggests that Tadelith might be a colour sport of Ohanes, or that sampling errors were made (either in previous studies or in the present work). These results underscore the need for experts to undertake sampling.
The techniques used in the present work have certain advantages. For example, the construction of mean leaves allows the variability in morphology between leaves of the same accession to be examined. Experience tells us that when sampling is properly performed, the leaves of plants from the same cultivar are similar (with the exception of the Spanish cultivar Godello), and that when mean leaves are being constructed, the coefficients of variation for the variables measured are usually low. The technique also allows for statistical comparisons to be made. In the present study, PCA was used, which permits the simultaneous comparison of many variables. The three-dimensional display of the results obtained (i.e. for the first three axes) shows the distribution of the different accessions in terms of leaf lateral sinus, the angle formed between the main veins and the shape of the leaf (more orbicular or more cuneiform). Figure 4 shows the accessions with similar leaves in these respects to be distributed close to one another, while others with different characteristics in one or more respects to lie more distant from the corresponding axes. The construction of mean leaves also allows comparisons to be made between leaves in terms of single variables.
In grapevine molecular analyses, SNP markers are not widely used while SSRs are commonly employed. In the present work, the genotyping results of SSR analyses and SNP analyses were compared (Supporting Information Table S1). The main difficulty in SSR data comparison is the different allele binning used by different authors, which in many cases precludes large-scale comparisons. Reaching definite conclusions about the identity of some alleles is therefore impossible. This is not the case when comparing SNP data because of the bi-allelic nature of SNPs. This makes comparisons much easier.
Conclusion
In the present work, the mean leaves of the main Moroccan and Algerian grapevine cultivars were constructed. SNP analysis and comparison of published microsatellite and ampelographic data highlighted the existence of new synonyms and homonyms. The existence of some errors of identification, misinterpretations and collection or sampling errors is suggested. Together, the present results contribute towards a better understanding of grapevine diversity in the Maghreb.
Acknowledgements
Lalla Hasna Zinelabidine was supported by a fellowship from AECI and a short-term scientific mission from COST FA1003. Iván González and Elena Zubiaurre are thanked for providing technical assistance and Adrian Burton for helping with the English version of the manuscript.