Effect of carboxymethyl cellulose on tartrate salt, protein and colour stability of red wine
Abstract
Background and Aims
Recent studies have confirmed a long-term effect of carboxymethyl cellulose (CMC) for tartrate salt stabilisation in white wine. It has been argued that CMC is not only less effective in red wine but also interacts with proteins and polyphenols generating turbidity and change in colour. In order to explain these effects, we studied in detail the impact of CMC on haze formation and colour stability of red wine.
Methods and Results
The influence of CMC concentration was tested with ten samples of red wine produced from several grape cultivars. The haze-forming material was analysed by sodium dodecyl-sulfate polyacrylamide electrophoresis and the protein composition by high-performance liquid chromatography-mass spectrometry. Colour alteration was documented by Vis-spectroscopy. Three samples of wine that were tested developed significant turbidity when treated with oenological doses of CMC. The haze formation coincided with a high-colour density and protein instability of the wine. The insoluble fraction contained pathogenesis-related wine proteins (thaumatin-like proteins, lipid transfer protein) and was associated with colouring matter.
Conclusions
Carboxymethyl cellulose is of value for tartrate salt stabilisation in red wine. Occasionally, it promotes development of protein haze and colour loss. At a first glance, this behaviour appears to limit the oenological suitability of CMC, but it might also be considered as a new tool to remove unstable wine proteins.
Significance of the Study
The results indicate the conditions under which red wine turbidity is triggered by CMC. Thus, they are important for the elaboration of recommendations for the optimal use of the polymer.
Introduction
Crystals in wine are caused naturally by the formation of insoluble salts of tartaric acid, and are not harmful. Nevertheless, solids in wine may cause complaints when the consumer assumes that dangerous splinters of glass are present in the wine bottle (Gerbaud et al. 2010, Salagoïty et al. 2011).
Potassium hydrogen tartrate and calcium tartrate crystallisation is governed by the solid–liquid equilibrium of potassium and calcium ions with L(+)-tartaric acid. Today, wine is frequently bottled before reaching solution equilibrium and oenological measures are needed to avoid tartrate salt crystal formation. There are basically three methods available: (i) induction of salt precipitation before bottling by means of cooling the wine; (ii) selective removal of excess potassium and/or calcium ions; and (iii) use of crystallisation inhibitors, such as metatartaric acid and yeast mannoproteins (Marchal and Jeandet 2009, Gerbaud et al. 2010, Lasanta and Gómez 2012). Recently, the Organisation Internationale de la Vigne et du Vin and the European Union (EU) authorised the use of carboxymethyl cellulose (CMC) for tartrate salt stabilisation in wine and sparkling wine at a concentration limit of 100 mg/L (Salagoïty et al. 2011).
Carboxymethyl cellulose is an inexpensive synthetic derivate of cellulose, which is easy to handle and already widely used as a food additive (E 466). Sodium CMCs are polymers of β-D-glucose units whose primary or secondary alcohol groups are etherified by sodium acetate groups. The degree of polymerisation and substitution varies among different CMC products (Marchal and Jeandet 2009). Initial studies have confirmed a long-term effect of CMC for tartaric stabilisation and no sensory impact on white wine (Bosso et al. 2010, Gerbaud et al. 2010, Salagoïty et al. 2011). The effect of CMC has been explained by the interaction of the negative charge of CMC and the positive charge of the crystals (Crachereau et al. 2001). It has been argued, however, that CMC may interact not only with tartrate but also with other wine components. Sediments of coloured matter or small crystals can arise in about 20% of red wines (Lasanta and Gómez 2012). It has been stated that CMC is not only less effective in red wine, but also interacts with polyphenols in general and anthocyanins in particular, generating turbidity and change in colour (Moutounet et al. 2010).
In order to explain these effects, we studied in detail the impact of CMC on haze formation and the colour of red wine. In particular, we investigated the protein composition of the precipitates. The results allow conclusions to be drawn under which conditions turbidity is accelerated, or may be slowed. Thus, they may be important for the elaboration of recommendations for the optimal use of CMC in winemaking.
Materials and methods
Wine samples
The influence of CMC was tested on ten samples of red wine produced from several grape cultivars (Table 1). They were obtained from commercial wineries, but the Dornfelder wine was experimentally produced by Erbslöh Geisenheim AG (Geisenheim, Germany). All wines were filtered with the exception of the Dornfelder and Lemberger wines. Some key features of the wines are listed in Table 1.
| Feature | Wine No., cultivar, year and origin | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Spätburgunder 2011 Germany | Prior Qba 2011 Germany | Merlot 2011 France | Tempranillo 2011 Spain | Spätburgunder 2011 Germany | Regent 2011 Germany | Spätburgunder 2012 Germany | St. Laurent 2011 Germany | Lemberger 2012 Germany | Dornfelder 2012 Germany | |
| pH | 3.52 | 3.58 | 3.51 | 3.70 | 3.77 | 3.87 | 3.84 | 3.74 | 3.26 | 3.94 |
| Colour density (Σ E420, E520, E620) | 4.69 | 7.54 | 8.97 | 7.16 | 4.97 | 11.4 | 6.16 | 7.19 | 8.03 | 12.26 |
| Colour hue (420/520 nm)† | 1.02 | 0.83 | 0.81 | 0.77 | 1.22 | 1.04 | 0.79 | 0.77 | 0.56 | 0.58 |
| Phenolic content (mg/mL)‡ | 2.17 | 1.69 | 1.91 | 2.46 | 2.09 | 3.01 | 0.96 | 1.64 | 0.84 | 1.23 |
| Turbidity (FNU) | 0.20 | 1.17 | 0.42 | 0.53 | 0.29 | 0.90 | 1.36 | 0.62 | 1.48 | 0.80 |
| Protein stability (FNU) 4 h, 80 °C | 3.34 | 2.89 | 2.70 | 1.36 | 1.17 | 7.85 | 7.08 | 0.71 | 2.33 | 185.4 |
| Crystal stability | ||||||||||
| Tsat (°C) | 18.6 | 20.3 | 21.7 | 23.1 | 17.7 | 21.5 | 18.1 | 18.3 | 21.3 | 22.5 |
| Δ μS/cm | 10.0 | 10.0 | 8.0 | 260.0 | 50.0 | 20.0 | 30.0 | 30.0 | 53.0 | 40.0 |
| Δ μS/cm§ | 10.0 | 0.0 | 6.0 | 20.0 | 0.0 | 10.0 | 0.0 | 0.0 | 0.0 | 0.0 |
- †<0.8: velvet; 0.8−1.2: red; >1.2: orange-red; ‡Ferulic acid equivalents; §After addition of VinoStab (=65 mg CMC/L). FNU, formazine nephelometric unit; Tsat, saturation temperature.
Concentration of phenolic substances
The concentration of phenolic substances of the wine samples was determined with the Folin–Ciocalteu reagent, using ferulic acid as standard (Gamella et al. 2006).
Turbidity and protein stability
Prior to the heat assay, the wines were filtered with a 0.45 μm cellulose nitrate membrane. The turbidity of the wine samples was measured with a nephelometer (Dr Lange Nephla LPG 239, 900 scattered light photometer, Hach Lange GmbH, Düsseldorf, Germany) and according to the standard method ISO 7027 recommended by the International Organization for Standardization (1999). Values are given as formazine nephelometric unit. The heat assay for verification of protein stability followed established methods (Jacobson 2006, Esteruelas et al. 2009).
Tartrate salt stability
The tartrate salt saturation temperature (Würdig et al. 1985) and the mini-contact process (Müller and Würdig 1978) was determined with a conductivity meter (EasyKristaTest, Erbslöh Geisenheim AG). To determine the saturation temperature, 200 mL of clear and degassed wine was adjusted to a temperature of above 18°C, and the initial conductivity (C1) was measured. Then 0.8 g of potassium hydrogen tartrate (Erbslöh Kali-Contact, Erbslöh Geisenheim AG) was added to the wine. After stirring and a short crystal settling time (5 min) the conductivity (C2) value was measured in the clear supernatant. From the difference in conductivity (ΔC = C2 − C1) at the actual wine temperature (T), the potassium hydrogen tartrate saturation temperature (Tsat) was calculated according to the formula: Tsat°C = T − (C2 − C1)/33 (ΔC of 33 μS corresponds to ΔTsat of 1°C). Red wines with a high-colloid concentration are regarded as tartrate salt stable when they exhibit a saturation temperature of below 18°C, whereas red wines with low-colloid concentration should display a value below 15°C, to be considered as stable (Würdig and Woller 1980).
Wines with increased saturation temperature should be tested by means of the mini-contact process. Therefore, 200 mL of clear and degassed wine were placed in a cooling bath and allowed to cool down to below 4°C. The initial conductivity (C1) value was measured. Then 0.8 g potassium hydrogen tartrate were added and stirred. After 2 h the conductivity (C2) was determined in the clear supernatant. The conductivity difference (ΔC = C1 − C2) was measured in μS/cm. If a decrease in conductivity occurs, then crystallisation has taken place in the wine. If the resulting ΔC is below 30 μS/cm, the wine is regarded as tartrate salt stable (Friedrich and Görtges 2004).
Effect of CMC on tartrate salt stability
VinoStab (Erbslöh Geisenheim AG) is a viscous solution of high-purity sodium CMC with a solid content of 5.0% (w/v). It is applied to the wine to prevent potassium hydrogen tartrate precipitation. The recommended addition of VinoStab is 0.75 to 2.0 mL/L. In practice, the optimal dosage must be determined in advance in order to minimise a possible impact on filtration by the application of CMC. This is possible by evaluation of the wine stability in respect to tartrate salt precipitation either by determining the saturation temperature or by conducting the mini-contact process.
In our experiments, VinoStab was added at 1.3 mL/L (=65 mg CMC/L) to ten red wines under constant stirring. The difference in conductivity was determined by means of the mini-contact method before CMC addition and after 7 and 14 days, respectively.
Effect of CMC on protein and colour stability
Fifty millilitres of each wine sample were clarified by centrifugation (20 000 × g, 30 min). VinoStab was added to the supernatants to achieve a CMC concentration of 37.5, 50.0, 65 and 100 mg/L. The control wines contained no CMC. Haze formation was monitored visually after 4 days at 4.0°C.
Electrophoretic analysis
Ten millilitres of each wine was centrifuged for 30 min at 9000 × g. The resulting precipitates were suspended in 1.0 mL deionised water, followed by a second centrifugation step for 5 min at 15 000 × g. This pellet was suspended in 60 μL of deionised water and mixed 3:1 with reducing sodium dodecyl sulfate (SDS) sample buffer (Roti-Load 1, Roth, Karlsruhe, Germany). After heating for 5 min at 100°C, 10 μL of the solution were applied to electrophoresis. A pre-stained protein ladder (PageRuler Plus, Fermentas, St Leon-Rot, Germany) served as the molecular mass standard. Discontinuous SDS-polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to Laemmli (1970) using the protocols of Hames and Rickwood (1981). Samples were separated in 12.5% (w/v) SDS-gels (10 × 10 mm) with a 4% (w/v) stacking gel. A voltage of 150 V was applied to the gel for 1 h at room temperature, and proteins were visualised by staining with Coomassie Brilliant Blue R-250 (Serva, Heidelberg, Germany).
Carbohydrates were detected with the periodic acid Schiff's reagent (PAS) (Merck, Darmstadt, Germany) applying a procedure modified from Segrest et al. (1972). The SDS gel was fixed in 40% (v/v) ethanol/5% (v/v) acetic acid /55% (v/v) water for 30 min, after which it was treated with 0.7 % (v/v) periodic acid in 5% (v/v) acetic acid for 30 min. The gel was then flooded with 0.2% (w/v) sodium bisulfite in 5% (v/v) acetic acid. After 30 min, the gel was stained with PAS until development of pink bands. Finally, the background was destained with 5% (v/v) methanol/7% (v/v) acetic acid/2% (w/v) sodium bisulfite /88% (v/v) water. Bovine serum albumin (Serva) served as a negative control and peroxidase from horseradish (Sigma-Aldrich, Taufkirchen, Germany) as a positive control. All incubation steps were done at room temperature and in the dark.
Spectrophotometry
The impact of CMC on wine colour was examined by recording the Vis spectra (Shimadzu UV-2450 Spectrophotometer, Shimadzu Corporation, Kyoto, Japan). The precipitates were removed by centrifugation for 10 min at 15 000 × g. The supernatants were measured spectrophotometrically according to Jacobson (2006). The sum of the absorption values at 420, 520 and 620 nm was taken as a measure of colour density and the ratio 420/520 nm as the parameter for colour hue (Jacobson 2006).
In-gel digestion and mass spectrometry
The red wines, Merlot, Regent and Dornfelder (Table 1), and the precipitates after CMC treatment were analysed by high-pressure liquid chromatography-mass spectrometry (HPLC-MS) to identify potential haze-forming proteins. The wines were prepared by dialysis (pore size 3.5 kDa) of 50 mL of wine for 4 days against deionised water and subsequent lyophilisation. The precipitates were obtained as described above (from 50 mL wine samples containing 100 μL VinoStab) and dialysed before further analysis. The samples were separated by SDS-PAGE and subjected to an in-gel tryptic digestion procedure (Wigand et al. 2009) before capillary liquid chromatography (LC)-MS/MS using either selected gel bands or alternatively the complete lane for shotgun analysis. The tryptic peptides were transferred to an autosampler vial, and were separated by LC with a Waters NanoAcquity ultra-performance liquid chromatography system (Waters Corporation, Milford, MA, USA) on a 100 μm × 100 mm CSH C18 column according to Wigand et al. (2009). The tryptic peptides were analysed with a Waters Xevo G2 quadropole time-of-flight (QToF) mass spectrometry system with a typical resolving power of R = 20 000, using a positive mode electrospray ion source with a NanoLockSpray source (Waters Corporation). The lock mass channel was sampled every 30 s. The mass spectrometer was calibrated with a [Glu1]fibrinopeptide solution (500 fmol/μL). The instrument was run in data-independent acquisition mode for fragment identification as described previously (Patzig et al. 2011). The integration time for the ToF analyser was 0.7 s with an interscan delay of 0.1 s.
The resulting MS/MS data were processed and searched by using the PROTEINLYNX GLOBAL SERVER, ver. 2.5.2. (Waters Corporation). Protein identifications were assigned by searching a hybrid database containing all known protein sequences from Vitis vinifera (containing 54 988 sequences from the TREMBL database, 904 sequences from the GenBank database and 158 sequences from the RefSeq database), Saccharomyces cerevisiae (6747 sequences from the Swissprot database), known possible contaminants (trypsin, human keratins) and typical fining reagents (casein, ovalbumin, lysozyme, collagens) with the precursor and fragmentation data afforded by the LC-MS/MS acquisition method. The maximum mass error tolerance values were typically 5 ppm for precursor ions and 15 ppm for fragment ions. Peptide identification was restricted to tryptic peptides with no more than one missed cleavage and cysteine carbamidomethylation, allowing for the following variable modifications [oxidation (Met), deamidation (Asn, Gln)]. The database search was repeated using a reversed database generated from the composite database described above to assess the false-positive rate of protein identification. The false-positive rate of peptide identification was calculated to be less than 1%.
Results and discussion
Tartrate salt stability
Four red wines revealed an elevated saturation temperature, but exhibited a low-conductivity difference in the mini-contact process, as shown for Spätburgunder wine no. 1, Prior wine, Merlot wine, and Regent wine (Table 1). These wines can be regarded as stable in respect of crystal precipitation. This result indicates that an elevated saturation temperature in red wines does not necessarily implicate tartrate salt instability. Due to different colloid levels, crystal stability in some wines is intrinsically good despite a high-saturation temperature (Würdig and Woller 1980).
For that reason, it is advisable to conduct the mini-contact process for red wines with a high-saturation temperature (Friedrich and Görges 2004). The Spätburgunder wines no. 5 and 7, St. Laurent wine, Lemberger wine, and Dornfelder wine, exhibit an elevated saturation temperature and a conductivity difference between 30 and 53 μS/cm; therefore they are regarded as unstable. Tempranillo wine, with a saturation temperature of 23.1°C and a conductivity difference of 260 μS/cm, is highly tartrate salt unstable. Addition of CMC achieved crystal stability in all red wines tested despite elevated saturation temperatures and high-conductivity differences.
Protein stability
Haze formation by the addition of CMC could be visually observed in three of the ten wines tested: Merlot wine, Regent wine and Dornfelder wine. The haze-forming fraction contains proteins in the range of 10–100 kDa, as visualised by SDS-PAGE (Figures 1, 2). Within the tested concentration range, there was apparently no effect of the CMC dose applied on the amount and nature of precipitated proteins.
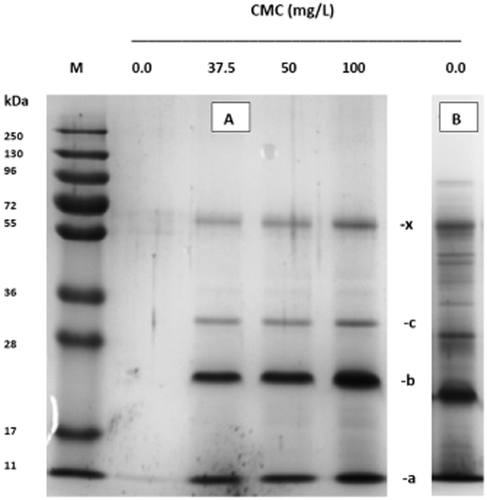
Sodium dodecyl sulfate polyacrylamide gel electrophoresis of the precipitates formed in clarified and nonclarified Dornfelder wine. Carboxymethyl cellulose (VinoStab) was added only to the clarified wine at a concentration of 37.5, 50 and 100 mg/L. Gels were stained with Coomassie Blue. A: Wine clarified by centrifugation (20 000 × g); B: nonclarified wine; M: protein molecular size standard (pre-stained); a: lipid transfer protein, b: thaumatin-like protein; c: glucan endo β-1,3 glucosidase; x: unknown protein (cf. Table 4 of protein characteristics).
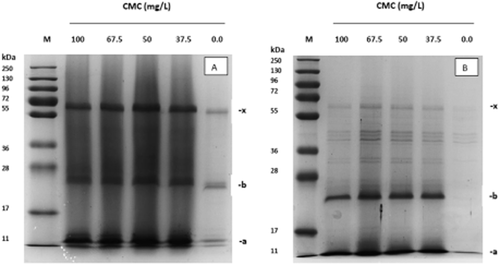
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the precipitate formed in A: untreated Merlot wine; B: untreated Regent wine; and in both wines after the addition of 37.5, 50, 67.5 and 100 mg/L carboxymethyl cellulose (VinoStab). Gels were stained with Coomassie Blue. M: protein molecular size standard (pre-stained); the characteristics of the main bands (a, b, x) correspond to the proteins listed in Table 4.
Intrinsic protein instability of a red wine might be one probable factor for the development of turbidity after CMC addition. The Dornfelder wine contained a high fraction of unstable protein, which had precipitated already without any CMC additions (Figure 1b). This protein instability is also reflected in the heat test (Table 1). Also the Regent wine displayed high-protein instability (Table 1) and was sensitive to CMC treatment. Other red wines, however, such as the Spätburgunder no. 7 displayed considerable protein instability in the heat test, but developed no haze formation after CMC addition. In contrast, the Merlot wine showed only medium protein instability in the heat test, but was sensitive to CMC. One explanation for this inconsistent behaviour is that there is no real evidence for protein involvement in the haze that develops after the heat treatment. High temperature likely also favours precipitation of non-protein wine compounds, such as phenolic substances. Unfortunately, common biochemical protein determinations are not applicable for red wines, because of interference with coloured materials.
As the haze-forming effect of CMC was detected only with the most highly coloured wine samples (Table 1), it can be argued that a red wine containing a large amount of certain phenolic substances is especially sensitive to CMC. Among the phenolic wine components, protein stability is mostly influenced by tannins, which form insoluble complexes with proteins (Santos-Buelga and de Freitas 2009). Several researchers have suggested a hydrophobic mechanism for the interaction between phenolic substances and proteins, in which the protein has a fixed number of phenolic binding sites. More of these sites are exposed when the protein is denaturated, e.g. by heat, ethanol and sulfate (Waters et al. 1992, Siebert and Lynn 2003, Pockock et al. 2006). In a wine-related approach, Gazzola et al. (2012) investigated the interaction of isolated wine proteins, polysaccharides and phenolic substances on haze formation in a white wine. The results demonstrated that the impact of phenolics on protein aggregation varies strongly with the individual wine protein, whereas polysaccharides have been reported as not contributing to haze formation. Some polysaccharide classes, such as yeast mannoproteins, are considered to have even a protective role with respect to wine protein hazing (Marchal and Jeandet 2009). A negatively charged polymer such as CMC, however, might behave quite differently to neutral polysaccharides in protein hazing. Indeed, in their recent study, Marangon et al. (2012) demonstrated the ability of anionic polysaccharides (carrageenan and pectin) to remove heat-unstable grape proteins from a Chardonnay juice. Gonçalves et al. (2011) reported that pectin can induce a competitive dissociation of tannin/protein complexes. Conclusively, the negative-charged CMC might interact with positive-charged wine proteins or tannin/protein complexes. As the CMC impact was especially pronounced with intensively coloured red wines, tannins, anthocyanins and derived pigments may represent the main targets for the polymer.
Protein identification
The protein composition of the haze-forming wine samples as well of the original product and of the sediments was analysed by LC-MS/MS in order to characterise proteins precipitated by CMC (Table 2, 3 and 4).
| Accession No. | Identified protein | Mass (kDa) | pI | Presence in precipitate (CMC) | Sequence Coverage (%) | No. of peptides detected |
|---|---|---|---|---|---|---|
| AAO33393.1 | Lipid transfer protein isoform 1 [Vitis vinifera] | 11.6 | 8.64 | − | 16.0 | 4 |
| AAO33394.1 | Lipid transfer protein isoform 4 [V. vinifera] | 11.7 | 8.63 | + | 43.7 | 9 |
| AAQ10092.1 | Thaumatin-like protein [V. vinifera] | 23.9 | 4.47 | + | 22.2 | 3 |
| AAB61590.1 | Thaumatin-like protein VVTL1 [V. vinifera] | 23.9 | 4.90 | + | 13.5 | 4 |
| NP_011770.3 | Phosphopyruvate hydratase Eno1p [Saccharomyces cerevisiae S288c] | 46.8 | 6.15 | + | 29.1 | 8 |
| NP_012044.1 | Phosphopyruvate hydratase Eno2p [S. cerevisiae S288c] | 46.9 | 5.59 | + | 22.4 | 6 |
| AAB47171.1 | Vacuolar invertase 1, GIN1 [V. vinifera] | 71.5 | 4.41 | + | 8.1 | 5 |
- CMC, carboxymethyl cellulose.
| Accession No. | Identified protein | Mass (kDa) | pI | Presence in precipitate (CMC) | Sequence coverage (%) | No. of peptides detected |
|---|---|---|---|---|---|---|
| AAO33393.1 | Lipid transfer protein isoform 1 [Vitis vinifera] | 11.6 | 8.64 | + | 43.7 | 8 |
| AAO33394.1 | Lipid transfer protein isoform 4 [V. vinifera] | 11.7 | 8.63 | + | 43.7 | 9 |
| CAB85636.1 | Thaumatin-like protein [V. vinifera] | 20.1 | 4.34 | − | 39.9 | 20 |
| A6ZQH4.1 | Cell wall mannoprotein CIS3 [Saccharomyces cerevisiae] | 23.0 | 4.30 | − | 11.6 | 8 |
| XP_002282964.1 | Thaumatin-like protein [V. vinifera] | 23.8 | 4.47 | + | 42.2 | 22 |
| CAA71883.1 | Osmotin-like protein [V. vinifera] | 23.9 | 4.36 | + | 26.2 | 5 |
| CAN66515.1 | Hypothetical protein VITISV_021587 [V. vinifera] | 23.9 | 4.36 | − | 42.2 | 21 |
| AAQ10092.1 | Thaumatin-like protein [V. vinifera] | 23.9 | 4.47 | + | 32.9 | 19 |
| AAB61590.1 | Thaumatin-like protein VVTL1 [V. vinifera] | 24.0 | 4.90 | − | 19.2 | 4 |
| CAN74833.1 | Hypothetical protein VITISV_004208 [V. vinifera] | 24.0 | 8.09 | − | 10.7 | 4 |
| CAN63010.1 | Hypothetical protein VITISV_005303 [V. vinifera] | 26.2 | 8.26 | − | 11.7 | 2 |
| AAB65776.1 | Class IV endochitinase [V. vinifera] | 27.2 | 5.29 | − | 19.2 | 4 |
| NP_011798.1 | Endo-beta-1,3-glucanase gl2p [S. cerevisiae S288c] | 34.1 | 4.13 | − | 31.3 | 9 |
| CBI37860.3 | Unnamed protein product [V. vinifera] | 36.5 | 5.87 | − | 24.3 | 5 |
| NP_014239.1 | Hypothetical proteinYgp1p [S. cerevisiae S288c] | 37.3 | 5.12 | − | 10.7 | 3 |
| CBI36906.3 | Unnamed protein product [V. vinifera] | 37.4 | 6.51 | − | 27.8 | 4 |
| CAN81263.1 | Hypothetical protein VITISV_028698 [V. vinifera] | 39.1 | 9.57 | − | 15.0 | 3 |
| NP_011770.3 | Phosphopyruvate hydratase Eno1p [S. cerevisiae S288c] | 46.8 | 6.15 | + | 36.8 | 10 |
| NP_012044.1 | Phosphopyruvate hydratase Eno2p [S. cerevisiae S288c] | 46.9 | 5.59 | + | 25.6 | 7 |
| CBI24585.3 | Unnamed protein product [V. vinifera] | 60.5 | 4.26 | + | 18.5 | 10 |
| CBI26778.3 | Unnamed protein product [V. vinifera] | 67.9 | 7.27 | + | 14.4 | 4 |
| AAB47171.1 | Vacuolar invertase 1, GIN1 [V. vinifera] | 71.5 | 4.41 | + | 10.6 | 4 |
| CAN72303.1 | Hypothetical protein VITISV_009715 [V. vinifera] | 128.6 | 7.99 | + | 2.2 | 3 |
- CMC, carboxymethyl cellulose.
| Accession No. | Identified protein | Mass (kDa) | pI | Banda |
|---|---|---|---|---|
| AAO33394.1 | Lipid transfer protein isoform 4 [Vitis vinifera] | 11.7 | 8.63 | a |
| AAB61590.1 | Thaumatin-like protein VVTL1 [V. vinifera] | 23.9 | 4.90 | b |
| XP_002277446.1 | Glucan endo 1,3-β glucosidase [V. vinifera] | 36.7 | 8.79 | c |
| CBI24585.3 | Unnamed protein product [V. vinifera] | 60.5 | 4.26 | x |
- a band assignment see Figure 1.
Most studies in the past have targeted the protein composition of white wines (Esteruelas et al. 2009, Vincenzi et al. 2011). Red wines are more complicated due to serious interference of the analysis by highly red-coloured components. These experimental problems were largely overcome in this study by separation of the red wines by SDS-PAGE followed by tryptic digest and LC-MS/MS shotgun analysis of the complete lane. With the same approach Wigand et al. (2009) identified the most abundant grape proteins of a Portugieser red wine as lipid transfer proteins [molecular mass (MM) ca. 12 kDa], thaumatin-like proteins (MM ca. 25 kDa) and vacuolar invertase (MM ca. 72 kDa). Such proteins were also detected by our shotgun LC-MS/MS analysis in the original wine as well as in the precipitates (Tables 2 and 3). Intensive protein bands of corresponding sizes in SDS-PAGE could be also regularly found in the precipitates of three red wines after CMC treatment (Figures 1 and 2). The four main bands found in the precipitate of the Dornfelder wine were identified by selective in-gel digestion and LC-MS/MS analysis (Table 4).
The present study confirms the results of earlier studies (Marchal et al. 1996, Wigand et al. 2009), which indicated that most wine proteins derived from V. vinifera appear to be glycosylated (Figure 3). In accordance with the previous work of Wigand et al. (2009), high-molecular mass (>100 kDa) compounds were detected, which could be only visualised by PAS- and not by Coomassie-staining (Figure 3, band h). This fraction, which may consist of yeast-derived glycoproteins and/or polysaccharides (Esteruelas et al. 2009, Vincenzi et al. 2011), was also detected in the precipitates, although in equal amounts in the untreated and CMC-treated samples. Conclusively, these molecules are probably not affected by CMC.
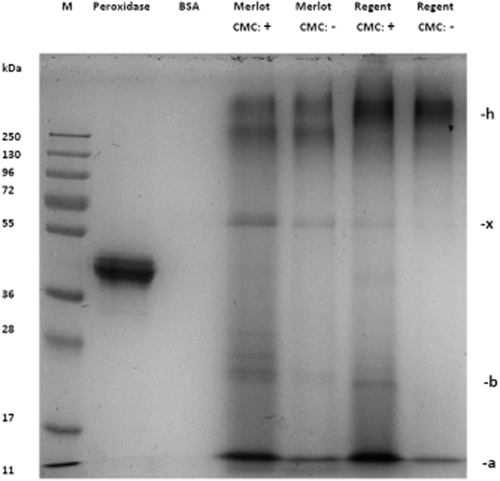
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the precipitates formed in the Merlot wine and the Regent wine before (−) and after (+) the addition of 100 mg carboxymethyl cellulose/L (VinoStab). Glycocompounds were visualised by periodic acid Schiff's reagent. Peroxidase from horseradish served as a positive, bovine serum albumin (BSA) as a negative control. M: protein molecular size standard (pre-stained); the size of the main bands (a, b, x) correspond to proteins listed in Table 4; band h represents high-molecular glycoproteins and/or polysaccharides.
Considering the mode of action of CMC, no obvious correlation exists between the molecular masses or charges of the precipitated wine proteins, because they differ considerably in these features (Tables 2, 3 and 4). Furthermore, at the average wine pH of 3.5 all identified wine proteins will be positively charged. Carboxymethyl cellulose seems to interact rather non-specifically with prominent red wine proteins being unstable per se (especially lipid transfer proteins and thaumatin-like proteins). In this context, it is interesting to note that we could not identify grape chitinases in the CMC-induced precipitates of the red wines. Chitinases and thaumatin-like proteins are the primary cause of heat-induced haze formation in white wine (Marangon et al. 2011, Vincenzi et al. 2011).
Colour stability
Carboxymethyl cellulose triggers a dose-dependent loss of colour density in six of the ten red wines investigated (Table 5 and Figure 4). The negative effects were mostly pronounced in the Merlot and Dornfelder wines, which also exhibited high-protein instability in the heat test. This indicates that decolourisation by CMC may not be primarily due to a direct interaction with phenolic substances, but the result of co-precipitation with proteins. Future experiments should elucidate the underlying mechanisms in more detail. That is a challenge regarding the manifold interactions of proteins, phenol substances and polysaccharides in red wine (Santos-Buelga and de Freitas 2009, Gazzola et al. 2012).
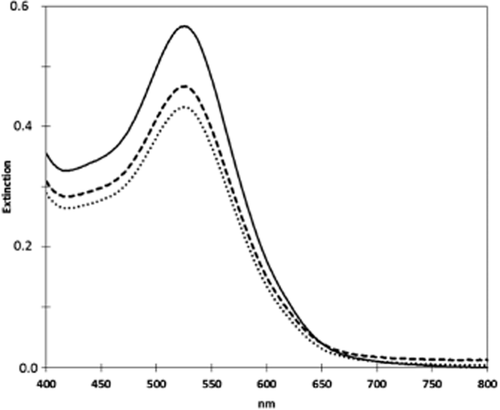
Visible spectrum of the supernatant of the Dornfelder wine (pre-clarified by centrifugation for 30 min at 20 000 × g) ( ) and of the wine after the addition of 50 (. . .) and 100 mg/L (- - -) carboxymethyl cellulose (VinoStab) and removal of the resulting precipitate by centrifugation for 30 min at 9 000 × g.
) and of the wine after the addition of 50 (. . .) and 100 mg/L (- - -) carboxymethyl cellulose (VinoStab) and removal of the resulting precipitate by centrifugation for 30 min at 9 000 × g.
| Concentration of CMC (mg/L) | Colour density (% increase) | ||||
|---|---|---|---|---|---|
| 3a | 6 | 7 | 9 | 10 | |
| 37.5 | 5.37 | 4.64 | 0.00 | 0.00 | 15.73 |
| 50.0 | 7.56 | 4.64 | 1.68 | 1.73 | 16.43 |
| 67.5 | 8.36 | 4.73 | 2.94 | 4.89 | 17.84 |
| 100.0 | 9.25 | 5.01 | 4.62 | 5.52 | 22.34 |
- a Wine No.
- All wine samples were clarified by centrifugation (20 000 × g) before adding VinoStab.
Conclusions
Although some red wines investigated delivered high-tartrate salt instability in different test systems, CMC addition prevented salt crystal formation in all wines. Three of the wines, however, developed significant protein turbidity when treated with CMC. As detected by SDS-PAGE and LC-MS/MS, the insoluble material contained typical pathogenesis-related wine proteins derived from V. vinifera, which are unstable per se and contribute to protein haze formation. Initially, this result appeared to be somewhat disappointing; however, when applied prior to wine-bottling, CMC may be of value as a new fining agent to improve protein stability of (red) wine. Such an observation accords with recent results of Marangon et al. (2012), which demonstrated the potential of anionic polysaccharides (like CMC) to remove heat-unstable grape proteins. In particular the removal of potential grape allergens, such as the lipid transfer protein (Wigand et al. 2009), represents an attractive application. An elevated level of PR proteins in wine may be induced by stress factors such as fungal attack of grapes. Girbau et al. (2004) demonstrated that powdery mildew infection of grapes resulted in an increased level of a thaumatin-like protein in wine. At a high level of infection (above 30% of berries) this had a significant impact on the level of haziness in wine following a heat test. In contrast, berries and must infected with Botrytis cinerea had a reduced protein level (Marchal et al. 1998, Girbau et al. 2004). Our next studies will focus on the relation between CMC-induced protein haze and the degree of fungal contamination of grapes. The results should facilitate recommendations for the optimal use of CMC for tartrate salt and/or protein stabilisation of red wines.
Acknowledgements
This work was partly supported by the German Science Foundation (H. König, DFG K0785/17-1) and the Rhineland-Palatinate Foundation for Innovation (H. König & H. Claus, Project-No. 961–386261/1051). Dr Stefan Tenzer was supported by the Research Centre Immunology (FZI) and the Natural Sciences and Medical Research Centre (NMFZ) of the Johannes Gutenberg-University Mainz.




