Assessment of wildlife populations trends in three protected areas in Tanzania from 1991 to 2012
Abstract
enWildlife populations have been experiencing declines across western, central and eastern Africa. In Tanzania, a national wildlife policy was instituted in 1998 to increase protection of wildlife. We assessed (i) the extent to which large herbivore populations have continued to decline in density and distribution within three representative protected areas (PAs) since the implementation of the wildlife policy; (ii) how consistent rates of decline were among the PAs, and between inside the PAs or land bordering these PAs; and (iii) how similar changes in abundance have been among herbivore species or groupings of species. We used aerial census data from 1991 to 2012 for the Tarangire, Ruaha-Rungwa and Katavi-Rukwa PAs for our assessment. Population densities of three of six species or groupings of species dropped by 7.3 ± 3.4% to 11.7 ± 5.8% per year across the three PAs, both inside and outside. Similarly, the extent of the range occupied by these species or groupings in these PAs decreased by 92.3 ± 103.0% to 95.7 ± 102.6% per year. These patterns suggest that the wildlife policy has yet to achieve its aim of reversing the habitat changes and illegal harvests affecting these species.
Résumé
frLes populations d'animaux sauvages ont connu des déclins à travers toute l'Afrique de l'Ouest, centrale et de l'Est. La Tanzanie a institué une politique nationale de la faune sauvage en 1998 pour améliorer sa protection. Nous avons évalué (i) la mesure dans laquelle les populations de grands herbivores ont continué à diminuer, tant en densité qu'en distribution, dans trois aires protégées (AP) représentatives depuis la mise en œuvre de cette politique, (ii) si les taux de déclin sont homogènes entre les AP, et entre celles-ci et les territoires qui les bordent et (iii) dans quelle mesure les changements en matière d'abondance sont semblables entre espèces d'herbivores ou entre groupes d'espèces. Pour notre évaluation, nous avons utilisé des données récoltées par recensements aériens entre 1991 et 2012 pour les Parcs de Tarangire, Ruaha-Rungwa et Katavi-Rukwa. Les densités de population de trois des six espèces ou groupes d'espèces ont chuté de 7,3 ± 3,4% à 11,7 ± 5,8% par an pour toutes les AP, à l'intérieur comme à l'extérieur. Parallèlement, l'étendue de la répartition de ces espèces ou groupes d'espèces a diminué de 92,3 ± 103,0% à 95,7 ± ;102.6% par an. Ces chiffres laissent penser que la politique en matière de faune sauvage est loin d'avoir atteint son but qui est d'inverser les changements d'habitat et les collectes illégales affectant ces espèces.
Introduction
Protected areas (PAs) have been established to help ensure the long-term persistence of wildlife species and habitats. However, the long-term effectiveness of PAs, particularly in Africa, Asia and Latin America, is threatened by habitat destruction through land clearing (Ottichilo, Leeuw & Prins, 2001; Clerici et al., 2007; Nagendra, 2008), fencing (Beale et al., 2013) and illegal hunting (Brashares et al., 2004). Such activities restrict movements of animals into their seasonal dispersal areas, potentially resulting in population decline or extirpation (Ogutu et al., 2009). Nevertheless, PAs have been promulgated worldwide to reduce the rate of loss of species (Sánchez-Azofeifa et al., 2003; Gaveau et al., 2009). As human population growth fuels increased demands for settlements, farmlands and income, fears mount that PAs might fail to achieve their long-term conservation goals (Wittemyer G., Bean W. T. & Burton O., 2008; Mora et al., 2011). Like many nations, Tanzania has demonstrated a commitment to protecting its wildlife resources by allocating 23.8% (224 958 km2) of its total land area to some form of PA for wildlife, including national parks (4.4%), game reserves (GRs) (13%), game controlled areas (GCAs) (5.5%) and conservation areas (0.9%).
Recently, wildlife populations have been declining in Tanzania's PAs (Stoner et al., 2007) and elsewhere in Africa (Blake et al., 2007; Western et al., 2009; Craigie et al., 2010; Hoppe-Dominik et al., 2011). In Tanzania, the primary reasons appear to be illegal hunting (Caro, 2008; Andimile & Caro, 2012) and farming activities (Msoffe et al., 2011). Wildlife populations in Tanzania are also threatened by clearing of vegetation for cultivation (Msoffe et al., 2011), and diversion of river flow for irrigation (Manase, Gara & Wolanski, 2010) especially in the land adjoining the PAs.
In 1998, the Tanzanian government established a national wildlife policy (amended in 2007) aimed at ensuring the survival of species classified as endangered, endemic or rare, and to promote community-based conservation outside the parks. Nevertheless, the Tanzania government retained overriding control over the management of wildlife outside PAs (see Sulle, Lekaita & Nelson, 2011; for details).
Given the changes in wildlife across PAs of Africa, we investigated (i) whether the new wildlife policy has alleviated the downward trends in large herbivore populations that were shown to be under way prior to 2006 (Stoner et al., 2007), (ii) how similar trends have been among selected PAs and between land bordering PAs and that within PA boundaries and (iii) how similar the trends shown by particular herbivore species or grouping of species have been. We anticipated that wildlife policy is effective, resulting in minimal habitat degradation around PAs and hence providing sufficient forage for large herbivore species inside and outside the PAs. Therefore, we expected the (i) populations of large herbivore and (ii) areas they occupied to show increasing trends inside and outside the PAs.
Materials and methods
For this assessment, we used aerial census data spanning 1991 to 2012 for the Tarangire, Ruaha-Rungwa and Katavi-Rukwa PAs and adjoining regions (Fig. 1). The Tarangire-Simanjiro ecosystem covers 12 749 km2 in northern Tanzania and comprises Tarangire National Park (TNP), Lake Manyara National Park, Lolkisale GCA, Mkunganero GR and Simanjiro plains. The TNP is the largest component of the ecosystem covering 2600 km2 at an elevation ranging from 1200 to 1600 m. The park receives short rains, usually between October and December, and heavy rains between February and May. Average total annual rainfall at the park's head office from 1979 to 2009 was 656 mm. The major types of vegetation are riparian woodland, wetlands and seasonal floodplain, Acacia-Commiphora woodland, riverine grassland, Combretum-Dalbergia woodland, Acacia drepanolobium woodland and grasslands with scattered baobab trees. Pastoralism has been the major land use in and around the Tarangire ecosystem over the past two centuries (Prins, 1987). However, in the past two decades, agricultural activities have been increasing on the north-east and eastern sides of the ecosystem (Mwalyosi, 1992).
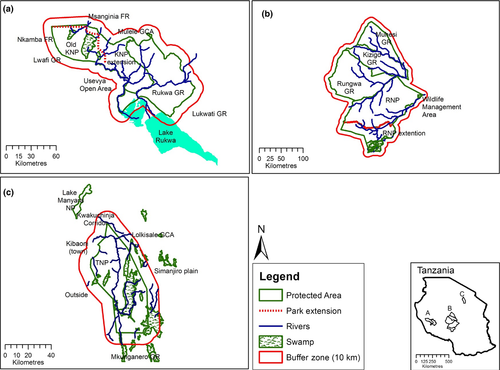
Located in south-western Tanzania, the Katavi-Rukwa ecosystem covers 13 378 km2. It comprises the Katavi National Park (KNP), Rukwa GR, Mlele GCA, Msanginia Forest Reserve (FR), Lwafi GR, Nkamba FR and Usevya Open Area. The KNP is the largest component of the ecosystem covering 4238 km2 at an elevation ranging from 800 m to 1600 m. The park was established in 1974 with 1816 km2 and enlarged in 1998 to the current size. The park receives rainfall once a year from November to May. Average annual rainfall at the park head office during 1997 to 2012 was 955 mm. The major types of vegetation are grassland interspersed with Brachystegia woodlands and mixed woodlands (Frost, 1996; Banda et al., 2006). Pastoralism and horticulture are the major land uses around the Katavi ecosystem (Caro, 1999). Wildfires and damming of the Katuma River for agricultural activities threaten the integrity of the park (Manase, Gara & Wolanski, 2010).
Located in the south-central Tanzania, the Ruaha-Rungwa ecosystem covers 43 641 km2 and comprises the Ruaha National Park (RNP), Rungwa, Muhesi and Kizigo GRs. The RNP is the largest component of the ecosystem covering 20 226 km2 at an elevation ranging from 750 m to 1830 m. The park was established in 1964 with 10 300 km2 and enlarged in 2008 to the current size. The park receives rainfall once a year from December to April. Average annual rainfall as per the data recorded at Msembe Headquarters from 1996 to 2011 amounts 470 mm. The major types of vegetation are Brachystegia woodlands, Acacia woodland, Commiphora-Combretum woodlands and bushed grassland and evergreen forest, dominated by Drypetes gerrardii (Bjørnstad, 1976). Wildfires and damming of the Great Ruaha River for farming irrigation and overgrazing by livestock threaten the integrity of the park (WWF Tanzania Country Office, 2010).
Animal density data
Animal density data from 1991 to 2012 were derived from aerial surveys conducted by the Tanzania Wildlife Research Institute (TAWIRI) using systematic reconnaissance flights (SRFs), following the guidelines designed by Norton-Griffiths (1978). The surveys were conducted during the dry season (September to October) every 1 to 3 years, using two aircraft, each with two parallel rods mounted on the wing struts. The aircraft were flown along parallel transects orientated in the east–west or north–south direction, 5 km apart from each other and about 106 m above ground. Each transect was divided into subunits defined by 30 s flying time, which translates to 1.8 km on the ground. Animals visible between the two parallel rods were counted in each subunit by two experienced observers positioned in the rear seats of the aircraft, while the pilot recorded the geographical position codes at the beginning and end of each transect and informed observers as the flights entered a new subunit. Species' density (individuals km−2) in each subunit was calculated from the number of animals observed and the strip width that covered 300 m per transect, using SISTA software, developed at TAWIRI's Conservation and Information Monitoring Unit specifically for SRFs surveys (TAWIRI, 2010a,b). The survey team also grouped the subunits into a 5 × 5 km grid system of cells covering the surveyed area Fig. 1.
Statistical analysis
Wildlife density data were selected to include only records that were within the boundaries of a PA or within a 10-km buffer region adjoining the park boundaries. The 10-km buffer was chosen to match that used by other researchers (Bruner et al., 2001). All grid cells inside or bordering each PA were considered for analysis. Wildlife density data for 22 species (Table 1) covering periods of six, seven and 5 years were available for a total of 774, 1261 and 3383 grid cells inside or adjoining the Tarangire, Katavi-Rukwa and Ruaha-Rungwa PAs, respectively.
| Scientific name | Common name | Ruaha | Katavi | Tarangire |
|---|---|---|---|---|
| Aepyceros melampus | Impala | ✓ | ✓ | ✓ |
| Alcelaphus buselaphus | Hartebeest | ✓ | ✓ | ✓ |
| Connochaetes taurinus | Wildebeest (blue) | ✓ | ||
| Damaliscus lunatus | Topi | ✓ | ✓ | ✓ |
| Equus burchellii | Zebra | ✓ | ✓ | ✓ |
| Gazella granti | Grant's gazelle | ✓ | ||
| Gazella thomsonii | Thomson's gazelle | ✓ | ||
| Giraffa camelopardalis | Giraffe | ✓ | ✓ | ✓ |
| Hippopotamus amphibius | Hippopotamus | ✓ | ✓ | |
| Hippotragus equinus | Roan antelope | ✓ | ✓ | |
| Hippotragus niger | Sable antelope | ✓ | ✓ | |
| Kobus ellipsiprymus | Waterbuck | ✓ | ✓ | ✓ |
| Kobus vardoni | Puku | ✓ | ||
| Loxodonta africana | Elephant | ✓ | ✓ | ✓ |
| Madoqua spp. | Dikdik | ✓ | ✓ | ✓ |
| Oreotragus oreotragus | Klipspringer | ✓ | ✓ | |
| Oryx gazella | Oryx | ✓ | ||
| Ourebia ourebi | Oribi | ✓ | ✓ | |
| Phacochoerus africanus | Warthog | ✓ | ✓ | ✓ |
| Potamochoerus larvatus | Bushpig | ✓ | ||
| Redunca spp. | Reedbuck | ✓ | ✓ | ✓ |
| Sylvicapra grimmia | Duiker | ✓ | ✓ | ✓ |
| Syncerus caffer | Buffalo | ✓ | ✓ | ✓ |
| Taurotragus oryx | Eland | ✓ | ✓ | ✓ |
| Tragelaphus imberbis | Lesser Kudu | ✓ | ✓ | |
| Tragelaphus scriptus | Bushbuck | ✓ | ✓ | |
| Total | 22 | 21 | 19 | |
Only species recorded in at least four grid cells per PA were selected for statistical analysis, and those recorded in less than four grid cells were grouped together, based on female body weight. Accordingly, elephant (Loxodonta africana), giraffe (Giraffa camelopardalis), buffalo (Syncerus caffer) and zebra (Equus burchellii) were analysed individually, and the remaining species were grouped as small or medium antelopes, respectively. If two or more species of the same grouping were encountered in the same grid cell, the sum of their densities was computed. The small antelopes included bovines with adult female body weight ranging between 40 and 100 kg (lesser kudu (Tragelaphus imberbis), bushbuck (Tragelaphus scriptus), impala, topi, puku (Kobus vardoni)), and Grant's gazelle) plus warthog. Medium antelopes included bovines with 100-500 kg female body weight range (waterbuck (Kobus ellipsiprymus), hartebeest (Alcelaphus buselaphus), wildebeest (Connochaetes taurinus), roan antelope (Hippotragus equines), sable antelope (Hippotragus niger), eland (Taurotragus oryx) and oryx (Oryx gazelle)). Small antelopes with female mean body weight less than 40 kg, hippopotamus (Hippopotamus amphibius) and bush pigs (Potamochoerus larvatus) were excluded from the analysis because they are difficult to detect using aerial surveys.
Average annual densities (individuals km−2) were computed for the six species or groups by taking the summation of their densities in grid cells where they were recorded and dividing it by (i) the total number of grid cells across the entire park area (inside or outside) or, alternatively, (ii) the total number of grid cells where the species or species grouping were present. The first procedure assumed that each of the grid cells in the PA was available to be utilized, whereas the second assumed that the animals would most likely be restricted to grid cells with suitable habitats. The number of grid cells occupied by large herbivore species or species grouping was also assessed, to determine whether species distributions were shrinking or expanding over time within the PAs.
Prior to the analyses, the average animal density (number of individuals per km2) and the number of 5 × 5 km2 grid cells (counts) occupied by large herbivore species or groups were examined using scatter plots and tested for normality using the Ryan–Joiner normality test. Both the average animal density and number of occupied grid cells were non-normally distributed. We therefore analysed trends in both variables using generalized linear models. The models assumed a lognormal error distribution and an identity link function. As some density estimates were zero, we added 1 to density prior to analysis. The model was fitted using GLIMMIX procedure in SAS software version 9.2 (SAS Institute Inc. 2010).
The trend analyses were used to: (i) test whether the average densities of the six large herbivore species or groups of species and the average number of grid cells occupied by each species or groups of species had changed significantly over time and (ii) compare the rates of change between matched locations inside and outside each of the three PAs. The models included three independent variables, year (n = 7), the PAs (n = 3) and location (inside vs. outside the PAs), and all their two- and three-way interactions as fixed effects. The year variable was treated as a quantitative rather than a categorical variable and location and PA as categorical variables with two and three levels, respectively. We contrasted the temporal trend slopes for density and grid cell occupancy for each species or group of species between locations inside and outside each PA and between PAs.
The estimated temporal trend slopes were back-transformed from the logarithmic to the original scale using the expressions for animal density and
for animal density and  for cell occupancy. The GLIMMIX procedure was also used to test for temporal change in annual rainfall in the PAs. The model included two independent variables, year [n = 17 (Tarangire), 14 (Katavi), 14 (Ruaha)] and the three PAs.
for cell occupancy. The GLIMMIX procedure was also used to test for temporal change in annual rainfall in the PAs. The model included two independent variables, year [n = 17 (Tarangire), 14 (Katavi), 14 (Ruaha)] and the three PAs.
Results
The annual rainfall did not change significantly over the period covered in any of the three PAs (Katavi: F1, 12 = 0.06, P > 0.05; Ruaha: F1, 12 = 0.25, P > 0.05; and Tarangire: F 1, 15 = 3.83, P > 0.05). Of the six herbivore species or groupings of species considered, three experienced significant change in density across the combined area of the three PAs between 1991 and 2012, including giraffe, medium antelopes and zebra (Table 2). Their average densities declined both inside the PAs and in the adjoining land between 1991 and 2012 by estimated annual rates ranging from 0.9 ± 4.0% to 7.3 ± 3.4% (giraffe), 7.3 ± 6.5 to 12.0 ± 7.2% (medium antelopes) and 4.4 ± 6.1% to 11.7 ± 5.8% (zebra) (Table 3). The average density for the small antelopes in Tarangire ecosystem declined by estimated rate of 7.7 ± 3.9% per year between 1994 and 2012, while not changing significantly in Katavi-Rukwa and Ruaha-Rungwa (Table 2 and 3).
| Species | Source | ||||||
|---|---|---|---|---|---|---|---|
| Year | Location | Year* location | PA | Year*PA | PA* location | Year* PA*location | |
| Buffalo | F1,24 = 0.81 | F1,24 = 0.81 | F1,24 = 0.79 | F2,24 = 0.78 | F2,24 = 0.78 | F2,24 = 1.5 | F2,24 = 1.5 |
| Elephant | F1,24 = 0.01 | F1,24 = 0.7 | F1,24 = 0.72 | F2,24 = 1.0 | F2,24 = 1.0 | F2,24 = 0.16 | F2,24 = 0.16 |
| Giraffe | F1,24 = 6.16* | F1,24 = 0.05 | F1,24 = 0.05 | F2,24 = 1.59 | F2,24 = 1.59 | F2,24 = 0.16 | F2,24 = 0.16 |
| Medium antelopes | F1,24 = 8.35* | F1,24 = 0.22 | F1,24 = 0.22 | F2,24 = 2.99 | F2,24 = 2.95 | F2,24 = 0.85 | F2,24 = 0.85 |
| Small antelopes | F1,24 = 1.90 | F1,24 = 0.01 | F1,24 = 0.00 | F2,24 = 4.23* | F2,24 = 4.21* | F2,24 = 0.09 | F2,24 = 0.09 |
| Zebra | F1,24 = 5.66* | F1,24 = 0.15 | F1,24 = 0.15 | F2,24 = 2.55 | F2,24 = 2.53 | F2,24 = 0.57 | F2,24 = 0.57 |
- F-values with an asterisk sign (*) are significant at P ≤ 0.05; and F-values without any signs mean that the corresponding effects were not significant at the 5% significance level.
| Species/group | Protected area | Slope ± Error (per year) | No. of years | DF | t-value | Pr > |t| |
|---|---|---|---|---|---|---|
| Buffalo | KNP | −0.020 ± 0.100 | 1991 – 2012 | 23 | −0.21 | 0.83 |
| RNP | −0.082 ± 0.120 | 1993 – 2011 | 23 | −0.75 | 0.46 | |
| TNP | −0.054 ± 0.112 | 1994 – 2012 | 23 | −0.52 | 0.61 | |
| Elephant | KNP | 0.051 ± 0.049 | 1991 – 2012 | 24 | 1.03 | 0.31 |
| RNP | −0.014 ± 0.059 | 1993 – 2011 | 24 | −0.26 | 0.80 | |
| TNP | 0.010 ± 0.055 | 1994 – 2012 | 24 | 0.2 | 0.85 | |
| Giraffe | KNP | −0.032 ± 0.031 | 1991 – 2012 | 24 | −1.07 | 0.30 |
| RNP | −0.009 ± 0.036 | 1993 – 2011 | 24 | −0.25 | 0.80 | |
| TNP | −0.073 ± 0.034 | 1994 – 2012 | 24 | −2.24 | 0.03 | |
| Medium antelopes | KNP | −0.073 ± 0.065 | 1991 – 2012 | 23 | −1.2 | 0.24 |
| RNP | 0.0098 ± 0.077 | 1993 – 2011 | 23 | 0.13 | 0.90 | |
| TNP | −0.120 ± 0.072 | 1994 – 2012 | 23 | −1.83 | 0.08 | |
| Small antelope | KNP | 0.005 ± 0.035 | 1991 – 2012 | 23 | 0.14 | 0.89 |
| RNP | 0.020 ± 0.042 | 1993 – 2011 | 23 | 0.49 | 0.63 | |
| TNP | −0.077 ± 0.039 | 1994 – 2012 | 23 | −2.09 | 0.05 | |
| Zebra | KNP | 0.005 ± 0.052 | 1991 – 2012 | 23 | 0.1 | 0.92 |
| RNP | −0.044 ± 0.061 | 1993 – 2011 | 23 | −0.76 | 0.46 | |
| TNP | −0.117 ± 0.058 | 1994 – 2012 | 23 | −2.22 | 0.04 |
- Pr > |t| is the probability of obtaining a t statistic greater in absolute value than that observed given that the true slope is equal to 0. If P ≤ 0.05 means, the slope is statistically different from 0.
The average number of grid cells occupied by giraffe and zebra declined significantly between 1991 and 2012 across the combined area of the three PAs (Table 4). In the case of buffalo and medium antelopes, their distributional range differed significantly among the PAs over time (Table 4). Elephants and small antelopes retained similar distributional ranges over the PAs over time (Table 4). Overall, the area occupied by giraffe and zebra decreased across the combined area of the three PAs, both inside and outside, by estimated rates ranging from 94.8 ± 102.7% to 99.2 ± 103.2% (giraffe) and 93.0 ± 102.1% to 98.1 ± 102.1% (zebra) grid cells per year between 1991 and 2012 (Table 5). The area occupied by buffalo both inside and outside the Katavi-Rukwa declined by an estimated annual rate of 95.7 ± 102.6% between 1991 and 2012, and 97.1 ± 31.0% in Ruaha-Rungwa between 1993 and 2011, but increased by 101.6 ± 102.9% in Tarangire between 1994 and 2012 (Table 5). The distributional range for buffalos in Tarangire and Ruaha-Rungwa expanded significantly faster than in Katavi-Rukwa with estimated annual rate of 88.0 ± 103.0% (Table S1). The area occupied by medium antelopes inside and outside the Katavi-Rukwa significantly declined with annual rate of 92.3 ± 103.0% between 1991 and 2012 while not showing significant changes in Ruaha-Rungwa and Tarangire ecosystems (Tables 5 and S1). On the other hand, the analysis of the average densities for the six large herbivore species or groups considering only areas where they were present across the PAs, both inside and outside, revealed no significant changes (P > 0.05) over the years.
| Species | Source | ||||||
|---|---|---|---|---|---|---|---|
| Year | Location | Year * location | PA | Year*PA | PA *location | Year* PA*location | |
| Buffalo | F1,23 = 0.14 | F1,23 = 1.43 | F1,24 = 1.34 | F2,23 = 9.56* | F2,23 = 9.49* | F2,23 = 3.15 | F2,23 = 3.17 |
| Elephant | F1,24 = 2.57 | F1,24 = 1.13 | F1,24 = 1.2 | F2,24 = 1.7 | F2,24 = 1.67 | F2,24 = 0.17 | F2,24 = 0.18 |
| Giraffe | F1,24 = 4.81* | F1,24 = 0.97 | F1,24 = 0.92 | F2,24 = 2.13 | F2,24 = 2.17 | F2,24 = 0.52 | F2,24 = 0.53 |
| Medium antelopes | F1,23 = 1.74 | F1,23 = 1.9 | F1,23 = 1.84 | F2,23= 4.52* | F2,23 = 4.56* | F2,23 = 0.17 | F2,23 = 0.17 |
| Small antelopes | F1,23 = 0.11 | F1,23 = 0.87 | F1,23 = 0.84 | F2,23 = 0.57 | F2,23 = 0.58 | F2,23 = 0.03 | F2,23 = 0.03 |
| Zebra | F1,23 = 11.76* | F1,23 = 0.77 | F1,23 = 0.70 | F2,23 = 0.47 | F2,23 = 0.49 | F2,23 = 1.98 | F2,23 = 2.00 |
- F-values with an asterisk sign (*) are significant at P ≤ 0.05; and F-values without any signs mean that the corresponding effects were not significant at the 5% significance level.
| Species/Group | Protected Area | Slope ± Error (per year) | No. of years | DF | t-value | Pr > |t| |
|---|---|---|---|---|---|---|
| Buffalo | KNP | −0.957 ± 1.026 | 1991 – 2012 | 23 | −1.71 | 0.05 |
| RNP | −0.971 ± 1.031 | 1993 – 2011 | 23 | −0.99 | 0.33 | |
| TNP | 1.016 ± 1.029 | 1994 – 2012 | 23 | 0.56 | 0.58 | |
| Elephant | KNP | 1.050 ± 1.030 | 1991 – 2012 | 24 | 1.62 | 0.12 |
| RNP | 1.011 ± 1.036 | 1993 – 2011 | 24 | 0.31 | 0.76 | |
| TNP | 1.051 ± 1.034 | 1994 – 2012 | 24 | 1.49 | 0.15 | |
| Giraffe | KNP | −0.948 ± 1.027 | 1991 – 2012 | 24 | −2.03 | 0.05 |
| RNP | −0.992 ± 1.032 | 1993 – 2011 | 24 | −0.27 | 0.79 | |
| TNP | −0.951 ± 1.030 | 1994 – 2012 | 24 | −1.71 | 0.10 | |
| Medium antelopes | KNP | −0.923 ± 1.030 | 1991 – 2012 | 23 | −2.76 | 0.01 |
| RNP | 0.996 ± 1.035 | 1993 – 2011 | 23 | −0.10 | 0.92 | |
| TNP | −0.979 ± 1.033 | 1994 – 2012 | 23 | −0.66 | 0.51 | |
| Small antelope | KNP | −0.963 ± 1.041 | 1991 – 2012 | 23 | −0.94 | 0.36 |
| RNP | −1.000 ± 1.049 | 1993 – 2011 | 23 | −0.01 | 0.99 | |
| TNP | −0.971 ± 1.046 | 1994 – 2012 | 23 | −0.67 | 0.51 | |
| Zebra | KNP | −0.981 ± 1.021 | 1991 – 2012 | 23 | −0.92 | 0.37 |
| RNP | −0.974 ± 1.025 | 1993 – 2011 | 23 | −1.07 | 0.30 | |
| TNP | −0.930 ± 1.024 | 1994 – 2012 | 23 | −3.09 | 0.01 |
- Pr > |t| is the probability of obtaining a t statistic greater in absolute value than that observed given that the true slope is equal to 0. If P ≤ 0.05 means, the slope is statistically different from 0.
Discussion
The distributional ranges of giraffe and zebra had decreased significantly between 1991 and 2012 both within the three PAs considered, and in land adjoining them, while elephant and small antelopes showed no significant reduction in range. The range occupied by buffalo decreased within Katavi-Rukwa and Ruaha-Rungwa but increased significantly within TNP and adjoining land. That occupied by medium antelopes decreased significantly within Katavi-Rukwa and adjoining land, but not in Ruaha-Rungwa and TNP. Nonetheless, the densities of the six species or groups of species within their occupied ranges did not change significantly. While declines in abundance were contemporaneous with range contraction, there was no evidence to indicate that habitat contraction caused the declines. The combined density of small antelopes decreased significantly within TNP and adjacent areas but not in Ruaha-Rungwa or Katavi-Rukwa.
The declining trend for giraffe is contrary to the findings of Stoner et al. (2007), who reported that giraffe numbers had remained stable between 1988 and 2001 in Tanzania's PAs that they considered. The declines by medium antelopes and zebra confirmed their population decreases in four of eight PAs in the country reported by Stoner et al. (2007). The stable trends for elephant are contrary to increasing population trends across Tanzania's PAs between 1989 and 2006 reported by CITES (2010) and also contrary to TAWIRI's (2014) report of 50% population declines across the country by 2014. Foley and Faust (2010) reported increasing trends for the elephant population in TNP between 1993 and 2005. Our analysis did not encompass the 2013 and 2014 aerial surveys.
Our results show similar patterns of decline in densities of large herbivore species or groups of species within the PAs and in the adjoining lands, contrary to our expectation that the densities of large herbivore would show increasing trends over time as a result of implementation of the new wildlife policy. These findings are similar to those of Stoner et al. (2007) who reported overall declining densities of large herbivores in PAs and nonprotected areas in Tanzania between 1988 and 2002. Likewise, Ogutu et al. (2011) found uniformly declining densities of large herbivore species within the Masai Mara National Reserve and the adjoining pastoral lands between 1977 and 2009. Our findings for Katavi-Rukwa ecosystem show different patterns from those of Caro (1998) who found higher densities of large herbivore species within the park than in the adjoining lands between 1988 and 1996 where human settlement and poaching were prevalent. In this study, we found no significant difference in densities of elephant within and outside the PAs, which is contrary to Kioko et al. (2013) who found a higher density of this species inside than outside the park in the adjoining Manyara Ranch.
We found declining distribution ranges for buffalo, giraffe, medium antelopes and zebra within one or all the three PAs and also in the adjoining lands, contrary to our expectation that area occupied by large herbivore would expand over time as a result of implementation of the new wildlife policy. This finding is similar to that of Kiffner, Stoner & Caro (2013) who found the occupancy of buffalo, zebra, giraffe, topi and waterbuck to have declined both inside and within a 5 km periphery of the Katavi National Park between 1987 and 2009.
In the Tarangire-Simanjiro ecosystem, buffalo, elephant, zebra and wildebeest migrate every year from the park to the Simanjiro plains at the start of the wet season (November/December) and return in the dry season (June/July) (Kahurananga & Silkiluwasha, 1997). However, wildlife migratory routes and dispersal areas have been affected by increased cultivation in these ecosystems (Msoffe et al., 2011). This habitat degradation may have contributed to the reduction of wet season ranges for buffalo, medium antelopes and zebra in this region. A similar situation occurred within areas adjacent to the Mara region in Kenya, where populations of large herbivores had declined between 1977 and 2009 because of habitat degradation, fragmentation and increased livestock grazing (Ogutu et al., 2011). In the Ruaha-Rungwa ecosystem, certain large herbivore species move seasonally over long distances searching for water (Epaphras et al., 2008), but this situation does not occur in Katavi-Rukwa ecosystem (Caro, 1999). However, during the dry season, elephant and buffalo are more frequently observed in the core area of this park than on its periphery, presumably driven by water availability (Kiffner, Stoner & Caro, 2013).
The shrinkage of ranges occupied by buffalo, giraffe, medium antelopes and zebra and the subsequent declines in the abundance of the latter three species and medium antelopes may also have been exacerbated by poaching. Escalating poaching has been suggested as the reason for the declines of small and medium antelopes, giraffe, buffalo and zebra between 1980s and 2009 in Katavi region (Andimile & Caro, 2012), and elephant (Dublin et al., 1990) buffalo and black rhinos (Metzger et al., 2007) in the western and northern portions of Serengeti National Park and Masai Mara National Reserve (Walpole et al., 2001).
Previous reports show that declines in abundances of large mammals across protected areas had been occurring prior to the establishment of the wildlife policy (Stoner et al., 2007). Our results confirm continued declines in abundances of large herbivores within PAs and in the adjoining land, indicating that the new wildlife policy has not yet been successful in reversing the habitat changes and illegal harvests affecting these species. The USAID (2013) evaluation report revealed weaknesses in the implementation of the wildlife policy component dealing with protection of wildlife outside PAs (WMAs) due to a lack of transparency and accountability among stakeholders, and incomplete devolution of responsibilities to WMA from the central government. To avoid further declines, we recommend rectification of these weaknesses. However, other factors such as climatic influences (Ogutu & Owen-Smith, 2003; Ogutu et al., 2007) might have contributed to the observed declines. We also recommend a further study to explore possible contribution of climate change to the declines of large herbivore populations in Tanzania's PAs.
Acknowledgements
We are grateful to the management team of TAWIRI particularly Simon Mduma, Honori Maliti, Machoke Mwita and Hadia Haji for provision of the wildlife count survey data; Andrew Taylor for advice on statistical analysis, the three anonymous reviewers for their comments, and the International Ford Foundation Fellowship Program, New York, USA for funding.




