Anatomical and surface ultrastructural investigation of the tongue in the straw-coloured fruit bat (Eidolon helvum, Kerr 1972)
Abstract
The morphology of tongue in straw-coloured fruit bat from tropical forests was evaluated in relation to frugivorous diets and in comparison with other species that consumes other food types. Gross, stereomicroscopy, scanning electron microscope and histological methods were used. The tongue was relatively long with round tip, which closely fitted into oral cavity. Five types of mechanical papillae included crown-like and trifid filiform papillae. Also bulky, cone-shaped papillae and long conical papillae were identified. These mechanical types also showed variations in shape, size and number of processes of papillae. Transitional forms of these mechanical papillae were present. Fungiform papillae with taste pores were interposed amongst filiform types in apex and body; three ovoid-shaped vallate papillae were in triangular arrangement on root and displayed taste pores. Some bulky, cone-shaped papillae surrounded the vallate papillae. Histologically, mechanical filiform types showed highly keratinized stratified squamous epithelium and dense connective tissue core with secondary papillae. Taste buds appeared in fungiform and vallate papillae. Neutral and acidic secretions were identified in lingual glands of root. The presence of prominent filamentous processes of filiform papillae and conical papillae of the tongue in conjunction with gustatory papillae ensures adaptation to copious fruit diets. The gross morphometric and histometric parameters of the tongue did not differ remarkably from previous values obtained for some fruit bats with comparable weight. This investigation showed similarities with fruit bats such as large flying fox and Egyptian fruit bat and reflect common diet and feeding habits but varied from insectivorous and nectivorous bats.
1 INTRODUCTION
The tongue plays essential role in acquisition, sorting of feed and suckling of new born in several mammals. It also has mechanical or gustatory functions by means of taste buds and additionally helps in the masticatory and swallowing role of the oral cavity along with other organs. Feeding habits and mechanisms are important factors that ensure the success of adaptations of many vertebrates to their environment (Darwish, 2012). The tongue and its papillae structures enhance the ability to feed on nectar and fruit and show significant morphological variations that reflect feeding ecology (Birt et al., 1997; Iwasaki, 2002; Trzcielinska-Lorych et al., 2009). In this regard, available literature indicates little attention to the morphology of the tongue of megachiropteran species of Africa origin.
The straw- coloured fruit bat, Eidolon helvum is a megachiropteran (Old world fruit bats or flying foxes) bat that belongs to the family Pteropodidae and is wide spread, being the most conspicuous migratory megachiropteran species in Africa (Cosson et al., 1996). The bat weighs about 250–310 g, with an average wingspan of 80 cm, and show little sexual dimorphism (Taylor, 2000). The megachiropteran bats feed on pollen, nectar, fruit, leaves and bark of native plants and therefore have been highly involved in the pollination and dispersal of seeds of several trees and plants (Izhaki et al., 1995; Ramteke, 2016; Valiente-Banuet et al., 1996). Approximately 75% of Chiroptera are insectivorous, feeding mainly on beetles and moths. About 20% are frugivorous (Pteropodidae and Phyllostomidae) and only a small number (about 2%) are predators of small mammals, reptiles, amphibians and fishes. Also only about three extant species (vampire bats) are haematophagous (Walldorf & Wehlhorn, 2014). In West Africa, Eidolon helvum (Pteropodidae) is a critically important seed dispersal agent for the African Iroko Milicia excelsa (timber tree), an economically important and threatened tree (Taylor, 2005).
The bats can switch between diet of fruit and nectar depending on availability. This variable mode of feeding, while it may suggest possible survival adaptation mechanism to seasonal food economy within the ecosystem, does not evidently indicate any functional role of a particular megachiropteran species within an ecosystem (Birt et al., 1997). It may be that the tongue of the fruit bat is not adapted for a diet of nectar as in some nectar-feeding pteropodid bats such as brush tips of the tongue (Birt et al., 1997). The mode of feeding in E. helvum is typical for pteropodid bats; whereby fruit is mashed between teeth by rapid tongue movements, stored in cheek pouches and juices are sucked from the mashed fruits. Small seeds are spit out as dry pellets (DeFrees & Wilson, 1988).
The general morphology of the tongue and its surface papillae in megachiropteran bats seem to display a strong relationship with the type of food consumed within the same geographical boundaries (Birt et al., 1997; Nishimura & Kato, 1993; Pastor et al., 1993; Yoshimura et al., 2009). For instance, the shape of the tongue has been shown to differ considerably in some Australian megachiropterans, suggesting subtle differences between individual feeding strategies (Birt et al., 1997), suggesting the need for understanding the tongue morphology of the various species. Previous studies on the tongue of some species of nectivory, frugivory and insectivory megachiropterans indicated significant variations in lingual papillae resulting from adaptations to the intake of liquid and semi-liquid food within the geographical environment (Abayomi et al. 2009; Abumandour & El-Bakary, 2013; Abumandour, 2014; Emura et al., 2001; Emura et al., 2012; Masuko et al., 2007; Mqokeli & Downs, 2013; Sharma et al., 1999; Taki-El-Deen et al., 2013; Trzcielinska-Lorych et al., 2009).
There is considerable information on the tongue structure of some Pteropodid bat species which have been relevant in the taxonomic and phylogenic applications (Emura et al. 2001; Jackowiak et al., 2009). Though, few basic morphometric and histologic data of the tongue have been reported on E. helvum (Abayomi et al. 2009; Igado et al. 2015). However, a comprehensive morphometric, histologic and surface ultrastructural information of the tongue is lacking on the straw-coloured fruit bat (E. helvum) species. The present work is designed to anatomically evaluate and document the anatomic and surface ultrastructural features of the dorsum of the tongue in the straw-coloured fruit bat by gross study, scanning electron microscopy (SEM) and histology. The results were compared with other fruit-eating and nectar-sucking micro- and megachiropteran bats.
2 MATERIALS AND METHODS
2.1 Experimental animals and procedures
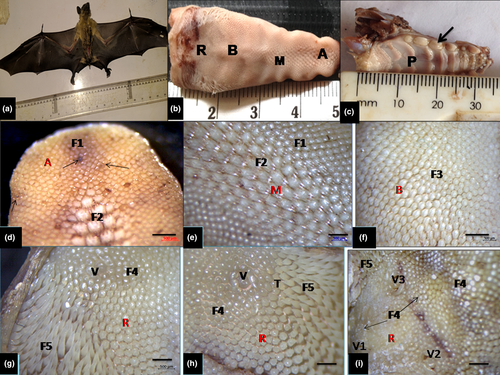
A total of fourteen (14) sexually matured E. helvum bats of both sexes (7 males and 7 females) were obtained by net-trapping from their roosting tree environment were utilized for this study (gross, light microscopy and scanning electron microscopy). They weighed between 230 and 340 g with wing spanning up to 82 cm (Figure 1a). The specimens were obtained in Nigeria in compliance with NIH Guideline for care and Use of Laboratory Animals, 8th edition (National Research Council (US) of the National Academies, 2011) as approved by the University of Nigeria (Nsukka).This bat is not under any conservation list in Nigeria and it is not in the Red List of threatened species according to International Union for Conservation of Nature (IUCN).They were examined to be in apparently good health and handled humanely. The bats were subsequently anaesthetized with intraperitoneal injection of sodium thiopental behind the last rib (20 mg/kg, Wellona Pharma) for adequate handling, weighing and further examination and thereafter euthanized by an overdose of the same drug following approved ethical procedures for use of animals in research (ASM Animal Care Committee, 1998). The tongues were dissected out for gross morphologic study and morphometric parameters (weight, length, width and volume). The length of the tongue (apex to root), width at the apex, body and root, and the thickness at apex, body and root were measured with digital Vernier callipers and the volume was obtained by water displacement method (Scherle 1970). The dorsal and ventral lingual epithelium was examined and photographed with a stereomicroscope (sub-microscopic) with camera attachment (Olympus SZ51). Gross photographs of the tongue placed on a white filter paper were obtained with a digital camera (Samsung 5.0 pixel) after gross observations and measurements.
2.2 Tissue processing and gross morphometry
For light microscopic study, 10 tongues of both sexes dissected out during the gross study were used. Subsequently, adequate tissue slices obtained from the apex, the body and the root of the tongues were fixed in 10% NBF for 36 hr and routinely processed. Briefly, they were dehydrated in graded series of ethanol concentration (70%–100%) and embedded in paraffin wax following routine histological procedures (Survana et al., 2013). Thereafter, serial sections of about 5–6 μm thick were obtained and stained alternatively with haematoxylin and eosin (H & E), Periodic acid Schiff (PAS) and counterstained with Fast green in place of Eosin, and diastase digestion was used as negative controls. Also Alcian Blue (AB, pH2.5) was also used for identifying acid mucopolysaccharides. Photomicrographs were obtained from mounted sections using AxioCamERC5S microscope (Zeiss). Histometric data relating to height and thickness of different lingual papillae and associated structures were obtained under Olympus microscope using ocular micrometre reticule calibrated with stage micrometre gauge using x10 objective lens and using adequately sampled slide sections. Approximately sixty H & E stained sections of the apex, body and root were measured at an average of 12 fields per section.
2.3 Scanning electron microscopy
Tongues of four (4) bats (2 males and 2 females) were each cut into apex, body and root and fixed immediately in 2.5% glutaraldehyde with phosphate buffer (pH 7.3 at 4°C) for surface ultrastructural details using the SEM. Adequately fixed tissues were post-fixed in 1% osmium tetraoxide (2 hr) and subsequently rinsed in same buffer solution. Specimens were dehydrated through graded series of ethanol concentration (60%–100%). Following satisfactory rinsing with phosphate buffer, a chemical drying agent hexamethyldisilizane (HMDS, Sigma Aldrich) was used following the manufacturer's instructions. It is an alternative to critical point drying (CPD). Well-dried tissue specimens were attached to aluminium stubs and coated with gold-palladium complex in a sputtering machine (Emscope SC 500). They were examined and photographed using TESCAN VEGA3 SEM operated at 6–10 KV at the Spectra Unit, University of Johannesburg, South Africa.
3 RESULTS
3.1 Gross observations, stereomicroscopy and measurements
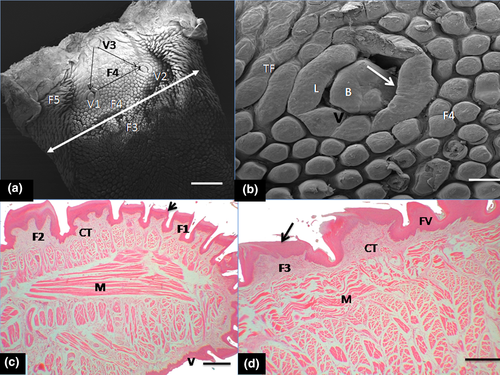
Observations of the gross morphology showed the tongue was relatively long and ended with rounded tip (Figure 1b). It was closely fitted within the oral cavity and palatal structures of about eight palatal rugae with other closely aligned dental structures (Figure 1b,c). It showed a rostral portion (apex or tip), followed by the robust middle portion (body) and the caudal aspect (root) which slopped ventrally towards the base of the epiglottis. Under the stereomicroscope, it showed that mucous membrane on the dorsal surface presented numerous backward-pointed papillae, amongst which were numerous mechanical papillae of mainly filiform with some conical papillae of different shapes and sizes. Few interspersed gustatory fungiform papillae were also identified in the apex, body and root of the tongue. In the middle part of the root, three oval-shaped gustatory vallate papillae surrounded by circular grooves were apparent. Five types of mechanical papillae of mainly filiform shape were present on the dorsum: first type (most numerous) was small crown-like were distributed on the tip, lateral boundaries of the apex and large portion of the body of the tongue. Fungiform papillae intermingled amongst them (Figure 1d); the second type had forked process (giant trifid shape) and were predominant on the mid-portion of the apex and it also extended to the rostral portion of the body (Figure 1e); the third type was large crown-like structures with processes and dominated the caudal portion of the body, and spread towards the root (Figure 1f); the fourth type showed short, bulky cone-shaped papilla with blunt surfaces which were located near to the root of vallate papillae (Figure 1g). The fifth type represented by long conical-shaped papillae with pointed tips was located on the lateral part of the root (base) of the tongue (Figure 1h). Three oval-shaped gustatory vallate papillae surrounded by circular grooves were present in the middle part of the root (Figure 1i). In addition, transitional forms of mechanical papillae mostly of filiform shape were apparent at the borders between small crown-like type and giant trifid type. Some gross morphometric parameters of the tongue relating to the weight, volume, length, width and thickness were presented in Table 1.
| Parameters of the tongue | Mean ± SE | Range |
|---|---|---|
| Weight of the tongue (g) | 2.41 ± 0.81 | 1.42–4.18 |
| Volume of the tongue (cm3) | 2.71 ± 0.40 | 2.62–2.77 |
| Length of the tongue (cm) | 3.60 ± 0.60 | 3.50–4.18 |
| Width of apex (cm) | 0.55 ± 0.20 | 0.40–0.60 |
| Width of body (cm) | 0.71 ± 0.30 | 0.90–1.20 |
| Width of root (cm) | 1.20 ± 0.40 | 1.10–1.50 |
| Thickness of apex (cm) | 0.28 ± 0.20 | 0.30–0.50 |
| Thickness of the body (cm) | 0.87 ± 0.30 | 0.80–1.10 |
| Thickness of root (cm) | 1.10 ± 0.30 | 0.90–1.20 |
3.2 Scanning microscopic features
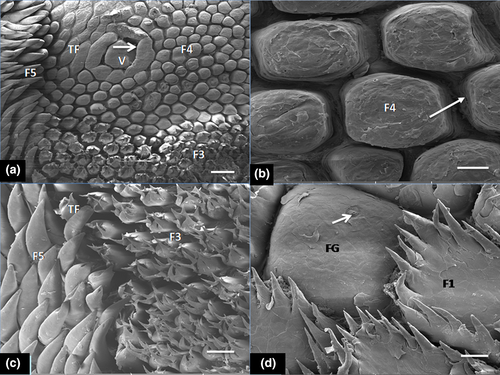
The first type of mechanical papillae ( small crown-like type) was arranged in scale-like fashion and showed a large basket-like base around the apex and also displayed several short filamentous processes (20–25 processes), directed caudally towards the root (Figure 2a–c). The second type had small base with three processes in form of a giant trifid filiform papillae and several additional short basal processes projected from it (Figure 2d). These processes were directed caudally. The third type of mechanical papillae (large crown-like shaped), showed a wide base and resembled the small crown-like type but displayed fewer short and thicker filamentous processes (about 6–8 processes) that tilted towards the root (Figure 2e). The fourth type was bulky, cone-shaped and clustered around the vallate papillae (Figure 3a). The single, blunt-surfaced non-filamentous process projected firmly in upright direction of the tongue surface (Figure 3b). The fifth recognizable mechanical papillae had a conical shape inform of a cornual (horn-like) process with wide base and displayed pointed tip and was classified as typical conical papillae. Each projected process tilted towards the mid-line of the root. The dense clusters of these papillae made contact with those of the fourth mechanical papillae type that surrounded the vallate with the transitional forms (of short bulky cone-shaped and long conical papillae) located between them (Figure 3c).
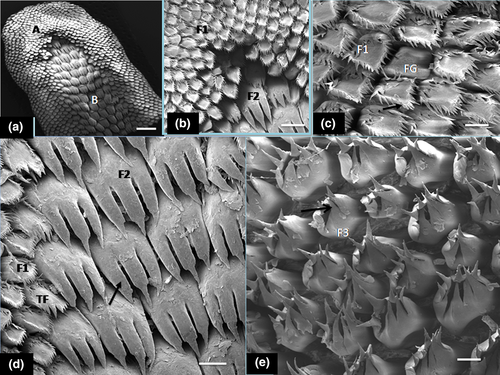
Generally, crowned-like filiform papillae were the most abundant form on the lingual surface and remarkably surrounded the fungiform papillae in the apex (tip) and lateral aspect of the body. Fungiform papillae seen in the apex and body were commonly surrounded by small crown-shaped filiform with wide base and pointed filamentous processes (20–25) but were absent in the zone occupied by giant trifid filiform types. The fungiform papillae showed microgrooves on the surfaces as taste pores (Figure 3d).
Also under SEM, three ovoid shaped and elongated vallate papillae were seen in the root in a typical ‘triangular formation’ (Figure 4a). The zone occupied by the base of these papillae showed the bulky, cone-shaped papillae (fourth type of mechanical papillae) that differed remarkably from those filiform mechanical papillae that were present in the apex and body. The vallate papillae were encircled by round deep grooves. Small elevations and some taste pores were identified on the epithelial surface (Figure 4b).
3.3 Histology and histometry
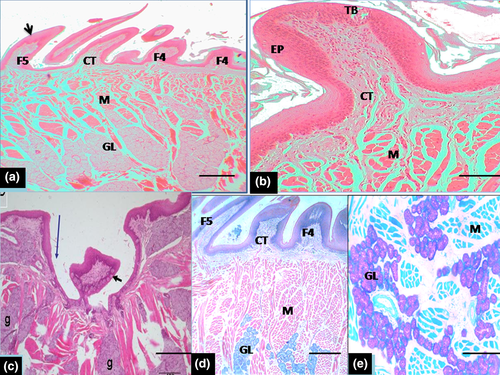
Histologically, the dorsal surface of the papillae (filiform, conical, fungiform and vallate) was covered by variably keratinized stratified squamous epithelium in the entire length with minor variations in thickness in regions (Table 2). The mechanical filiform and conical types were the most numerous with variable forms predominantly represented in the apex, body and root (Figure 4c,d). The various types of papillae differed in size such that the lamina propria was a thick layer made up of dense irregular connective tissue that extended into the core of the projecting papillae and formed large, central connective tissue core in large crown-shaped filiform and fungiform papillae. Small connective tissue core of lamina propria also projected into the base of surface epithelium bearing short and bulky cone-shaped papillae (fourth type of mechanical papillae) and typical long conical papillae (fifth type of mechanical papillae) situated in the root (Figure 5a). Fungiform papillae were commonly seen in the apex and body of the tongue. They were poorly keratinized and slightly raised above the surface, with barrel-shaped taste buds apparent on the surface (Figure 5b). The taste buds diameter of the taste buds is indicated in Table 2. The vallate papillae were elongated structures with ovoid central bulb encapsulated by round deep grooves (Figure 5c). The depth of the circular grooves and the cross-sectional diameter of the adjacent taste buds of the vallate papillae measured are indicated in Table 2. Some taste buds were apparent on the lateral walls of the papillae. The intrinsic muscular layer of the tongue consisted of outer thin and inner longitudinal muscles fibres. Serous and mucous glands which were positive to neutral and acid carbohydrates secretions (PAS and AB) intermingled with the muscular layer in the regions of the body and root close to the vallate papillae but not in the apex. The ducts of these glands appeared to open on the surface epithelium (Figure 5d,e). Also other histometric measurements relating to the height of various forms of mechanical papillae (from the basal epithelial surface of the tongue including the keratinized layer and connective tissue core of lamina propria) which varied according to the type of mechanical papillae were presented on Table 2. In addition heights of the fungiform and vallate papillae, thickness of the stratified squamous epithelium (without keratin layer) and the thickness of ventral non-keratinized epithelium were also shown in Table 2
| Measured parameter | Mean ± SE (μm) | Range (μm) |
|---|---|---|
| (a) Height of mechanical papillae with keratin layer | ||
| i. Small crown-like in apex | 417.01 ± 31.50 | 385.72–423.78 |
| ii. Giant trifid | 425.88 ± 28.15 | 404.43–437.51 |
| iii. Large crown-like | 426.76 ± 30.07 | 411.32–435.63 |
| iv. Bulky and cone-shaped | 401.88 ± 18.15 | 389.87–404.76 |
| v. long conical | 869.16 ± 45.79 | 866.12–872.86 |
| (b) Height of gustatory papillae with keratin layer | ||
| i. Fungiform papillae | 348.74 ± 45.79 | 339.45–352.21 |
| ii. vallate papillae | 354.91 ± 20.45 | 346.74–364.97 |
| (c) Lingual epithelium and taste buds | ||
| i. Thickness of epithelium with keratin layer (apex) | 81.26 ± 7.50 | 77.8–83.27 |
| ii. Thickness of keratin layer (apex) | 8.29 ± 0.80 | 7.97–9.22 |
| (d) Taste buds and epithelia invaginations | ||
| i. Diameter of taste buds (fungiform papillae) | 39.40 ± 2.50 | 36.95–41.23 |
| ii. Diameter of taste buds (vallate papillae) | 40.56 ± 10.61 | 38.33–42.75 |
| iii. Depth of invaginations (vallate papillae) | 435.76 ± 23.76 | 429.67–439.54 |
4 DISCUSSION
During the evolutionary process, functional adaptations are crucial for species to be appropriately suited to their ecological niches. From morphologically consideration, these adaptations are reflected in the anatomical specializations of different organs. Structural specializations of the tongue (lingual organ) are a crucial adaptation of mammals such as bats that allows their nutritional needs to be met (Iwasaki, 2002; Iwasaki et al., 2019).
Several mammals have developed an outstanding diversity of feeding strategies and associated morphological specializations and behavioural adaptations commonly linked to environmental needs (Santana & Cheung, 2016). The food habits vary greatly amongst the flower-eating, fruit-eating and insectivorous, nectarivorous and carnivorous feeding species of bats and these varieties have given rise to different distinctive features of the lingual surface according to their geographical distribution (Akbari et al., 2018). Earlier studies on the tongue of some species of nectarivory, frugivory and insectivory showed remarkable variations in lingual papillae resulting from adaptations to the intake of liquid and semi-liquid food relative to varying seasonal environmental needs (Abumandour & El-Bakary, 2013; Abumandour, 2014; Emura et al., 2001; Emura, 2009; Heiss et al., 2017; Kobayashi & Shimamura, 1982; Mqokeli & Downs, 2013; Sharma et al., 1999; Taki-El-Deen et al., 2013). The present report showed some structural features similar to those seen in fruit-eating bats previously studied (Birt et al., 1997; Emura et al., 2001; Gunawan et al., 2019; Jackowiak et al., 2009; Shindo et al., 2009; Trzcielinska-Lorych et al., 2009). Such remarkable similarities as shape and size, variable forms of mechanical papillae (filiform and conical); location, distribution and number of gustatory fungiform and vallate papillae, and with their taste buds (and taste pores) seem to reflect the adaptation of the E. helvum to fruit diets and their ability to switch between diet of fruit, nectar and/or leaves depending on seasonal availability of food type in the challenging tropical West African rain forests habitat. These features also support their role in pollination and seed dispersal which is consistent with that report on megachiropteran fruit bats (Izhaki et al., 1995; Ramteke, 2016).
The relatively long and tapered tongue with rounded tip facilitates its movement within the oral cavity while piercing and extracting fruits as previously reported in many species of fruit bat (Birt et al., 1997; Jackowiak et al., 2009). Furthermore, our findings showed morphological adaptations in the palatal ridge and dental structures in E. helvum suggestive of effective feeding on fruit and nectar, indicating that diversity of food habits can influence modifications of teeth and palatal structures (Mqokeli & Downs, 2013). This is consistent with previous reports on other fruit bats such as dog-faced fruit bat Cynopterus brachyotis, Indian flying fox Pteropus gigantus and Wahlberg's epauletted fruit bat Epomophorus wahlbergi and (Emura et al., 2001; Mqokeli & Downs, 2013; Shindo et al., 2009). In addition, the tongue has been reported to work with the palatal structures and teeth to enhance the feeding mechanisms during the process of extraction of fruit juices and nectar in many megachiropteran fruit and nectar-eating bats (Dumont & O’Neal, 2004).
The tongue of straw-coloured fruit bat is robustly structured for efficient consumption of varieties of fruits and nectar including such special fruits as mango (Magnifera indica), paw-paw (Carica papaya) and Ficus spp amongst other plants in the West African forests and the domestic environment (Richter & Cumming, 2006). Additionally, when feeding on fruits such as bananas and plantain (Musa spp), the tongue is designed for effective manoeuvring against the palate to move and compress the bolus, thereby enhancing swallowing (Freeman, 1998). Several fruit-eating bats use their elongated, tapering and protruding tongue to eat both fruits and nectar (Gonzalez-Terrazas et al., 2016). The rapid passage of food that exists in this bat is enhanced by the conformation of the tongue and small size of the mouth. It is also likely that body size plays significant role in the partitioning of fruit resources (Dumont & O’Neal, 2004). This is because body size of bats is significantly correlated with the maximum forces that bats produce during biting (Aguirre et al. 2003), and thus defines an upper limit to the hardness of foods that can be eaten by bats.
In the present study, five variable forms (types) of highly keratinized mechanical papillae that comprised of filiform and conical papillae along with its transitional forms at the borders were tilted towards the caudal (posterior) aspect of the tongue. Similarly, about 5–6 variable forms of mechanical papillae that comprised filiform and conical forms have been reported in other nectar and fruit-eating bats (Emura et al., 2001, 2002; Greenbaum & Philips, 1974; Jackowiak et al., 2009; Mqokeli & Downs, 2013; Shindo et al., 2009), and their keratinized processes were also seen to be directed caudally towards the root. Four types of filiform papillae were described in the frugivorous bat Carollia perspicillata and three forms in the insectivorous Common European bat Pipistrellus pipistrellus (Pastor et al., 1993; Masuko et al., 2007). The three-pronged (trifid) filiform papillae observed in the mid-line of the rostral (apex and body) of the tongue of E. helvum may be utilized in grabbing and puncturing into ripe fruits and flowers. These pronged (forked) papillae are common to the frugivorous, nectarivorous and some insectivorous bats (Abumandour & El-Bakary, 2013; Masuko et al., 2007; Shindo et al., 2008), with minor variations in the number of their projections (processes) for grabbing and crushing their food. In addition, the function of the giant trifid filiform papillae has been speculated to increase the surface area during collection of nectar (Gunawan et al., 2019). The mechanical crowned-filiform type with filamentous secondary processes assists in trapping fruit juices and other fluids by increasing surface tension (Sharma et al., 1997). In the insectivorous bats, crowned-filiform papillae with keratinized rugged surfaces engage in manipulating insects towards the dental surfaces for crushing. The comparatively tall conical papillae on the lateral aspect of caudal extremity of the tongue serve protective function against abrasion of the underlying lingual mucosa (Sharma et al., 1999).
The caudally directed processes of mechanical papillae (filiform forms) enable the gradual movement of fruit contents from the apex and mid-line of the tongue to the caudal aspect of the tongue and finally into the pharynx as postulated for other fruit-eating bat species (Emura et al., 2012; Jackowiak et al., 2009; Mqokeli & Downs, 2013). In addition, the densely packed filiform papillae in the lingual surface increase the surface area of the tongue during the consumption of nectar and fruit juice. Three-pronged giant filiform (so-called trifid, bifid or horny papillae) are necessary for piercing through the tough outermost layer as in most frugivorous bats (epicarp) of the fruit (Birt et al., 1997; Mqokeli & Downs, 2013; Sharma et al., 1997). It has also been suggested that the processes of the giant trifid papillae help in conjunction with canine teeth to direct food materials into the caudal aspect of the tongue for swallowing (Abumandour & El-Bakary, 2013). The secondary processes (filaments) of the crowned- filiform in E.helvum help in trapping fluid and fruit juices by increased surface tension (Birt et al., 1997). The copious keratinization of the filiform papillae serves as protective measure during mastication of fruits.
The fungiform papillae observed in the apex, body were commonly surrounded by small crown-shaped filiform with wide base and pointed secondary processes. The fungiform in most fruit and insectivorous bats studied are predominantly found in the apex and body of the tongue similar to the present report (Abumandour & El-Bakary, 2013; Ramteke et al., 2012; Shindo et al., 2008; Sharma et al., 1999).The presence of fungiform papillae with taste buds and pores that were interspersed amongst filiform papillae of the apex and in the body of tongue provides for greater involvement in taste sensation of the variable fruit diets in the tropical forests. It may specifically enhance the initial selection of fruit diets by its preponderance in the apex of the tongue.
Three triangularly arranged vallate papillae were observed in the current report. The distribution and microstructure of the vallate papillae depend upon the different types of food consumed by bat species. It appears that there is no relationship between diet and the number of vallate papillae in bats and that this trait is more closely related to phylogeny than function. Generally many fruit-eating bats have shown to possess three vallate similar to the present report (Emura, 2009; Emura et al., 2001, 2002; Ramteke et al., 2012; Trzcielinska-Lorych et al., 2009) However, several insectivorous bats have two vallate papillae such as the long-fingered Miniopterus schreibersi fuliginosus and Common European bat Pipistrellus savii (Kobayashi & Shimamura, 1982; Park & Lee, 2009; Pastor et al., 1993) and two small vallate papillae in insectivorous Hipposiderous cervinus (Sharma et al., 1999) have been observed. Also four vallate was reported in the frugivorous Carollia perspicillata and nectarivorous Glossophaga soricina, while the blood-sucking (haematophagous) bat Desmodus rotundus has none (Greenbaum & Philips, 1974; Masuko et al., 2007). However, studies may be needed to ascertain the specific relationship between species of bats and the number of vallate papillae. The lingual glands which showed neutral and acid mucopolysacharides is similar to such reactions identified in frugivorous Rousettus aegyptiacus and insectivorous bat Rhinopoma hadrwicke but differ from acid mucous secretions of tomb-inhabiting insectivorous Taphozous perforatus (Taki-El-Deen et al., 2013). Neutral mucopolysacharides are major constituents of mucous secretions. It is likely that the secretions of these glands facilitate the sensation of taste by cleansing the area of the gustatory papillae during feeding on predominantly fruit and flowering trees as suggested by some investigators (Nagato et al., 1997).
In conclusion, the present morphological investigation in the tongue of fruit bat Eidolon helvum showed marked similarities with those of other fruit bats such as lesser dog-faced bat, large flying fox and Egyptian fruit bat and this reflect similar kind of fruit diet and feeding habits in their diverse environment. In addition, the variable forms and abundance of mechanical papillae (filiform and conical) scattered on the dorsum of the tongue indicate its protective role in feeding ecology. The number, size and distribution of gustatory fungiform and vallate papillae highlighted the relevance of taste in the fruit diet during foraging and migration. These features of the tongue when taken along with other adaptive features of this bat such as: large eyes, good sense of smell, small size of the mouth to prevent juice from dribbling out and large wings for flying long distances enhances the adaptation of this bat to the tropical habitat with its abundant varieties of fruits.
ACKNOWLEDGEMENT
We thank the Spectra Unit (SEM Unit) of University of Johannesburg and the Histology Division of University of Witwatersrand, Johannesburg for their technical assistance.
CONFLICT OF INTEREST
The authors do not have any potential conflicts of interest concerning this research to declare.