Influence of the solvent system on the composition of phenolic substances and antioxidant capacity of extracts of grape (Vitis vinifera L.) marc
Abstract
Background and Aims
Grape marc (GM), a byproduct of winemaking, contains significant amounts of substances that are not efficiently used. This study investigated the effect of solvent systems on the extraction of phenolic substances and antioxidant capacity (AC) from the Carignan grape (Vitis vinifera L.) marc, as well as from the peduncle, skin and seed of Carignan.
Methods and Results
The solvent systems tested were (v:v): methanol (MeOH) : water (6:4, 7:3 and 9:1), MeOH : water : acetone (ACE) (3:3.5:3.5) and ethanol (EtOH) : water (6:4, 7:3 and 9:1). The highest extraction of phenolic substances and flavonoids was obtained with EtOH : water (6:4 and 7:3) and MeOH : water : ACE (3:3.5:3.5) followed by MeOH : water (7:3). The highest AC was obtained with MeOH : water : ACE (3:3.5:3.5) followed by EtOH : water (6:4 and 7:3). The concentration of phenolic substances varied from 4.72 to 7.05 g catechin equivalents (CE)/kg fresh mass (FM) for seed extracts, 2.72–3.55 g CE/kg FM for skin extracts and 2.03–2.11 g CE/kg FM for peduncle extracts. Catechin, epicatechin, rutin, myricetin, quercetin, epicatechin gallate, quercetin 3-O-glucuronide, quercetin 3-O-hexoside and malvidin 3-hexoside were found in the marc.
Significance of the Study
The best solvent systems for extracting phenolic substances with high AC from Carignan GM were MeOH : water : ACE (3:3.5:3.5) and EtOH : water (6:4 and 7:3). The main phenolic substances that remained in wine marc after fermentation were myricetin, catechin and epicatechin. The results of this study suggest that Carignan GM, considered an industrial waste, is a natural resource of antioxidants.
Introduction
Grape marc is rich in phenolic substances (PS) but typically is regarded as a waste byproduct from the winemaking industry (De Campos et al. 2008). From grape production, by-products represent approximately 13% w/w (Torres et al. 2002). In 2011, 6 million tonnes of grape waste was produced (Organisation Internationale de la Vigne et du Vin 2012). This by-product is produced after crushing the grapes or after fermentation in winemaking. The fermented product is usually distilled in wineries to recover ethanol (EtOH), which is used further to produce spirituous liquors, leaving large amounts of distilled grape marc (GM) without efficient use after the winemaking process (Rivera et al. 2007). Fortunately, there is an increased interest in the processing of agricultural wastes as a low-cost source of antioxidants (Spigno and De Faveri 2007). In the north-western region of México, the red grape cultivar Carignan is mostly used in the production of fermented beverages, which contributes to the generation of agro-industrial waste.
During the winemaking process, the transfer of PS from grapes to must occurs mainly from the grape skins during the maceration step. Then the sensory attributes of the wines are to a large extent ascribable to the PS located in the cell walls of the grape skin. Phenolic substances in the grape can be classified into three main groups: (i) phenolic acids (mainly benzoic and hydroxycinnamic acids); (ii) simple flavonoids (flavanols, flavonols and anthocyanins); and (iii) tannins and proanthocyanidins (Pinelo et al. 2006b). Grape marc contains three constituents of the grape: seed, skin and peduncle.
Studies have shown GM as a health promoter combating hyperglycemia and inflammatory diseases, as result of its anti-oxidant capacity (AC) (Hogan et al. 2010, Nishiumi et al. 2012). Marc from red grapes has been studied more often than that from white grapes for the extraction of PS. Red GM is one of the best resources of natural antioxidants, the presence of anthocyanin pigments being the main difference between the phenolic profile of white and red grapes (Luque-Rodríguez et al. 2007, Zhang et al. 2011).
Methanol (MeOH) is one of the most widely used solvents for extracting PS from solid grape wastes (Song et al. 2010, Zhang et al. 2011). Moreover, the extraction of PS using alcoholic solvents, in different proportions, has been reported in numerous studies by the affinity of the PS with alcoholic solvents (Spigno and De Faveri 2007). It is not clear which solvent system is the most effective in extracting PS from different materials and consequently, in extracting the AC (Li et al. 2008). A simple procedure (one solvent-one step extraction), however, can give comparable results to that reported in other literature obtained with similar or more complex extraction systems (Spigno and De Faveri 2007).
The aim of this study was to investigate the effect of solvent systems on the extraction of PS and AC from winery GM and from the individual constituents of the marc: peduncle, skin and seed.
Materials and methods
Raw material
A sample of Carignan grape (Vitis vinifera L.) marc (WM) was obtained in July 2010 from the winery Casa Pedro Domecq (Pernod Ricard México) located in Sonora, México. The WM was collected at the end of the primary fermentation (72 h, 8.5°Brix). This marc was separated manually into its individual constituents, seed, skin and peduncle. A sample of marc (GM) from Carignan grapes after crushing and before the start of fermentation (from the same batch of grapes from which the WM was prepared) was used as the control. The samples of WM and GM were stored in hermetically sealed bags at −20°C.
Chemicals
All reagents and solvents employed for the extraction were analytical grade. All reagents, solvents and standards were acquired from Sigma Chemical Co. (St Louis, MO, USA). The solvents used for high-performance liquid chromatography (HPLC) analyses were prepared with MeOH, formic acid and Milli-Q water (EMD Millipore Corporation, Billerica, MA, USA). Calibration curves were prepared for catechin, epicatechin, rutin, myricetin and quercetin.
Extraction of phenolic substances
The solvent systems tested for WM are expressed in volume ratios (v:v or v:v:v) and were as follows: MeOH : water (6:4), MeOH : water (7:3), MeOH : water (9:1), MeOH : water : acetone (MeOH : water : ACE) (3:3.5:3.5), EtOH : water (6:4), EtOH : water (7:3) and EtOH : water (9:1). Phenolic substances from the WM constituents (seed, skin and peduncle) were extracted using systems containing only MeOH. The solvent system EtOH : water (6:4) was selected to obtain extracts of WM and GM because it is considered a less toxic solvent. The PS in the marc were extracted as described by Molina-Quijada et al. (2010), with some modifications. The temperature (25°C), pressure (1 MPa) and particle size (3–5 mm) remained constant. The ground sample (5 g) was extracted twice with 10 mL of each solvent system. The extracts were filtered with Whatman paper No. 2 (GE Healthcare UK Limited, Little Chalfont, Buckinghamshire, England) for subsequent analysis.
Determination of phenolic substances
A sample of 0.05 mL of extract was mixed with 3 mL of distilled water and 0.25 mL of 1N Folin–Ciocalteu solution. After 5 min, 0.75 mL sodium carbonate (20%) followed by 0.95 mL distilled water were added. Absorbance at 765 nm was measured after 30 min with a Cary Model 100 UV-VIS Spectrophotometer (Varian Australia Pty Ltd, Belrose, NSW, Australia) (Tundis et al. 2011). A calibration curve with catechin as the standard was prepared for determination of the sample concentration (Göktürk-Baydar et al. 2007). The concentration of PS in the samples was expressed as g catechin equivalents (CE)/kg fresh mass (FM) GM.
Total flavonoids assay
Total flavonoids (TF) were determined according to Molina-Quijada et al. (2010). One millilitre of extract was added to 4 mL distilled water, 0.3 mL NaNO2 (5%) and 0.3 mL AlCl3 (10%). After mixing well, 2 mL NaOH (5% w/v) were added, and the resulting complex was read at 352 nm (Cho et al. 2004), with quercetin dehydrate as a standard. The results were expressed as g quercetin dihydrate equivalents (QE)/kg extract FM (Zamani et al. 2011).
Identification and quantification of individual PS
Individual PS were identified with a HPLC–mass spectrometer (Agilent 1100 Series LC/MSD ion trap/electro-spray, Agilent, San Jose, CA, USA). A loop of 20 μL and a flow rate of 1 mL/min were used. The relation m/z was obtained from the samples where the signals of the respective substances were most intense and pure. Phenolic substances were quantified with a HPLC (Varian 9012, México D.F., México) equipped with a Supelcosil column LC18 (30 cm × 0.4 cm × 5 μm) (Supelco, Bellefonte, PA, USA) and a diode array detector (Varian Prostar 335 México, D.F., México) at 280, 320, 360 and 370 nm. Mixtures of formic acid 5% (solvent A) and MeOH (solvent B) were used as the mobile phase at a flow rate of 1.2 mL/min in the following gradient: 98:02 to 68:32 in 30 min, 68:32 to 60:40 in 10 min and 60:40 to 05:95 in 10 min. The system returned to 98:02 in 10 min at the same flow rate (Cantos et al. 2000).
The mean peaks of the extracts were identified by comparison with the retention time of commercial standards. Then, fractions of the extracts were collected by HPLC and identified by its ultraviolet-visible (UV-VIS) absorption spectra. Calibration curves (5–30 μg/mL) were used for quantification under the same chromatographic conditions as above. Catechin and epicatechin were quantified with the same HPLC equipped with a fluorescence detector (Varian 9075) at 280 nm of excitation wavelength and 310 nm of emission wavelength (Figure 1). A Microsorb C18 column 100 × 4.6 mm (Varian) was employed, and an isocratic elution with the same mobile phase as above (90:10) at a flow rate of 1 mL/min was used. Phenolic substances were expressed as mg/kg FM.
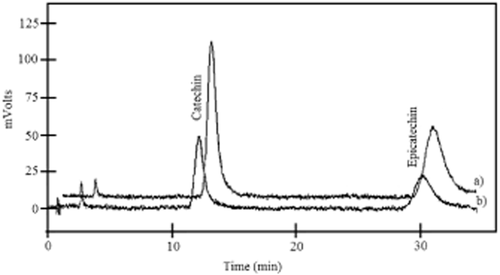
High-performance liquid chromatograms of catechin and epicatechin in (a) a standard mixture of 15 μg/mL each and in (b) an extract of winery Carignan grape marc determined with a fluorescence detector at 280 nm and 310 nm of emission.
Antioxidant capacity
Antioxidant activity analysed with different methods has different reaction characteristics and mechanisms. Therefore, the antioxidant properties of marc extracts were evaluated with two assays: 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2'-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS).
Antioxidant capacity by radical cation (ABTS●+)
This method is based on the reduction of the green/blue colour by ABTS●+ according to the ability of the antioxidant molecules to quench the long-lived ABTS●+, compared with Trolox, a water-soluble vitamin E analogue. A stable stock solution was prepared with 19.2 mg ABTS●+ in 5 mL distilled water. A volume of 0.088 mL potassium persulfate solution (37.8 mg K2SO4 in 1 mL distilled water) was mixed with the stock solution. The mixture was allowed to stand in the dark at room temperature for 12–16 h before use (Pellegrini et al. 2003). The ABTS solution was obtained by the dilution of the stock solution in EtOH to 0.70 ± 0.1 absorbance units (734 nm) at 1 min of reaction in a UV-VIS spectrophometer (Cary 100, Varian Australia Pty Ltd). The results were expressed as μmol Trolox equivalent (TE)/g of extract FM (Molina-Quijada et al. 2010).
DPPH● radical scavenging capacity
This method is based on the ability of the antioxidant to scavenge the DPPH● cation radical. Briefly, 0.1 mL aliquot of sample extract was added to 3.9 mL DPPH● reagent (0.025 mg/mL) and shaken vigorously in a vortex. The reaction was incubated in the dark for 30 min at room temperature. The change in colour was read at 515 nm with a Cary 100 UV-VIS (Molina-Quijada et al. 2010). The radical scavenging activity of the test samples was expressed as μmol Trolox equivalent AC (TE)/g FM (Sreeramulu et al. 2009, Xu et al. 2010).
Statistical analysis
Data were analysed by one-way analysis of variance using SigmaPlot (Systat Software, San Jose, CA, USA). Significant differences among the means were tested using Tukey's test (P < 0.05). Seed, skin and peduncle extracts were compared using the same solvent systems. Experimental data were the means ± standard deviation (SD) of three parallel extractions and analyses.
Results and discussion
Concentration of phenolic substances and total flavonoids
The concentration of PS in the marc extracts range from 1.21 to 5.29 g CE/kg FM (Table 1). The highest extraction of PS was obtained with EtOH : water (6:4 and 7:3) and MeOH : water : ACE (3:3.5:3.5) followed by MeOH : water (7:3).
| Solvent system | PS | TF | DPPH● | ABTS●+ |
|---|---|---|---|---|
| (g CE/kg FM) | (g QE/kg FM) | (μmol TE/g) | (μmol TE/g) | |
| MeOH : water (6:4 ) | 3.22 ± 0.09§ | 2.32 ± 0.09§ | 80.16 ± 0.07‡ | 40.06 ± 1.70‡ |
| MeOH : water (7:3) | 4.05 ± 0.02¶ | 2.55 ± 0.10§¶ | 89.38 ± 0.43§ | 42.72 ± 0.45§ |
| MeOH : water (9:1) | 1.21 ± 0.14† | 1.02 ± 0.02† | 8.01 ± 1.44† | 19.43 ± 1.42† |
| MeOH : water : ACE (3:3.5:3.5) | 4.96 ± 0.14†† | 2.72 ± 0.07¶ | 101.81 ± 2.84¶ | 78.74 ± 1.88†† |
| EtOH : water (6:4) | 5.29 ± 0.25†† | 2.39 ± 0.07§ | 87.13 ± 1.26§ | 76.34 ± 1.71†† |
| EtOH : water (7:3) | 5.18 ± 0.10†† | 2.44 ± 0.06§ | 89.39 ± 4.42§ | 78.24 ± 0.24†† |
| EtOH : water (9:1) | 1.73 ± 0.07‡ | 1.52 ± 0.02‡ | 6.96 ± 0.11† | 18.31 ± 1.83† |
- Values in the same column followed by the same symbol are not significantly different (P > 0.05). Average of three determinations ± standard deviation. ABTS, 2,2'-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid; CE, catechin equivalents; DPPH, 2,2-diphenyl-1-picrylhydrazyl; FM, fresh mass; PS, phenolic substances; QE, quercetin equivalents; TE, Trolox equivalent; TF, total flavonoids.
The constituents of WM were extracted with MeOH : water (6:4 and 7:3) and MeOH : water : ACE (3:3.5:3.5) (Table 2). The concentration of PS among the different extracts varied from 4.72 to 7.05 g CE/kg FM for seed extracts, 2.72 to 3.55 g CE/kg FM for skin extracts and 2.03 to 2.11 g CE/kg FM for peduncle extracts (Table 2). The highest concentration of PS was found in the seeds and skin when MeOH : water : ACE (3:3.5:3.5) was the solvent. Methanol is one of the most widely used solvents for extracting PS from solid grape wastes (Song et al. 2010, Zhang et al. 2011). Therefore, the extraction of PS using MeOH, in different proportions, has been reported in numerous studies by the affinity of the PS to MeOH (Spigno and De Faveri 2007). There are, however, few reports of the food industry employing methanolic extraction systems.
| Constituent | Solvent system | PS | TF | DPPH● | ABTS●+ |
|---|---|---|---|---|---|
| (g CE/kg FM) | (g QE/kg FM) | (μmol TE/g) | (μmol TE/g) | ||
| S | MeOH : water (6:4) | 4.72 ± 0.07†† | 2.75 ± 0.04¶ | 77.19 ± 0.37¶ | 75.15 ± 0.57‡‡ |
| S | MeOH : water (7:3) | 6.00 ± 0.06‡‡ | 3.70 ± 0.02†† | 133.76 ± 1.87§§ | 76.32 ± 0.29§§ |
| S | MeOH : water : ACE (3:3.5:3.5) | 7.05 ± 0.10§§ | 3.71 ± 0.07†† | 126.72 ± 0.36‡‡ | 77.08 ± 0.88§§ |
| C | MeOH : water (6:4) | 2.86 ± 0.07§ | 2.40 ± 0.01§ | 81.37 ± 0.06†† | 40.01 ± 0.49¶ |
| C | MeOH : water (7:3) | 2.72 ± 0.01‡ | 2.32 ± 0.01‡ | 73.56 ± 1.12‡ | 37.44 ± 0.63§ |
| C | MeOH : water : ACE (3:3.5:3.5) | 3.55 ± 0.01¶ | 2.72 ± 0.02¶ | 66.73 ± 2.23§ | 42.86 ± 0.37†† |
| P | MeOH : water (6:4) | 2.11 ± 0.01† | 2.08 ± 0.07† | 27.04 ± 1.19† | 15.70 ± 0.81‡ |
| P | MeOH : water (7:3) | 2.07 ± 0.05† | 2.00 ± 0.02† | 26.57 ± 0.29† | 11.11 ± 0.32† |
| P | MeOH : water : ACE (3:3.5:3.5) | 2.03 ± 0.08† | 2.00 ± 0.09† | 20.99 ± 1.07† | 13.99 ± 0.27† |
- Values in the same column followed by the same symbol are not significantly different (P > 0.05). Average of three determinations ± standard deviation. ABTS, 2,2'-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid); C, skin; CE, catechin equivalents; DPPH, 2,2-diphenyl-1-picrylhydrazyl; FM, fresh mass; P, peduncle; PS, phenolic substances; QE, quercetin equivalents; S, seed; TE, Trolox equivalent; TF, total flavonoids.
In contrast, PS were in the range of 1.02–2.72 g CE/kg FM in the methanolic and ethanolic extracts of WM (Table 1). The highest concentration of PS was found in the WM seed extracts (Table 2).
Pinelo et al. (2006b) published a compilation of several studies of the grape and its constituents, reporting the concentration of PS in the range of 0.37–4.88 g/kg for GM, 3.58–6.20 g/kg for seed, 0.60–4.01 g/kg for skin and 0.22–1.11 g/kg for peduncle. These values were obtained with different solvent systems. Other studies report PS to range from 1.5 to 85.8 g gallic acid equivalents/kg dry mass (DM) (Spigno and De Faveri 2007, Zhang et al. 2011), while Göktürk-Baydar et al. (2007) reported 24 g CE/kg for marc and 704 g CE/kg grape seed, using ACE : water : acetic acid (90:9.5:0.5) in exhaustive extractions. Some PS values reported for winemaking residues range from 18.8 to 33.6 g gallic acid equivalents/kg DM (Zhang et al. 2011) and from 31.5 to 83.4 g QE/kg DM (Negro et al. 2003).
During maceration of grapes before fermentation, the seeds generally remain intact. Therefore, in the primary fermentation, mainly PS contained in the skin are transferred to the wine (Pinelo et al. 2006b).
Individual phenolic substances
The substances identified in WM were the flavonoids catechin, epicatechin, rutin, myricetin, quercetin, epicatechin gallate, quercetin 3-O-glucuronide, quercetin 3-O-hexoside and malvidin 3-hexoside (Figure 2). Peaks, compounds, retention times, m/z in negative mode and m/z fragments are presented in Table 3, where the corresponding peaks from Figure 2 are identified. Myricetin varied from 12 to 51 mg/kg FM in the extracts of WM with all the solvent systems used. Catechin and epicatechin were found at a concentration of 20–38 and 9–27 mg/kg FM, respectively. A lower concentration of rutin was found in the WM with all the solvent systems used (<10 mg/kg FM).
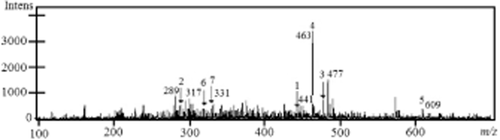
Mass spectra of phenolic substances in an extract of winery Carignan grape marc.
| Peak | Compound | Retention time (min) | M-H (m/z) | Fragments (m/z) |
|---|---|---|---|---|
| 1 | Epicatechin gallate | 3.7 | 441 | 289 |
| 2 | Catechin/epicatechin | 7.3 | 289 | 245 |
| 3 | Quercetin 3-O-glucuronide | 26.5 | 477 | 301 |
| 4 | Quercetin/quercetin 3-O-glucoside | 26.5 | 463 | 301 |
| Peonidin 3-hexoside | 26.9 | 463 | 301 | |
| 5 | Rutin | 27.4 | 609 | 301 |
| 6 | Myricetina | 30.7 | 317 | 300 |
| 7 | Malvidin 3-hexoside | 30.8 | 493 | 331 |
- Mobile phase: formic acid 5% (solvent A), methanol 5% formic acid (solvent B) (Cantos et al. 2000, modified).
Cantos et al. (2000) reported the presence of quercetin and traces of catechin in skins of grapes of cv. Napoleon extracted with MeOH : formic acid (9.07:0.03). In another study by Molina-Quijada et al. (2010), gallic acid, resveratrol and rutin were reported in an extract of Carignan grapes obtained with MeOH : water (6:4).
Table 4 shows a comparison of the concentration of PS in the WM constituents extracted with methanolic solvent systems MeOH : water (6:4 and 7:3) and MeOH : water : ACE (3:3.5:3.5). A significant difference (P < 0.05) among the extracts of seed, skin and peduncle was found. Epicatechin was the main compound found in seeds with the three solvent systems used (52.2–99.7 mg/kg seed FM), whereas myricetin was the main compound in marc skins. Myricetin and catechin in peduncles were the main compounds (Table 4). It is known that peduncles and seeds are rich in monomers of PS (Pinelo et al. 2006b). Li et al. (2008) reported a concentration 230 mg of gallic acid/kg, 1150 mg of catechin/kg and 1230 mg epicatechin/kg in methanolic extracts of seeds from Cabernet Sauvignon grapes. Kammerer et al. (2004) also reported 10 mg of gallic acid, 220 mg of catechin, 13 400 mg of epicatechin and 350 mg of quercetin 3-O-glucoside/kg in skin extracts; and 100 mg of gallic acid, 790 mg of catechin, 670 mg of epicatechin and 32 mg of quercetin 3-O-glucoside/kg in MeOH : HCl (9.09:0.01) extracts of seeds from Weisser Riesling GM. Molina-Quijada et al. (2010) reported 52 mg/kg of resveratrol and 1040 mg/kg of rutin in MeOH : water (6:4) extract of Carignan grape skins. Other PS from grapes extracted with MeOH (5:5) include gallic acid, protocatechuic aldehyde, gentisic acid, catechin, vanillinic acid, caffeic acid, vanillin, epicatechin, syringaldehyde, p-coumaric acid, ferulic acid, sinapic acid and resveratrol (Kammerer et al. 2004). In these reports, the values were higher than those reported in the present study because a large proportion of extractable PS present in the Carignan grapes migrate during the fermentation into the wine.
| Constituent | Solvent system | Rutin | Myricetin | Quercetin | Catechin | Epicatechin |
|---|---|---|---|---|---|---|
| S | MeOH:water (6:4) | 1.70 ± 0.40† | 10.60 ± 1.02† | 1.60 ± 0.30† | 35.40 ± 0.30‡ | 52.20 ± 0.50† |
| S | MeOH:water (7:3) | 2.20 ± 0.20† | 10.40 ± 2.71† | 2.80 ± 0.90†‡§ | 55.50 ± 1.00¶ | 99.70 ± 0.13§ |
| S | MeOH:water:ACE (3:3.5:3.5) | 2.90 ± 0.20‡ | 14.80 ± 0.92§ | 5.50 ± 1.50¶ | 49.90 ± 0.20§ | 92.40 ± 0.13‡ |
| C | MeOH:water (6:4) | 3.90 ± 0.05§ | 74.10 ± 4.85¶ | 2.40 ± 0.14‡ | nd | nd |
| C | MeOH:water (7:3) | 7.10 ± 1.30†† | 66.70 ± 2.60¶ | 6.90 ± 0.01¶ | nd | nd |
| C | MeOH:water:ACE (3:3.5:3.5) | 5.06 ± 0.01¶ | 106.40 ± 2.00†† | 2.90 ± 0.16§ | 15.70 ± 0.30† | nd |
| P | MeOH:water (6:4) | 2.80 ± 0.04‡ | 10.70 ± 0.20† | 2.70 ± 0.32‡§ | 15.70 ± 0.32† | nd |
| P | MeOH:water (7:3) | 3.50 ± 0.35§ | 17.10 ± 2.40§ | 3.00 ± 0.51§ | 16.60 ± 0.55† | nd |
| P | MeOH:water:ACE (3:3.5:3.5) | 2.10 ± 0.50† | 12.50 ± 0.51‡ | 6.90 ± 0.50¶ | 15.90 ± 0.21† | nd |
- Values in the same column followed by the same symbol are not significantly different (P > 0.05). Average of three determinations ± standard deviation. C, skin; nd, not detected; P, peduncle; S, seed.
Rubilar et al. (2007) identified quercetin galactoside, kaempferol, epicatechin, luteolin and gallic acid derivatives in GM from Garnatxa cultivar, and myricetin, quercetin and several gallic acid derivatives in Cabernet Sauvignon GM. Therefore, not only the concentration, but also the type of compound may vary according to the grape cultivar.
Antioxidant capacity
The highest values of AC were found in the WM extracts obtained with MeOH : water : ACE (3:3.5:3.5) followed by EtOH : water (6:4 and 7:3) and MeOH : water (7:3) (Table 1). Floegel et al. (2011) suggested that DPPH and ABTS assays estimate better the AC in fruits. These methods have different reaction characteristics and mechanisms. The ABTS assay is based on a single-electron transfer, end point assay whereby the antioxidant compound is able to donate one or two electrons to reduce the radical cation, while the DPPH assay is mainly based on the normal hydrogen atom transfer reaction between anti-oxidants and the peroxyl radical (Huang et al. 2005). Xu et al. (2010) reported an AC in a range from 76.33 to 649.85 μmol TE/g by ABTS and 52.42 to 422.18 μmol TE/g by DPPH in several grape cultivars, while for Cabernet Sauvignon GM values of 324.62 μmol TE/g by DPPH and 488.86 μmol TE/g by ABTS were obtained.
A significant correlation between the AC and PS was found. Among the constituents of WM, seed extracts showed the highest AC in the range from 77.19 to 133.76 μmol TE/g FM by DPPH and from 75.15 to 77.08 μmol TE/g FM by ABTS (Table 2). The lowest AC values were found for the peduncle extracts. The AC was closely related to the PS concentration in the constituents of WM of the Carignan grape. The values of AC obtained in this study were consistent with those reported for other grape cultivars, where the AC was higher in the seed than in the skin (Yilmaz and Toledo 2004).
Comparison between pre-fermentation and post-fermentation marc
Table 5 shows the comparison of PS, TF and AC between WM and GM extracts. During fermentation, PS are only partially transferred to the wine, and as a result a significant quantity of PS remain in the marc (Pinelo et al. 2006a). In this study, approximately 62% of PS and ∼42% of TF remain in the WM. In contrast, the decrease in the AC of WM obtained after the fermentation process is not large, about 36% by DPPH and about 2% by ABTS, which could be due to the PS remaining in the marc after fermentation.
| Sample | PS | TF | DPPH | ABTS |
|---|---|---|---|---|
| (g CE/kg FM) | (g QE/kg FM) | (μmol TE/g) | (μmol TE/g) | |
| Grape marc | 8.50 ± 0.14‡ | 5.71 ± 0.35‡ | 135.17 ± 0.81‡ | 77.36 ± 0.17‡ |
| Winery marc | 5.29 ± 0.25† | 2.39 ± 0.07† | 87.13 ± 1.26† | 75.83 ± 1.00† |
- Values in the same column followed by the same symbol are not significantly different (P > 0.05). Average of three determinations ± standard deviation. ABTS, 2,2'-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid); CE, catechin equivalents; DPPH, 2,2-diphenyl-1-picrylhydrazyl; FM, fresh mass.; PS, phenolic substances; QE, quercetin equivalents TE, Trolox equivalent; TF, total flavonoids.
Figure 3 shows the individual PS present in the GM. The original phenolic composition in grapes changed after the fermentation process (72 h fermentation, 8.5°Brix); some of the PS, especially catechin and epicatechin, were more easily extracted by the alcoholic liquid, which in this case gives the characteristic odour and flavour to fermented grape beverages.
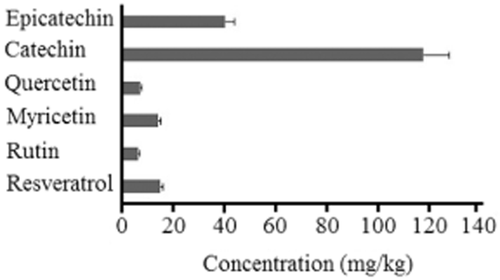
Concentration of individual phenolic substances in a ethanol (EtOH) : water (6:4) extract of Carignan pre-fermentation grape marc.
Conclusions
The best solvent systems for extracting PS with high AC from Carignan GM were MeOH : water : ACE (3:3.5:3.5) and EtOH : water (6:4 and 7:3). It is recommended that more environmentally friendly solvents such as EtOH are used. The main substances that remained in WM after fermentation were myricetin, catechin and epicatechin. Seeds were rich in flavanols. The results of this study suggest that Carignan GM, considered an industrial waste, is a natural resource of antioxidants.
Acknowledgements
The authors thank Casa Pedro Domecq (Pernod Ricard México) for providing the grapes and winery marc, and CONACYT México for financial support (2008-CB-106224 project). Finally, the authors thank María del Refugio Robles Burgueño for her support in the HPLC-mass spectrometer analysis.




