Historical arthropod diversity patterns direct rehabilitation targets for Robben Island, South Africa, a continental island in a biodiversity hotspot
Abstract
enIsland species are susceptible to extinction through disturbances such as habitat transformation. Due to the small size and isolation of islands, species have limited options for refuges and recolonization, making their rehabilitation a conservation priority. Robben Island is a continental island, isolated from the mainland ca. 15 000 years ago, and has been degraded by humans and alien species for nearly 400 years. Mainland areas with similar vegetation should be good reference sites for the biological restoration of the island due to historical connectedness. However, very little information exists as to which species were lost. Here we aim to identify the best mainland sites to use as reference sites for Robben Island based on remaining arthropod diversity on the island. Sites found to be most similar in terms of arthropod diversity to Robben Island were sites north of Robben Island (Elandsbaai and Dwarskersbos) rather than the geographically closest locations. These sites therefore represent ideal reference sites for biological restoration of the island. We do not suggest the reintroduction of species from these localities, but rather Robben Island should be restored to match their vegetation height and cover.
Résumé
frLes espèces insulaires risquent l'extinction en raison de perturbations telles que la transformation de l'habitat. Vu la petite taille et l'isolement des îles, les espèces n'y ont que peu d'options de refuge ou de recolonisation, ce qui fait de leur réhabilitation une priorité de la conservation. Robben Island est une île continentale, isolée du continent il y a quelque 15 000 ans, et elle est dégradée par les hommes et les espèces exotiques depuis près de 400 ans. Les régions du continent qui possèdent une végétation similaire devraient être de bons sites de référence pour la restauration biologique de l'île vu la connectivité historique, mais il existe très peu d'informations quant aux espèces qui auraient disparu. Nous cherchons ici à identifier les meilleurs sites de référence continentaux pour Robben Island, en nous basant sur la diversité des arthropodes qui subsistent encore sur l'île. Les sites identifiés comme les plus comparables à Robben Island en termes de diversité des arthropodes sont situés plus au nord (Elandsbaai et Dwarskersbos), contrairement à des lieux plus proches. Ces endroits sont donc des sites de référence idéaux pour la restauration biologique de l'île. Nous ne suggérons pas de réintroduire des espèces de ces localités, mais de restaurer Robben Island de façon à ce qu'elle reproduise cette hauteur et cette couverture de végétation.
Introduction
Island animal populations are particularly vulnerable to extinctions because of their small ranges and isolation (Gillespie & Roderick, 2002). Extinction probability is greatly amplified by anthropogenic influences such as urbanization, forestry plantations, alien introductions and pollution (Richardson et al., 1996). Of great concern is that island endemics are often lost due to reduction in natural habitat and the subsequent disturbance of any remaining natural areas (Whittaker, 1998). One method of mitigating the loss of species is to rehabilitate altered habitats on islands. The rehabilitation of islands is of particular conservation priority, as islands can act as refugia for some species, as well as preserve island endemics (Whittaker, 1998; Kelly & Samways, 2003; Samways, 2005).
Robben Island is a 507 ha island located 10 km off of the west coast of South Africa and falls within the Core Cape Subregion (previously Cape Floristic Region) (Manning & Goldblatt, 2012) that is well known for its high levels of floral diversity and endemism, and corresponding high arthropod diversity (Wright & Samways, 1998; Goldblatt & Manning, 2000; Mittermeier et al., 2004; Proҁhes & Cowling, 2006; Proҁhes et al., 2009). Robben Island was historically covered by an endangered vegetation type known as Strandveld (Mucina & Rutherford, 2006). This vegetation type is found all along the west coast of South Africa up to the Western Cape/Northern Cape border. However, presently only 60% of Strandveld vegetation remains due to urbanization and land transformation, and of this only 5% is formally protected (NSBA, 2004; Rebelo et al., 2011).
Robben Island, a UNESCO natural and cultural World Heritage Site (Bouchenaki, 2003), has a long history of disturbance, including use as a prison, an asylum, housing a leper colony, and the introduction of alien flora and fauna, particularly sheep, European rabbits and fallow deer, which have degraded the natural vegetation (Brooke & Prins, 1986; Smith, 1997; Deacon, 2004; Roets & Pryke, 2013). These influences have led to the local extirpation of many plant species on the island (Adamson, 1934; Thomb, 1954; Deacon, 2004). Despite these issues, the island is a viable candidate for conservation as a result of the presence of large African penguin populations, which is being a breeding site for eight seabird species (six of which are endemic to South Africa) and the high natural arthropod diversity present on the island (Pastor Makhurane, 2005; Lloyd & David, 2008; ICMP, 2009; Roets & Pryke, 2013).
Little is known of Robben Island's native arthropod species, particularly what was present before human occupation, making it difficult to set a restoration target. However, arthropod populations on islands have been shown to exhibit greater resilience to disturbance than expected from other faunal groups, with rapid reestablishment after alien removal (Priddel et al., 2003; Lawrence et al., 2011; St Clair et al., 2011; Watts et al., 2011; Roets & Pryke, 2013). Determining which arthropod species are native to islands, however, remains a major challenge to conservationists as homogenization and anthropogenic habitat degradation often alter these systems (Gillespie & Roderick, 2002). In this study, we aimed to identify a reference site for Robben Island by conducting comparative studies between arthropod communities associated with its remaining natural habitats and those found at mainland sites. This research gives an indication of what historical arthropod communities on the island may have been, as arthropod communities are usually strongly correlated to specific vegetation types (Labandeira et al., 1994; Johnson, 2004), and only areas with similar Strandveld vegetation were considered (Mucina & Rutherford, 2006; Rebelo et al., 2011). Here we compare the Araneae (spiders), Coleoptera (beetles) and Hymenoptera (bees, ants and wasps) communities of Robben Island to those of seven locations along the west coast of South Africa. These taxa were selected based on their high species richness and abundance on Robben Island (Roets & Pryke, 2013). Identifying mainland areas found to be most similar to Robben Island in terms of these three arthropod groups allows us to set possible restoration targets for Robben Island, as well as providing an idea as to the arthropod composition on the island when it was last connected to the mainland.
Materials and methods
Study area and site selection
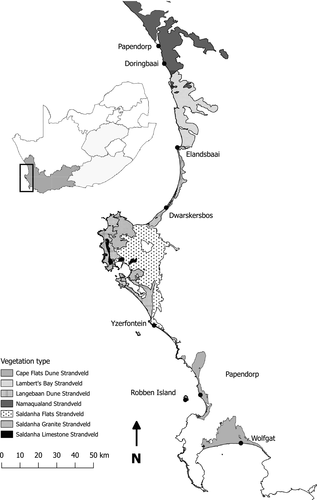
Robben Island (18°22′E 33°49′S) was separated from mainland South Africa between 12 000 and 18 000 years ago due to rising sea levels (Dingle & Rogers, 1972; Tankard, 1976). It is dominated by Cape Flats Dune Strandveld vegetation which is characterized by succulents, geophytes, annuals, and broadleaved shrubs (Goldblatt & Manning, 2000; Mucina & Rutherford, 2006; Rebelo et al., 2011). The closest mainland site to the island is Bloubergstrand (18°27′E 33°47′S) (Fig. 1). An additional six sites along the west coast (ca. 50 km apart) were selected based on the similarity of their vegetation type to that of Robben Island. These sites differ in terms of annual precipitation in the form of a south–north moisture gradient, with the southernmost site, Wolfgat Nature Reserve (18°37′E 34°04′S), receiving ca. 500-650 mm. precipitation p.a., and the northernmost site, Papendorp (18°11′E 31°42′S), receiving less than 350 mm. precipitation p.a. (Fig. 1) (Breedlove & Fraser, 2000).
Sampling and weather data
Sampling was conducted during summer, from January to early March, as arthropod numbers are particularly high during this time (Pryke & Samways, 2008). At each site, four sampling plots were selected opportunistically at 400 m apart, to avoid pseudoreplication, and at approximately 100 m from the coastline. To maximize the number of taxa collected, a range of different sampling techniques were used (Standen, 2000; Hyvarinen, Kouki & Mrtikainen, 2006). At each plot, three yellow pan traps were placed at two-metre interspersing distances to collect anthophiles. These pan traps were constructed from translucent plastic tubs, 115 mm by 50 mm, painted with yellow high gloss enamel paint (True Yellow, Dulux, Alberton, South Africa) as previously shown to be most efficient in collecting anthophiles in similar habitats (Vrdoljak & Samways, 2012). Pan traps were half filled with sea water (to prevent animals from drinking the water) and contained a small amount of detergent to reduce surface tension. To collect epigaeic arthropods, four pitfall traps were set at an interspersing distance of two metres at each plot. These consisted of clear plastic honey jars (60 mm diameter) that were buried in the soil, with their rims level to the soil surface, and filled to approximately one-fifth with preservative (ethylene glycol/water solution in a 50:50 ratio). All traps were set for 3 days, after which all collected arthropods were preserved in 70% ethanol solution for later sorting and identification. In addition, a vacuum sampler was used to collect arthropods associated with vegetation. The vacuum sampler was constructed from a Stihl SH 86 leaf blower/vacuum (Stihl, Germany) with a 150-mm-diameter nozzle fitted with a collection net as described by Stewart & Wright (1995). The nozzle of the vacuum sampler was inserted into the vegetation for approximately 2 s at a time, repeated 100 times per plot. The first plant for sampling was chosen at random, and a comprehensive selection of the vegetation present was sampled, including maximizing the number of plant species sampled, collecting from different heights of the vegetation as well as collecting from both external and internal branches.
All arthropods were identified to family level and assigned to a morphospecies (Samways, McGeogh & New, 2010). Spiders were identified to species level by a spider taxonomist as it is notoriously difficult for nonspecialists to differentiate individuals of the same species for those that are highly colour and/or sex polymorphic. Other focal taxa were much easier to correctly assign to species using the morphospecies approach (Samways, 2005). By identifying spiders to species level, we minimized biases in differences of arthropod assemblages from different sites due to artificial increases in spider morphotypes. All species were classed into functional feeding groups, flying or nonflying and plant or animal associated using Scholtz & Holm (1985). Mobility grouping was ‘flying’ if they either belonged to spider taxa known to disperse via ballooning or were winged for at least one life stage and ‘nonflying’ if not. Plant or animal association was assigned to determine dependence on local vegetation; species were classed as ‘plant associated’ if they depended on plants as their main food source, and ‘animal associated’ if they depended on animals as their main food source. All spider vouchers are housed in the South African National Collection of Arachnida, ARC, Pretoria, South Africa, while the other vouchers are housed in the Stellenbosch University Entomological Collection, South Africa.
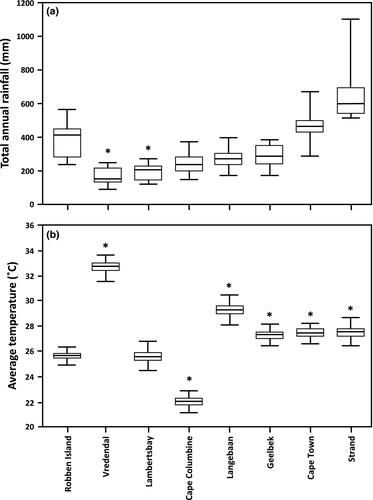
Plant height and cover were measured along a 400-metre transect in each plot, with height and cover measurements taken every 10 m. These data were then averaged per plot. The five most dominant plant species per plot were also recorded. Weather data were obtained from the South African Weather Service (SAWS). Data for total yearly rainfall since 2000 and monthly average maximum temperature for the hottest months (summer, December–February) since 2000 were sourced from stations in closest proximity to arthropod collection sites. These included the following: Strand 00056098 (close Wolfgat), Robben Island 0020618, Cape Town 00208053 (close to Blouberg), Geelbek 00401924 (close to Yzerfontein), Cape Columbine 00606209 (between Yzerfontein and Dwarskersbos), Langebaanweg 00612988 (close to Dwarskersbos), Lambert's Bay 00835728 (close to Elandsbaai) and Vredendal 0106880A2 (close to Papendorp and Doringbaai). Rainfall data were non-normally distributed as determined by a Shapiro–Wilk test in Statistica 12 (Statsoft Corporation, U.S.A.). Median total yearly rainfall data were therefore compared between Robben Island and other sites using a Kruskal–Wallis ANOVA in Statistica. Data for monthly average maximum temperature was normally distributed for all 3 months, and subsequently a one-way ANOVA was performed to compare mean monthly temperature between Robben Island and other sites using the Statistica software.
Data analyses
Alpha-diversity measures were calculated for each of the eight sites for all arthropods collected and per arthropod group separately, using the BAT package (Cardoso, Rigal & Carvalho, 2015) in R (The R Foundation for Statistical Computing, 2015). Alpha-diversity was calculated based on the observed data rarefied using 100 rarefication runs and the maximum number of individuals permitted (the number of individuals found within the least abundant site type). This was needed as species estimators did not reach asymptotes as determined by the slope function in BAT (Walther & Moore, 2005).
Arthropod beta-diversity (as calculated in the BAT package) was compared between Robben Island assemblages and those at the mainland sites using the Sørensen's beta-diversity measure with abundance data for all arthropods combined, and for the different groups separately. Total beta-diversity (βtotal) was deconstructed into βrepl = replacement component of this diversity (i.e. beta-diversity due to species turnover) and βrich = the richness component (beta-diversity due to species loss or gain) (Carvalho, Cardoso & Gomes, 2012). In addition, assemblage composition data were analysed using canonical analysis of principal coordinate (CAP) analyses using PRIMER 6 (PRIMER-E 2008). This method is appropriate for data where a fixed relationship between response and explanatory relationships does not exist, and it allows any dissimilarity measure to be used for constrained ordination (Anderson & Willis, 2003). For these analyses, Bray–Curtis similarity measures were used (with 9999 permutations) with data square-root transformed to reduce the weight of abundant species (Curtis & Bray, 1957; Anderson, 2001).
Results
Overall, 332 species were sampled here with the ants and wasps (Hymenoptera) having the highest number of observed species of all taxonomic groups and the predators the most species-rich functional group (Tables 1 & 2). Rarefied data suggested that Robben Island has fairly similar numbers of species captured compared to the northernmost sites sampled (Doringbaai and Papendorp) (Fig. S1). The sites that were in closest proximity to Robben Island (Blouberg and Wolfgat) had higher species numbers than Robben Island based on rarefied data (Fig. S1). However, Robben Island had similar numbers of species for most arthropod groupings compared to the geographically closest sites. Other sites varied with regard to their similarity in species numbers for the different groupings to Robben Island with most being similar (Fig. S1). Robben Island shared most arthropod species with Blouberg (20%), Dwarskersbos (21%) and Elandsbaai (21%) (Table 1). The high percentage of species shared between Robben Island and Blouberg is likely due to Blouberg having the highest overall species richness (108 species). Robben Island had 21 unique species, 40% of its total species count, but only 6% of the total species count for both Robben Island and the mainland.
| Mainland site | Number of species per site | Shared species between mainland site and RI | Species unique to Robben Island | Species unique to mainland Site |
|---|---|---|---|---|
| Papendorp | 68 | 19 | 49 | 49 |
| Doringbaai | 64 | 20 | 48 | 44 |
| Elandsbaai | 92 | 28 | 40 | 64 |
| Dwarskersbos | 59 | 20 | 48 | 39 |
| Yzerfontein | 108 | 25 | 43 | 83 |
| Blouberg | 108 | 30 | 38 | 78 |
| Wolfgat | 90 | 21 | 47 | 69 |
| All/Robben Island | 68 | 46 | 22 | 272 |
| All | Spiders | Coleoptera | Hymenoptera | Detritivores | Herbivores | Parasitoids | Predators | Non-flying | Flying | Animal dependant | Plant dependant | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S(obs) | Total | 332 | 74 | 95 | 163 | 19 | 41 | 52 | 182 | 39 | 293 | 241 | 90 |
| Papendorp | βtotal | 0.82 | 0.70 | 0.92 | 0.86 | 1.00 | 0.67 | 0.77 | 0.80 | 0.89 | 0.83 | 0.80 | 0.93 |
| βrepl | 0.78 | 0.38 | 0.25 | 0.51 | 0.63 | 0.47 | 0.40 | 0.56 | 0.78 | 0.78 | 0.60 | 0.39 | |
| βrich | 0.04 | 0.32 | 0.68 | 0.35 | 0.37 | 0.21 | 0.37 | 0.24 | 0.11 | 0.05 | 0.20 | 0.54 | |
| Doringbaai | βtotal | 0.82 | 0.85 | 0.86 | 0.76 | 0.65 | 0.97 | 0.64 | 0.79 | 0.59 | 0.82 | 0.79 | 0.91 |
| βrepl | 0.65 | 0.10 | 0.64 | 0.40 | 0.12 | 0.27 | 0.64 | 0.76 | 0.48 | 0.70 | 0.75 | 0.29 | |
| βrich | 0.16 | 0.75 | 0.22 | 0.36 | 0.53 | 0.70 | 0.00 | 0.03 | 0.10 | 0.12 | 0.03 | 0.62 | |
| Elandsbaai | βtotal | 0.66 | 0.71 | 0.85 | 0.58 | 0.59 | 0.78 | 0.82 | 0.68 | 0.73 | 0.69 | 0.69 | 0.74 |
| βrepl | 0.53 | 0.41 | 0.40 | 0.48 | 0.48 | 0.73 | 0.82 | 0.47 | 0.21 | 0.46 | 0.48 | 0.60 | |
| βrich | 0.13 | 0.30 | 0.45 | 0.11 | 0.10 | 0.05 | 0.00 | 0.21 | 0.52 | 0.23 | 0.21 | 0.13 | |
| Dwarskersbos | βtotal | 0.82 | 0.82 | 0.57 | 0.87 | 0.60 | 0.53 | 0.83 | 0.83 | 0.52 | 0.82 | 0.83 | 0.57 |
| βrepl | 0.38 | 0.41 | 0.49 | 0.17 | 0.34 | 0.11 | 0.46 | 0.43 | 0.29 | 0.43 | 0.44 | 0.25 | |
| βrich | 0.44 | 0.41 | 0.08 | 0.70 | 0.26 | 0.42 | 0.37 | 0.40 | 0.24 | 0.39 | 0.40 | 0.32 | |
| Yzerfontein | βtotal | 0.82 | 0.79 | 0.87 | 0.83 | 0.75 | 0.80 | 0.91 | 0.84 | 0.69 | 0.84 | 0.84 | 0.72 |
| βrepl | 0.51 | 0.40 | 0.44 | 0.21 | 0.67 | 0.78 | 0.24 | 0.59 | 0.51 | 0.57 | 0.57 | 0.57 | |
| βrich | 0.31 | 0.40 | 0.43 | 0.63 | 0.08 | 0.02 | 0.67 | 0.25 | 0.18 | 0.27 | 0.27 | 0.15 | |
| Blouberg | βtotal | 0.81 | 0.78 | 0.87 | 0.83 | 0.71 | 0.97 | 0.87 | 0.82 | 0.73 | 0.82 | 0.82 | 0.77 |
| βrepl | 0.46 | 0.28 | 0.54 | 0.19 | 0.64 | 0.27 | 0.23 | 0.54 | 0.35 | 0.53 | 0.52 | 0.52 | |
| βrich | 0.35 | 0.50 | 0.33 | 0.64 | 0.07 | 0.70 | 0.64 | 0.28 | 0.37 | 0.29 | 0.30 | 0.26 | |
| Wolfgat | βtotal | 0.76 | 0.76 | 0.88 | 0.72 | 0.62 | 1.00 | 0.89 | 0.76 | 0.78 | 0.77 | 0.77 | 0.79 |
| βrepl | 0.67 | 0.32 | 0.57 | 0.34 | 0.38 | 0.94 | 0.51 | 0.76 | 0.67 | 0.75 | 0.75 | 0.74 | |
| βrich | 0.09 | 0.44 | 0.31 | 0.38 | 0.24 | 0.06 | 0.37 | 0.00 | 0.11 | 0.01 | 0.01 | 0.05 |
Comparisons of total beta-diversity values obtained when comparing Robben Island arthropod assemblages to mainland sites indicated that for overall assemblages, Elandsbaai was most similar to Robben Island (lowest βtotal value) (Table 2). Overall assemblages from all other sites, including sites geographically closest to Robben Island (Blouberg and Wolfgat), were markedly different from the assemblages collected at Robben Island. Differences in total beta-diversity for overall assemblages between Robben Island and other sites considered were driven more by species turnover (βrepl) than by differences in species richness (βrich) (Table 2). When considering total beta-diversity of the different groupings, Dwarskersbos and, to a lesser extent, Elandsbaai had the lowest βrep, suggesting that these are naturally most similar sites to Robben Island (Table 2). This included the fairly mobile flying arthropod assemblage. Arthropod assemblages from the geographically closest sites (Blouberg and Wolfgat) differed substantially from those collected at Robben Island (Table 2). Most of the differences between Robben Island, Elandsbaai and Dwarskersbos in terms of total beta-diversity were again driven more by species turnover than by differences in species richness.
Canonical analysis of principal coordinates showed no clustering of Robben Island assemblages with other sites for the overall assemblage, predators, spiders, flying species or hymenopterans (Fig. S2). Robben Island beetle assemblages clustered with Dwarskersbos; detritivore clustered with Doringbaai, Wolfgat and Papendorp; herbivores with Dwarskersbos and Papendorp; nonflying species with Wolfgat, Papendorp and Doringbaai; parasitoids with Wolfgat and Papendorp; ants and bees with Elandsbaai, Yzerfontein and Doringbaai; and, most strongly, plant-dependent species with Elandsbaai.
Robben Island had similar vegetation cover as the two most northern sites (Papendorp and Doringbaai), and vegetation height was similar to these two sites and Wolfgat (Fig. 2). Few dominant plants were shared between the sites with only two sites sharing two species with Robben Island (Dwarskersbos and Doringbaai) (Table 3). In terms of total monthly rainfall, an obvious aridity gradient was found from south to north, with rainfall on Robben Island statistically similar to all sites in fairly close proximity (df = 7, N = 109, H = 83.07401, P = <0.001; Fig. 3). Mean average maximum temperature for the three hottest months gave similar results with temperature on Robben Island significantly different to all other stations except Lambert's Bay 00835728 (closest to Elandsbaai) (December: df = 7, N = 115, F = 76.88, P < 0.001; January: df = 7, N = 115, F = 105.89, P < 0.001; February: df = 7, N = 115, F = 142.69, P < 0.001). Only data for February are presented (Fig. 3).

| Plant species | RI | PA | DB | EB | DK | YZ | BB | WG |
|---|---|---|---|---|---|---|---|---|
| Asparagus capensis | 3 | 4 | ||||||
| Calobota sericea | 3 | |||||||
| Carpobrotus edulis | 2 | |||||||
| Cassampebs capensis | 3 | |||||||
| Cissampelos capensis | 1 | |||||||
| Cladoraphis cyperoides | 3 | |||||||
| Conicosia pugioniformis | 2 | |||||||
| Crassothonna cylindrica | 4 | 2 | ||||||
| Cynodon dactylon | 2 | 2 | 4 | |||||
| Ehrharta villosa | 2 | |||||||
| Euclea racemosa | 4 | 4 | 1 | 4 | ||||
| Euphorbia mauritanica | 4 | 4 | 4 | |||||
| Lycium afrum | 2 | |||||||
| Lycium tetrandrum | 1 | 4 | 4 | 4 | ||||
| Olea exasperata | 3 | |||||||
| Oncosiphon sabulosum | 4 | 2 | ||||||
| Oncosiphon suffruticosum | 4 | |||||||
| Osteospermum moniliferum | 4 | 4 | 2 | 4 | ||||
| Pelargonium capitatum | 4 | |||||||
| Searsia crenata | 2 | 4 | ||||||
| Searsia laevigata | 4 | |||||||
| Stoeberia utilis | 4 | |||||||
| Tetragonia fruticosa | 4 | 4 | 4 | 4 | 4 | 2 | ||
| Zygophyllum morgsana | 4 | 4 | 4 | 4 | ||||
| Number of plants in common with RI | 1 | 2 | 1 | 2 | 0 | 1 | 1 |
Discussion
This study shows that the current arthropod assemblage composition of Robben Island is most similar to that of Elandsbaai and Dwarskersbos. The two geographically closest sites to Robben Island (Wolfgat and Blouberg) were dissimilar to Robben Island in terms of arthropod assemblage composition. This dissimilarity is likely the result of current and past climates. After Robben Island's isolation from the mainland, climatic shifts (wetter conditions in the south) probably caused the mainland arthropod assemblage to shift up the west coast to around the Elandsbaai/Dwarskersbos area, whereas the arthropod fauna on Robben Island has remained stranded in situ. Importantly, this result is seen in the flightless arthropods, which supports the idea of an historic climate shift as the primary mechanism dictating contemporary assemblage composition on the island. The current climate of Robben Island is cooler than that of the nearby mainland and most similar to Elandsbaai, which is much drier than Robben Island. This drier state is presumably similar to past precipitation patterns on the island prior to separation from the mainland.
Robben Island's plant-dependent arthropod community is most similar to that of Dwarskersbos and these sites also shared two dominant plant species. However, this area currently has very different vegetation composition, cover and height compared to Robben Island. Host plants affect herbivorous insect demography, and composition of herbivorous insect assemblages associated with a particular plant species depends on geographic location and the plant's architecture and chemistry (Schoonhoven, Jermy & van Loon, 1998). Herbivorous arthropods form the basis of the food chain and so affect primary and secondary predators (Roets & Pryke, 2013). The clustering of Robben Island, Dwarskersbos and Elandsbaai herbivorous species, when all of these sites have comparatively lower summer temperatures, supports arthropod dependence on both vegetation and climatic variables. Additionally, the differences of arthropod assemblages between Robben Island and Blouberg Wolfgat and Yzerfontein indicate that direct transmission between the island and its nearest mainland sites is unlikely. This is exemplified by the flying arthropod assemblage that was most similar to Elandsbaai and highly dissimilar to Blouberg, Wolfgat and Yzerfontein. This, including the geographic isolation of Elandsbaai and Dwarskersbos from both Robben Island and other mainland sites, offers further support for faunal introductions to the island before an historic climate shift.
The second mechanism that could be engendering these results is human-mediated invasion. Artificially higher richness of arthropods species found on Robben Island might be directly associated with human induced introductions or indirectly through associated human disturbance of the environment, which contributes to invasion success (Groves & di Castri, 1996). Invasion and the consequent presence of a subset of species could lead to skewed food webs and thus further disturbance (Whittaker, 1998). The large number of arthropod species unique to Robben Island may also be a result of introductions or alien invasion.
The arthropod assemblage composition on Robben Island, whatever the original colonization mechanism, has no doubt been further altered by the alien plant and animal species present and continued anthropogenic disturbance. Native plants have more herbivorous insect species, which will attract a greater diversity of predators and parasitoids (Schoonhoven, Jermy & van Loon, 1998). Bezeng (2012) found that the phylogenetic diversity of native vegetation on Robben Island was lower than that of invasive alien species on the island. Alien plants, such as those present to a large extent on Robben Island, host fewer herbivorous insect species, and so fewer of the predators and parasitoid wasps that depend upon herbivorous insects (Schoonhoven, Jermy & van Loon, 1998). Environmental conditions are known to affect arthropod development, and all three taxonomic groups included in this study are critical for the maintenance of ecological processes and systems (Strand, 2000; New, 2012). It is possible that a positive feedback system is occurring, whereby the poor vegetation structure on Robben Island is causing altered arthropod richness, which in turn negatively impacts the vegetation present on the island. Thus, habitat restoration of Robben Island and further limitation of further disturbance is imperative.
As arthropod assemblage compositions on Robben Island were most similar to those at Elandsbaai and Dwarskersbos, and less similar to closer mainland sites even though these sites are drier than Robben Island, it is suggested that habitat restoration practices should attempt to match these communities. However, this does not imply that species should be brought in from these areas to be reintroduced, and compound the problems of invasion discussed above, but rather that the local vegetation should match these areas in terms of vegetation structure, height and cover. The vegetation structure on Robben Island is currently most similar to the low and sparse vegetation at Papendorp and Doringbaai, and we suggest restoring the island back to levels closer to Elandsbaai and Dwarskersbos (i.e. average cover > 70% and average vegetation height > 60 cm). The removal of alien invasive plant species from Robben Island is also supported, as these species are homogenizing the island's remaining arthropod habitats. Most importantly, the rehabilitation of Robben Island's biological diversity is recommended.
Acknowledgements
We thank A.S Dippenaar-Schoeman from the University of Pretoria and ARC-PPRI for spider identification, K. C. Oberlander, Sabelo Madlala, Daniel Kandan, Mihynu Qagana, Lyle Claassen, Yaron Truter and Theunette van Heerden for assistance in the field and the Blouberg Nature Reserve, Wolfgat Nature Reserve and Cape Nature for collection permits and permission to work on conserved land. The South African Weather Service (SAWS) is acknowledged for providing the relevant weather data. We also thank two reviewers and the associate editor in charge of our manuscript for their valuable recommendations that improved the quality of the work presented here.




