Computed tomography and cross-sectional anatomy of the head in the red fox (Vulpes vulpes)
Abstract
The red fox (Vulpes vulpes) is a universally widespread wild carnivore in the world. It has an important impact on human interests and plays a vital role in conservation biology. There is no report to describe computed tomography (CT) of the red fox head and its correlation with gross anatomy. Therefore, the intent of the present study was to document, by CT, the basic anatomical structures in the head region of the red fox to lay the groundwork of an appropriate anatomical description of the head structures comparable with traditional dissection technique. Three red fox heads were imaged sagittally and transversely using CT scanner. Six red fox heads were then serially cut, sagittally and transversely, by an electric band saw, and the obtained slices were cleaned and photographed. Different anatomical structures on CT images and the corresponding anatomic sections were identified and labelled. CT images revealed an excellent three-dimensional definition of bony structures of the entire craniofacial region. Moreover, CT scans revealed different contrasts between bones, oral and nasal cavities, paranasal sinuses and pharynx which enabled good visualization of those structures. The paranasal sinus system in red fox was poorly developed and represented by a frontal sinus and a small sphenoid sinus, in addition to a maxillary recess. In conclusion, the series of CT images of the normal red fox head presented in the current study will serve as an anatomical reference for the normal head of the red fox, which in turn will improve knowledge for clinical interpretation.
1 INTRODUCTION
The red fox (Vulpes vulpes, order Carnivora, family Canidae, tribe Vulpini) is a universally, widespread wild carnivore in the world (Statham et al., 2014). It is the largest true fox present in the Middle East and North Africa being distributed in hill areas close to human habitats and agricultural land. In Egypt, red fox can be found along the Egyptian coast, Nile Delta and Nile Valley up to Sudanese borders (Stuart & Stuart, 2017). Its spreading is somewhat related to human distribution (Osborn & Helmy, 1980). Red foxes have an important impact on human interests since they play an integral role in conservation biology (Virgós & Travaini, 2005). Red foxes have been reported as a reservoir for rabies virus and have an important role in the trans-governmental spread of rabies virus in Egypt with a possibility of spreading to neighbouring countries via uncontrolled movement of foxes across the boundaries (El-Tholoth, El-Beskawy, & Hamed, 2015). Moreover, red foxes are considered as a potential reservoir of canine distemper virus (Aničić et al., 2018). Additionally, red foxes have an economic importance in traditional fur countries, such as the Soviet Union, Mongolia and from North America (Stubbe, 1980).
Modern imaging modalities, such as computed tomography (CT), enable fast non-invasive visualization of animal anatomy in live animals (Lauridsen et al., 2011). CT provides differential appearance of bone, soft tissues, sinuses and cavities according to their radiodensity (Alsafy, El-Gendy, & El Sharaby, 2013; Kaminsky, Bienert-Zeit, Hellige, Rohn, & Ohnesorge, 2016) which in turn enables easy identification of those structures within CT slices. Although CT is an X-ray-based imaging technique, it is superior than conventional radiography, because it is not affected by superimposition or the thickness of tissue. In addition, CT is considered as a standard imaging technique for assessing bones of skull, teeth, osseous canals, nasal passages, paranasal sinuses (Manso-Díaz, Taeymans, García-López, & Weller, 2019), orbit (Salgüero et al., 2015) and intracranial, as well as, extracranial lesions (Ohlerth & Scharf, 2007). Additionally, three-dimensional (3D) reconstructed CT images are considered a valuable tool in the teaching and practice of medicine for the diagnosis of a variety of animal diseases (Keane, Paul, Sturrock, Rauch, & Rutland, 2017).
Several studies have investigated normal CT scans of the head of domestic animals and the correlation of these scans with gross specimens, including the dog (Burk, 1992; George & Smallwood, 1992), goat (Shojaei, Nazem, & Vosough, 2008), buffalo (Alsafy et al., 2013), horse (Morrow, Park, Spurgeon, Stashak, & Arceneaux, 2000), donkey (El-Gendy, Alsafy, & El Sharaby, 2014) and camel (Alsafy, El-Gendy, & Abumandour, 2014). These studies serve as useful anatomical references for clinical applications and diagnosis of head disorders using CT (Alsafy et al., 2014; Burk, 1992; Morrow et al., 2000). Previous studies have investigated the craniometric measurement of the red fox head (Jaslow, 1987; Onar, Belli, & Owen, 2005). Moreover, the morphology of the red fox skull has previously been studied by CT (Jurgelėnas, Daugnora, Monastyreckienė, & Balčiauskas, 2007), but there are no studies to our knowledge that have compared 3D reconstructed CT scans of the red fox head and evaluated their correlation with gross anatomy from similar planes of section. It is important for veterinarians and surgeons of zoo animals and wildlife to have a basic anatomical knowledge of the animals that they are responsible for treating, including the red fox. Lack of this knowledge not only results in substandard care for these patients but can potentially put the veterinary care team at risk if these animals were infected with rabies. To accurately interpret CT scans of the red fox head, a thorough knowledge of the anatomical structures of the normal fox head is necessary. Therefore, the aim of the current study was to investigate the details of red fox's head by means of CT and correlates these scans with anatomical cross-sectional images. The data of the present study will be useful as an anatomic reference of the red fox head. This study also provides a basis for interpreting of CT images in clinical applications of the red fox head.
2 MATERIALS AND METHODS
The current study was conducted using nine adult red fox (Vulpes vulpes) cadaver heads of both sexes. Foxes were collected from local hunters in Qena Governorate, Egypt. After euthanizing the animals by intravenous injection of thiopental sodium, heads were disconnected between cervical vertebrae 3 and 4. Cadavers had no evidence of facial trauma. All procedures were conducted according to the standards of the Animal Ethics Committee of the South Valley University for Veterinary Research, Qena, Egypt.
2.1 Computed tomography scans
Serial CT scans of three heads were obtained using a 16-slice Optima CT520 scanner (GE Healthcare). CT scans were acquired, at 0.625 mm-thickness (Soukup et al., 2015), from the atlanto-occipital joint at the caudal margin to the tip of nostrils rostrally on transverse and sagittal planes. Scans were done perpendicular to the hard palate with a scanning setting of 120 kV and 130 mA, and window width and length were 2,000 and 350 Hounsfield units, respectively. CT images were used to reconstruct volume-rendered 3D CT-reformatted images for the skull using multiplanar reconstructions.
2.2 Anatomic sections
The scanned red fox heads were frozen then serially cut transversely at about 1 cm intervals using an electric band saw to observe the gross morphology. The cuts were done from the level of the canine teeth rostrally to the external occipital protuberance caudally. The slices were gently cleaned of debris using water and light brushing. Three additional heads were sagittally cut to describe the anatomical structures identified on the sagittal CT scans. Slices were photographed using a digital camera. The cheek teeth and bony parts of the skull were used as landmarks to explore and define the location and extension of the structures and cavities within the skull due to their easy identification within the cross sections (El-Gendy et al., 2014). To investigate the anatomy and communications of the paranasal sinuses, three additional heads were macerated. The frontal sinus was exposed after removal of the osseous plate of the frontal bone (Jurgelėnas et al., 2007). To expose the maxillary recess, resection of the zygomatic arch was done followed by removal of the osseous plate at the junction of the lacrimal, zygomatic and maxillary bones. Labelling of identifiable anatomical structures on CT images and corresponding anatomic sections was done with the help of the anatomic literature (Done, Goody, Evans, & Stickland, 2009; George & Smallwood, 1992; Thrall & Robertson, 2015). Nomenclature was adopted according to the official anatomic terminology (Nomina Anatomica Veterinaria, 2017).
3 RESULTS
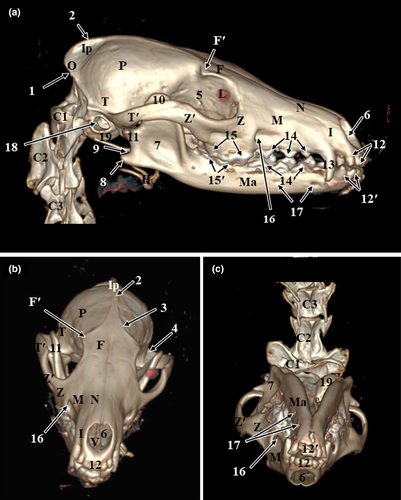
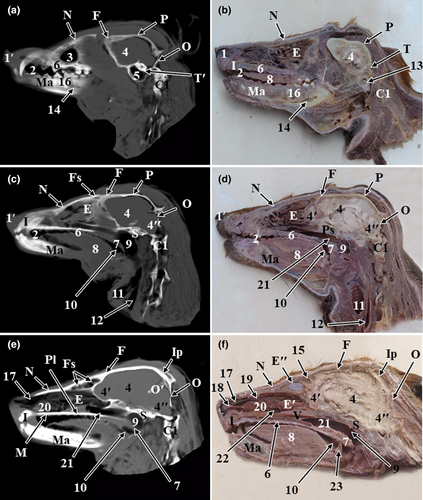
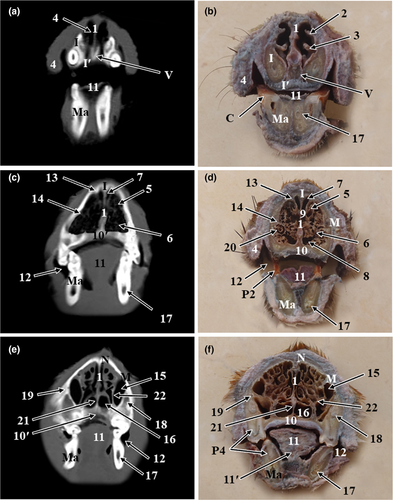
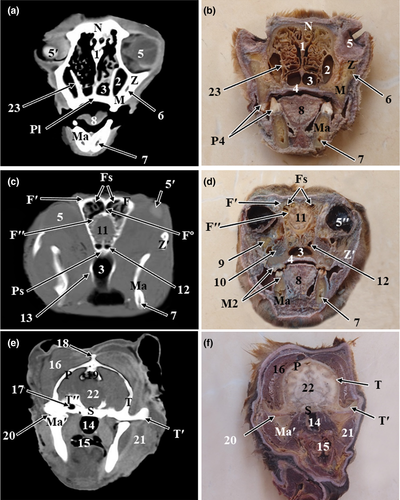
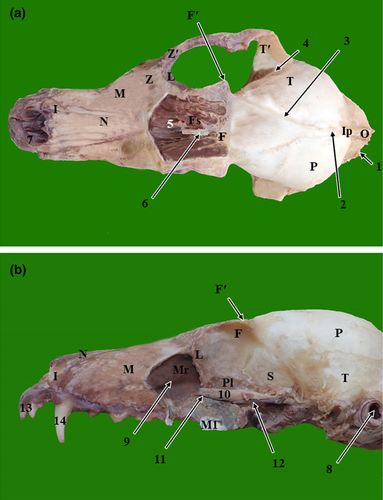
The results of the present study were presented as 3D reconstructed images representing the lateral, rostral and ventral views of skull (Figure 1), three sagittal CT scans (Figure 2) and a series of six representative transverse CT images displayed in sequential order in a rostral to caudal direction from the level of canine tooth rostrally to the level of temporomandibular joint caudally (Figures 3 and 4). The sagittal and transverse CT scans were displayed with the most closely corresponding anatomic sections. In addition, macerated skulls with exposed sinuses were presented to show their anatomy and communication (Figure 5). Identifiable structures on anatomic sections, as well as, CT scans were labelled.
3.1 Computed tomography scans
3.1.1 Volume-rendered 3D-reconstructed images
On the volume-rendered 3D-reconstructed images of the red fox head, various cranial and facial bones of the skull, as well as the anatomical structures of these bones, could be identified. The identified cranial bones included the occipital bone, parietal bone, interparietal bone, temporal bone and frontal bone. While the identified facial bones included the nasal bone, zygomatic bone, maxillary bone, incisive bone, lacrimal bone, mandible, vomer bone and hyoid bone. Moreover, bony projections, such as the nuchal crest of the occipital bone, external sagittal crest, zygomatic process of the temporal bone, temporal process of the zygomatic bone and the zygomatic process of the frontal bone were identified. Furthermore, the lateral surface of the ramus of the mandible with its coronoid process at the dorsal part, condylar process caudally at the middle part and angular process caudally at the ventral part were recognized. Additionally, bony depressions, such as the orbital cavity, temporal fossa, masseteric fossa, mandibular notch, and infraorbital and mental foramina, were thoroughly identified. Moreover, dental structures, such as incisors, canines and upper and lower cheek teeth could be identified clearly (Figure 1).
3.1.2 Sagittal and transverse CT scans
On the sagittal and transverse CT scans, numerous bony and soft structures associated with the nasal, oral and cranial cavities could be identified (Figure 2). The identified bony structures were the occipital bone, parietal bone, interparietal bone, frontal bone, sphenoid bone, nasal bone, incisive bone, horizontal plate of maxilla, palatine bone and mandible. In addition, the nasal cavity with its conchae, frontal sinus, maxillary recess, the orbital cavity containing the eye with its lens, the oral cavity with its divisions and parts of the pharynx were visible. Parts of the brain, such as the olfactory lobe, cerebrum, and cerebellum and mental canal and tympanic bulla, could be distinguished.
3.2 Anatomic sections
On the sagittal and cross anatomic sections, most structures of the oral cavity, nasal cavity, paranasal sinuses, pharyngeal cavity, ear and parts of the brain were easily identified (Figures 2-4).
3.2.1 The cranial cavity
The bones forming the cranial cavity were well-identified on CT scans and confirmed on anatomic sections. The roof of the cranial cavity was formed by the frontal, parietal and interparietal bones. The lateral wall was formed by the frontal, parietal, squamous part of temporal and sphenoid bones. The caudal wall was formed by the occipital bone. The rostral wall was formed by the cribriform plate of ethmoid bone. The base of cranium was formed by the sphenoid bone rostrally and the occipital, tympanic and petrous part of temporal bone caudally (Figures 1 and2).
3.2.2 The nasal cavity
The nasal cavity of red fox was divided by the median nasal septum into two symmetrical cavities. The nasal septum was extended from the external nares rostrally to the choanae caudally. It was formed of a cartilaginous plate in the rostral two thirds, and a thin osseous plate, the perpendicular plate of the ethmoid bone, in the caudal third of the nasal cavity (Figures 3 and 4). Both right and left nasal cavities were occupied by three sets of nasal conchae: dorsal, ventral and ethmoidal conchae. The dorsal and the ventral nasal conchae were attached to the nasoturbinate crest and maxilloturbinate crest of the maxilla, respectively. The dorsal nasal concha was single and long, it continued rostrally by the straight fold. While the ventral nasal concha was branched and short, it continued rostrally by the alar fold. The ethmoidal conchae occupied the caudal part of the nasal cavity and were attached to the cribriform plate caudally. In between the nasal conchae, the nasal meatuses were present. The dorsal and middle nasal meatuses were narrower than the ventral nasal meatus, which enlarged caudally to continue as the nasopharyngeal meatus (Figure 2e,f). The three nasal meatuses communicated through the common nasal meatus medially.
3.2.3 The paranasal sinuses
The frontal sinus was located rostrodorsal to the olfactory bulb of the brain. Grossly, the frontal sinus had the shape of a small compressed triangle dorsoventrally with its base located dorsally. It was bounded dorsally by the frontal bone, rostrally by the nasal bone, ventrally by the ethmoturbinates and the caudal part of the dorsal nasal concha and caudoventrally by the cranium and olfactory lobe of the brain (Figure 2e,f). The frontal sinus extended from the level of the caudal limit of zygomatic process of frontal bone caudally to the caudal surface of the 3rd upper premolar tooth rostrally. The frontal sinus was divided by a bony frontal septum into right and left sinuses. The sinus showed thin radiopaque partitions which subdivided it into smaller radiolucent compartments of various sizes (Figure 4c). Grossly, each sinus was subdivided by bony partitions into rostral, medial and lateral compartments. The lateral compartment was the largest compartment while the medial compartment was the smallest one. The frontal sinus was invaded by ethmoidal ectoturbinates and communicated ventrally with the ethmoidal meatuses (Figure 5a).
The sphenoid sinus appeared as a small radiolucent cavity excavated the body of the presphenoid bone and divided by a longitudinal sphenoidal septum (Figure 4c,d).
The maxillary recess appeared as a radiolucent cavity between the maxilla laterally and ventrally, orbital lamina of ethmoid bone medially and lacrimal bone caudally. It was located medial to the infraorbital and lacrimal canals and extended from the level of the middle of the 1st upper molar tooth caudally to the level of the middle of the 3rd upper premolar tooth rostrally (Figure 4e,f). It has the shape of an irregular triangle with its apex directed cranially. The maxillary recess communicated with the ventral nasal meatus through a wide nasomaxillary opening which was located at the level between the 3rd and 4th upper premolar teeth (Figure 2f).
3.2.4 The oral cavity
The oral vestibule, tongue, hard palate, soft palate and oropharynx were distinguished in the CT scans. Moreover, dental structures, such as incisors, canines and upper and lower cheek teeth, were very clear. In addition, teeth roots were easily identified on sagittal CT images while the infraorbital and mental canal were easily identified on transverse CT images (Figures 2 and 3). The permanent dental formula was I 3/3, C 1/1, P 4/4, M 2/3.
3.2.5 The pharyngeal cavity
The pharynx appeared as a radiolucent space located at the base of cranium. Divisions of the pharynx, soft palate and root of tongue were easily identified in sagittal CT scans by their different contrasts, as well as, in anatomic sections. The rostral part of the pharynx was divided by the soft palate into the nasopharynx dorsally and the oropharynx ventrally. The caudal part, the laryngopharynx, was located dorsal to the larynx (Figure 2).
3.2.6 The orbital cavity
Osseous structures that formed the orbit were identified on CT scans and confirmed on anatomic sections. The orbit was a conical cavity with its apex formed by the optic canal. It contained the eyeball and its accessory structures. Its medial wall was formed by the orbital part of frontal, lacrimal and orbital process of the palatine bone. The orbital cavity was related dorsomedially to the frontal sinus, rostromedially to the maxillary recess and caudomedially to the cranial cavity. The orbital rim was formed by the frontal, lacrimal, zygomatic and maxillary bones. In macerated samples, the orbital rim was incomplete dorsolaterally except for the small zygomatic process of frontal bone that projected laterally (Figure 5a). The optic canal, orbital fissure, rostral alar foramen opened in the caudal part of the orbit. Rostrally, the orbital wall had fossa lacrimalis dorsally and maxillary foramen and sphenopalatine foramen ventrally. In CT scans, the lens appeared as a biconvex hyperdense structure, while both aqueous and vitreous humours appeared as hypodense structures (Figure 4a,c).
3.2.7 The middle ear
The air-filled tympanic cavity and tympanic bulla of the middle ear were easily identified as radiolucent spaces located within the petrous temporal bone (Figures 2a and 4e).
4 DISCUSSION
Affections of the head region are common among animals, and radiographic imaging is used as a mean of diagnosis (Thrall, 2018). However, it is challenging for a veterinary practitioner to diagnose skull lesions by conventional radiography due to its complex anatomy and superimposition of its bony structures (Forrest, 2018). Therefore, many practitioners use CT to overcome this disadvantage since it provides good details and contrast, which are not affected by superimposition (Thrall & Robertson, 2015). Moreover, CT is the preferred imaging method to evaluate lesions of the nose, paranasal sinuses, periorbital region, middle ear and brain (Stickle & Hathcock, 1993). The morphometric measurement of the red fox's head has been previously investigated (Jaslow, 1987; Onar et al., 2005). Moreover, the morphology of red fox skull has previously been studied by CT (Jurgelėnas et al., 2007). To our knowledge, there is lack of studies that have investigated the diagnostic imaging anatomy of the head of the red fox. Therefore, we focused our attention on providing a description of the normal anatomy of the red fox's head using CT scans. The main structures of the head were identified, and the results were compared with the findings that have been reported in the red fox and other carnivores.
The 3D reconstruction of CT images is an advantage of a traditional CT cross-sectional image because it enables the examiner to visualize a 3D orientation of the head. This added detail of the bony structures of the head and can be used as an anatomic reference for the anatomical, as well as, clinical CT imaging studies of this region (Arencibia & Jáber, 2014). We created a 3D-model of the red fox skull in which various bones of the skull, the anatomical features of these bones, including bony projections and depressions, and dental structures, were identified and labelled. In the present study, bony structures of the red fox head were well-defined and labelled. Moreover, the nasal cavity, paranasal sinuses, orbital cavity and teeth structures were well documented in sagittal and transverse CT scans. The 3D-reconstructed images of the red fox skull were comparable to those of the canine head as reported by Arencibia and Jáber (2014). To obtain reconstructions of excellent quality and high detail, it is important to reduce the CT scan slice thickness as much as possible, particularly with single-slice CT scans. The newest multi-slice CT scanners permit submillimetre collimation slice width (Larguier et al., 2019). In the present study, we used 0.625 mm-thick slices, which is considered in other carnivora CT scans protocol (Soukup et al., 2015). The 3D images can also be used as novel teaching tools for veterinary anatomy (Jaber et al., 2018) and may help in planning of surgical approaches because it delineates congenital bone defects, fractures and bony pathology from different angles (Ram, Joshi, Debnath, & Khanna, 1998).
Air space in the nasal cavity, pharynx and paranasal sinuses provides good contrast in CT images (Probst, Henninger, & Willmann, 2005). Therefore, CT imaging is a favourable definitive diagnostic method of upper airway obstruction and nasal diseases in dogs (Lefebvre, Kuehn, & Wortinger, 2005; Stadler, Hartman, Matheson, & O'brien, 2011). CT can be used to determine the size and extension of benign and malignant neoplasms of the nasal cavity and paranasal sinuses in dogs (Auler et al., 2015) and detect nasal foreign bodies (Moreno-Aguado et al., 2019). Moreover, CT is more sensitive than conventional radiography for diagnosing nasal aspergillosis in dogs (Saunders & Van Bree, 2003). In this respect, De Rycke, Saunders, Gielen, Van Bree, and Simoens (2003) showed that the transverse plane can be used as a reference plane to evaluate the pathologic changes of the nasal region of dogs. In the current CT study of the red fox head, the bony support of the nasal cavity, nasal conchae, nasal meatuses, divisions of the pharynx and paranasal sinuses could be readily identified by their different contrasts in transverse CT scans. Importantly, the cartilaginous nasal septum is not visible in conventional radiographs (Harvey, 1979). However, transverse CT scans in the current study provided good visualization of the cartilaginous nasal septum, demonstrating the advantage of CT and the utility body of work as a clinical reference.
Similar to the domestic dog (Holt, 1998; Singh, 2018), our findings showed that the red fox frontal sinus was small and divided by a bony septum into right and left sinuses and each frontal sinus was divided into rostral, lateral and medial compartments by osseous partitions. It has been shown that the frontal sinus of the Japanese wolf (Endo et al., 1997) is divided into small divisions by osseous partitions. In this respect, Jurgelėnas et al. (2007) reported that the frontal sinus of red fox is divided by a very thin osseous partition while that of raccoon dog is divided by two well-defined osseous partitions. The latter authors added that the frontal sinus of red fox does not extend into the zygomatic process of frontal bone. In contrast, the frontal sinus of raccoon dog fully occupies the zygomatic process. On the other hand, the right and left frontal sinuses in the domestic cat are undivided (Hedlund, 2014). Similar to our findings, the frontal sinus in the domestic dog is filled with ethmoidal ectoturbinates (Singh, 2018). The three compartments of the frontal sinus are connected together; therefore, single trephination site is needed to treat all compartments of frontal sinus in the domestic dog (Caudal, Snead, Starrak, Sathya, & Feng, 2017). The frontal sinus can be trephined in the middle between the midline and the zygomatic process of the frontal bone (Holt, 1998). In this regard, we suggest that trephination site of the frontal sinus in red fox should be 1 cm rostral to the site described by Holt (1998) in domestic dogs to avoid penetration of the cranial vault.
It has been shown that the sphenoid sinus is a component of paranasal sinuses of the head of the domestic dog (Burk, 1992; De Rycke et al., 2003). In consistent, the present study demonstrated a small sphenoid sinus excavated the body of the presphenoid bone and was divided by a longitudinal sphenoidal septum. The sphenoid sinus of the domestic dog is largely occupied by endoturbinate IV (De Rycke et al., 2003; Hermanson, De Lahunta, & Evans, 2020; Singh, 2018); therefore, it has not been considered as a sinus in previous literatures (Hermanson et al., 2020). On the other hand, the maxillary recess appeared as a radiolucent cavity between the maxilla, orbital lamina of ethmoid and lacrimal bone. The maxillary recess is not considered as a true sinus because it is formed between individual bones and not between the two plates of the maxillary bone (Singh, 2018). It has been reported that abscesses of the upper cheek teeth roots spread infection to the maxillary recess through lysis of the osseous plate separating them. The maxillary recess is drained by removal of the upper carnassial tooth in the domestic dog (Kumar, 2015).
The orbit was related dorsomedially and rostromedially to the frontal sinus and maxillary recess, respectively. Therefore, infection may spread from these cavities to the orbital structures (Singh, 2018). In agreement to our results, the orbital rim of the crab-eating fox is incomplete dorsolaterally (Lantyer-Araujo et al., 2019). Consequently, the orbit is accessible for surgery without bone resection (Singh, 2018). Our findings are in agreement with those of Salgüero et al. (2015) who reported that the canine lens appears as hyperdense structure while aqueous and vitreous humours appear as hypodense structures.
The observed permanent dental formula of the red fox was similar to that reported in the red fox (Gingerich & Winkler, 1979) and the domestic dog (Evans & De Lahunta, 2010). CT scan images can help in diagnosis of dental diseases (Campbell, Peralta, Fiani, & Scrivani, 2016). In support to this notion, dental structures and teeth roots were easily identified on sagittal CT images suggesting that the sagittal plane images may be helpful in defining the direction and description of canine and cheek teeth roots of the red fox.
5 CONCLUSION
In conclusion, the present study provided the first detailed comparison of 3D-reconstructions, sagittal and transverse CT scans of the head of the red fox with their corresponding anatomic sections, as well as a general view of the entire red fox head, which was comparable to the anatomy of other canine heads. This study provided information on anatomic characteristics of the red fox head which will help to improve the knowledge for clinical interpretation of the pathological conditions affecting the head region.
ACKNOWLEDGEMENTS
Authors are grateful to Dr. Mohamed Ashour, Qena Scan Radiology Center, for performing CT scans.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
M. Mahdy: Conceptualization, design, investigation, acquisition of data, data analysis, interpretation, drafting of the manuscript, critical revision of the manuscript and approval of the final article; M. Zayed: Interpretation, analysis, drafting of the manuscript, critical revision of the manuscript and approval of the final article.
Open Research
DATA AVAILABILITY STATEMENT
Data are available from the corresponding author upon request.




