Mechanoreceptors in the Anterior Horn of the Equine Medial Meniscus: an Immunohistochemical Approach
Summary
Lameness due to stifle and especially meniscal lesions is frequent in equine species. In humans, mechanoreceptors involved in proprioceptive function are well studied. Given the high incidence of meniscal injuries in horses, and the lack of information concerning them in equine menisci, our objective was to study these corpuscles in six healthy anterior horns of the equine medial meniscus, which is the most common localisation reported for equine meniscal injuries. Immunohistochemical stainings were performed using antibodies against high molecular weight neurofilaments and glial fibrillary acidic proteins. From a purely fundamental point of view, our work highlights for the first time the presence of Ruffini, Pacini and Golgi corpuscles in equine meniscus. They were found, isolated or in clusters and always located at the vicinity of blood vessels, at the level of the anterior horn of the equine medial meniscus. This morphological approach could serve as a basis for clinical studies, to evaluate the impact of these corpuscles on the poor sportive prognosis in equine meniscal tears.
Introduction
In horses, diseases leading to lameness are of major importance due to the loss of performance and the economic concerns (Olivier et al., 1997). In the hindlimb, stifle lesions are an important source of lameness due to the large and complex joint (Stashak, 2002) and meniscal tears have been identified as the most common soft tissue injuries in this joint (Walmsley, 2005; Fowlie et al., 2011b).
Menisci can be described as pairs ‘semilunar fibrocartilaginous structures between the femoral condyles and the tibial plateau’ that ‘play central load-bearing roles in the knee joint including joint stabilisation, shock absorption and protection of articular cartilage from excessive stress’ (Chevrier et al., 2009).
Mechanoreceptors (MCR) are ‘encapsulated sensory end organs’ involved in proprioceptive function. They are innervated by nerve fibres that detect mechanical stimuli and transform them into neural excitation, called transduction. Primary afferents transmit the signal to the dorsal horn of the spinal cord via the dorsal root. Nervous interconnections allow a rapid control of the joint position via the efferent alpha-motoneurons as well the transmission of proprioceptive information via the dorsal column and/or the spinocerebellum tract, the cerebellum, the thalamus and the somatosensory-motor cortex for complete integration of sensorimotor functions (Møller, 2002). These MCR are found in tissues around joints, including skin, muscles, fascias, tendons, joint capsules and ligaments (Proske and Gandevia, 2012). According to Freeman and Wyke's criteria (1967), MCR can be classified into three morphologically different types: Type 1 or Ruffini corpuscles, Type 2 or Pacini corpuscles and Type 3 or Golgi corpuscles. These different morphologies perform different functions: Ruffini and Golgi corpuscles are considered to be slowly adapting receptors and Pacini corpuscles are rapidly adapting receptors (Freeman and Wyke, 1967).
Because of the high frequency of knee injury in humans, various morphological and clinical studies about these MCR have been carried out in humans as well as in animal models such as rats (Delgado-Baeza et al., 1999), rabbits (Aydog et al., 2006) and sheep (Raunest et al., 1998). Morphological studies, both structural and ultrastructural, have been performed to localise these corpuscles and to describe them. In humans, these studies have particularly concerned the anterior cruciate ligament (Lee et al., 2009; Dhillon et al., 2010; Bali et al., 2012; Kim et al., 2012) and the menisci (Mine et al., 2000).
Despite the clinical importance of meniscal injuries in equine species (Walmsley, 2005; Fowlie et al., 2011b), to the best of our knowledge, no descriptive study of these MCR was performed in this area. Indeed, morphological studies led to the animal stifle are few and old and concern mainly the cat (Freeman and Wyke, 1967; Halata, 1977) and the dog (O'Connor, 1984).
These studies, which used different technical approaches, frequently ended in various descriptions for the same type of MCR in the same species (Freeman and Wyke, 1967; Halata, 1977). One explanation of these differences is that morphological studies using 2D light microscopy are limited by the fact that it is not possible to obtain a complete representation of the mechanoreceptor (Rein et al., 2013a). Considering this, we proposed to localise MCR inside the equine meniscus with classical morphological approach, and then to identify the different types, we suggested to use immunohistochemical stainings highlighting the localisation of Schwann cells and nerve fibres, criteria which appears to be more relevant than those of size and for which authors in literature describing MCR were frequently in accordance.
Using this original approach, three different types of MCR were localised in the anterior horn of the equine medial meniscus, which is the most common localisation reported for equine meniscal injuries (Walmsley et al., 2003; De Busscher et al., 2006; Fowlie et al., 2012).
Materials and Methods
Sample collection
Horses' menisci were taken from euthanised or deceased horses, whose death was not related with any locomotor problem and that had not presented any macroscopical injuries at their stifle. Choices for sex, breed and age were made randomly.
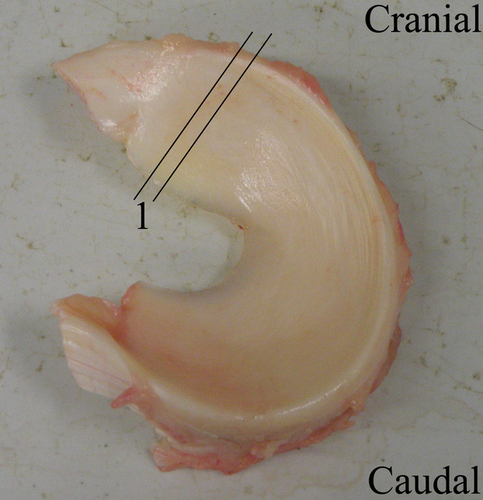
Specimens (n = 6) from medial menisci were collected at the level of the anterior horn of the meniscus (Fig. 1) in a local autopsy room at the Faculty of Veterinary Medicine (University of Liège). Time between death and dissection did not exceed 48 h.
Tissue preparation and cryosections
Samples were immersed in tissue optimal cutting temperature (OCT) embedding medium (LaboNord S.A.S, Templemars, France), snap-frozen and stored at −80°C until processed. Cryosections of about 10 μm were cut at −12°C with a microtome (MICRON HM 500 OM), deposited on glass slides coated with poly-L-lysine (Sigma, St Louis, CA, USA) and stored at −20°C until use. Sections were air-dried and fixed in acetone for 10 min at 4°C.
Simple immunoperoxidase staining
After rehydration, menisci cryosections were incubated for 20 min at room temperature with Protein Block (Serum-Free, Ready-to-Use, Dako, Carpinteria, CA, USA) to prevent unspecific staining. Cryosections were then incubated in a range of primary antibodies. Briefly, Schwann cells were stained with a rabbit polyclonal anti-glial fibrillary acidic protein (GFAP) antibody (Dako, Glostrup, Denmark) diluted to 1/2000 in phosphate-buffered saline (PBS) 0.1 m, pH 7.4 (Sigma), applied overnight on the sections at 4°C in a humidity chamber.
To detect nerve fibres and especially neurofilaments with a high molecular weight (200 kDa), a rabbit anti-bovine neurofilament H (NFH) antibody diluted to 1/200 (AbD Serotec, Düsseldorf, Germany) in PBS was used. Incubation was carried out for approximately 3 h at 4°C in a humidity chamber.
Primary antibodies were revealed with a species-specific secondary antibody peroxidase-labelled polymer (Amplification EnVision®System-HRP, Dako, Denmark) for 45 min at room temperature and with a substrate chromogen, 9-ethyl-3-aminocarbazole (AEC, Zymed, San Francisco, CA, USA), used for 12 min in the dark. The peroxidase activity was stopped in distilled water for 3 min.
The specimens were counterstained with Mayer's haematoxylin and Light Green. All samples were mounted in Glycergel medium (Dako, Denmark). Observations were made with a light microscope (Olympus Bx51,Olympus Belgium N.V., Aartselaar, Belgium).
The specificity of the antibodies was tested using an isotype control antibody, and negative staining controls were obtained by omitting the primary antibody.
Double immunofluorescent staining
Horses' menisci cryosections were first incubated at room temperature (20 min) with Protein Block (Serum-Free, Ready-to-Use, Dako, USA) to prevent unspecific staining. Then, the sections were incubated, as described above, in a range of primary antibodies directed against Schwann cells and nerve fibres. These antibodies were revealed with species-specific secondary antibodies bearing a fluorescent label to perform fluorescent microscopy observations (Olympus Bx51,Olympus Belgium N.V., Aartselaar, Belgium).
To detect Schwann cells, a secondary goat anti-rabbit IgG antibody (heavy + light chains) conjugated to Alexa Fluor 568 and diluted to 1/4000 in PBS was applied 1 h at 4°C in the dark. Intermediate neurofilaments (200 kDa) were revealed with an Alexa Fluor 488 goat anti-mouse IgG (H + L) antibody (Molecular Probes, Leiden, the Netherlands), diluted to 1/4000 in PBS and applied to the sections for 1 h at 4°C in the dark.
Sections were mounted with a specific Dako Fluorescent Mounting Medium (Dako, USA).
The specificity of the antibodies was tested using an isotype control antibody, and negative staining controls were obtained by omitting the primary antibody.
Results
Anatomical location of mechanoreceptors in the anterior horn of the equine medial meniscus
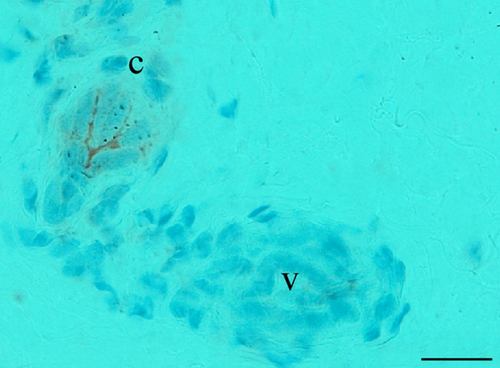
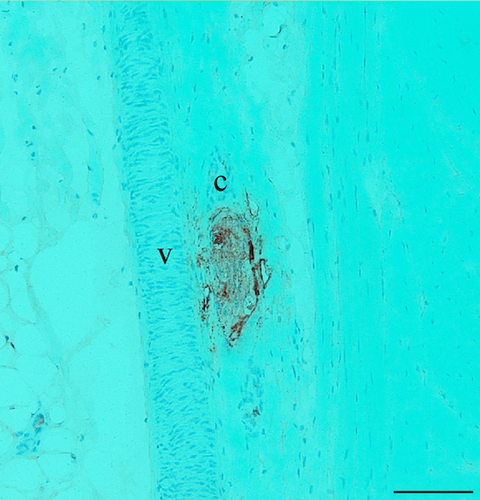
MCR were observed between the abaxial quarter and the abaxial third of the meniscus (Fig. 2). Isolated or linked in clusters, these corpuscles were frequently observed in the vicinity of blood vessels. Even if the orientation of the different samples before sectioning was standardised, the size and the shape of MCR showed great variability. Their position in the meniscus being unpredictable, the same corpuscle can present a fusiform, cylindrical or globular appearance. On all mechanoreceptors observed (n = 21), their lengths and widths ranged between 30 to 290 μm and 13 to 130 μm, respectively, estimated with image j software (National Institutes of Health (NIH),Rockville Pike, Bethesda, Maryland, USA).
These two morphological criteria are not thus relevant to differentiate the three types of MCR.
Identification of mechanoreceptors: an immunoperoxidase approach
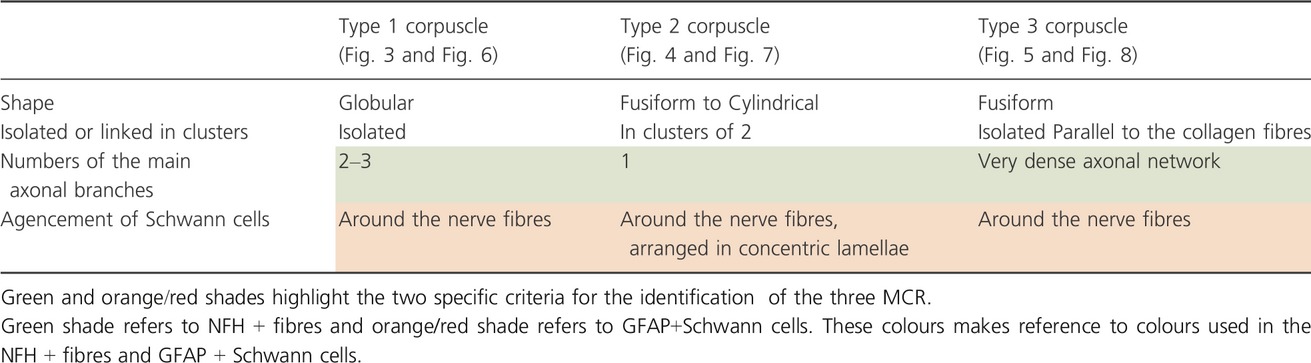
In addition to the anatomical location, the immunoperoxidase approach by simple immunostaining allowed us to (1) highlight the axonal architecture, revealed by NFH-positive fibres; (2) visualise the arrangement of GFAP+ Schwann cells and (3) determine the size of the capsule (Table 1). The size of the capsule, estimated by considering the number of cell layers (GFAP-) surrounding the external part of the MCR, was not relevant to identify the MCR. In view of these criteria, three particular morphotypes of MCR were identified. Figure 3 illustrates a Type 1 corpuscle, characterised by sparse axonal arborisation. Frequently, the principal afferent axon breaks up into two or three main axonal branches. It was found always isolated. The Type 2 corpuscles are distinguishable from Type 1 corpuscles by their organisation in clusters (generally linked by two) and by their axonal arborisation. Indeed, only a single main axon was observed inside (Fig. 4). Unlike the two previous copuscles, the Type 3 corpuscle, as illustrated on Fig. 5, is composed of a dense axonal arborisation and exhibit a fusiform-shaped oriented parallel to collagen fibres.

Intimate relationship between Schwann cells and nerve fibres: double immunofluorescent staining
To consolidate the results obtained by immunoperoxydase and to procure a more precise vision of the spatial organisation of Schwann cells with regard to the network fibres, we performed double immunofluorescence immunostainings (Table 1). GFAP+ Schwann cells surround neurofilaments highlighted with antibody directed against NFH. Three types of MCR were also able to be highlighted by this approach. In Type 1 corpuscles, as illustrated in Fig. 6, some Schwann cells were organised around three main axonal branches and established locally intimate contacts. The second type of MCR exhibit only one NFH+ fibre inside its axis. This axon is surrounded by Schwann cells organised in concentric lamellae. Intimate contacts were found especially between the axon and the innermost lamellae (Fig. 7). Due to the important network of nerve fibres in the Type 3 corpuscles (Fig. 8), Schwann cells established numerous close contacts with axonal branches.
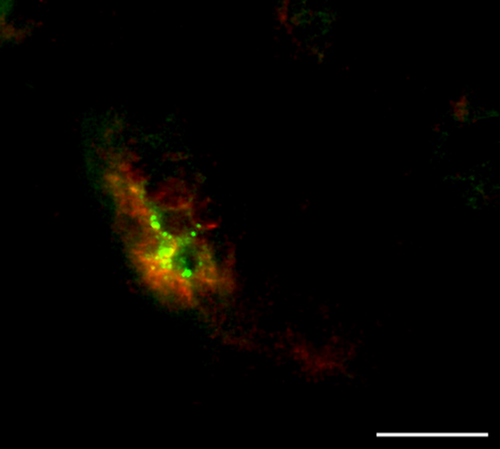
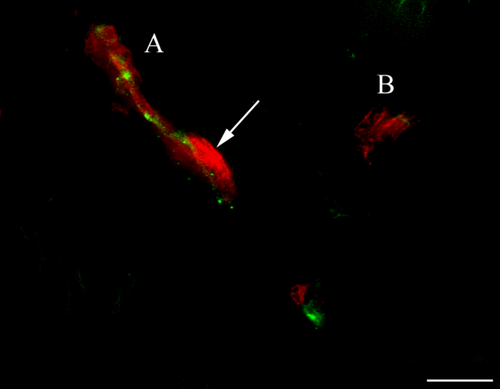

Frequency of the different mechanoreceptors observed
Although no quantification was performed, it would appear that Type 1 corpuscles were more numerous. Indeed, on all mechanoreceptors observed (n = 21), we observed 15 corpuscles of Type 1, 4 of Type 2 and only 2 of Type 3.
Discussion
In equine species, meniscal tears are the most common soft tissue injuries in the stifle (Walmsley, 2005; Fowlie et al., 2011a). Even with treatment, sports prognosis remains poor with meniscal injuries (Walmsley et al., 2003; De Busscher et al., 2006). Indeed, several studies (Walmsley et al., 2003; Cohen et al., 2009; Ferris et al., 2014) reported that, overall, only 37 to 47% of affected horses returned to full use, even if additional mesenchymal stem cell treatment may be more promising (Ferris et al., 2014). This could arise from a lack of meniscal repair resulting in particularly poor vascularisation in some areas, for example the body of the meniscus (Chevrier et al., 2009), or could be due to early-stage osteoarthritis derived from biomechanical alteration (Heijink et al., 2012). But proprioceptive disabilities due to the loss of MCR might also contribute to the development of osteoarthritis and thus may also be responsible for a poor sportive prognosis, the role of the meniscus in joint stabilisation being not only mechanical (Karahan et al., 2010).
Although the localisation, the description of MCR and their function in the menisci have already been reported in humans and other species, such as rabbits, to the best of our knowledge it is poorly documented in equine menisci (O'Connor and McConnaughey, 1978; Assimakopoulos et al., 1992; Moon and Kim, 1997; Gray, 1999; Mine et al., 2000; Saygi et al., 2005; Akgun et al., 2008).
Because of unconvincing results using conventional gold chloride staining to identify MCR and the misinterpretation resulting of the lack of standardisation of morphological criteria for their identification (Freeman and Wyke, 1967; Hogervorst and Brand, 1998; Rein et al., 2013b), we used an immunohistochemical approach to characterise the MCR by highlighting the axonal architecture and the Schwann cells' arrangement. Indeed, authors in literature were frequently in accordance with these two morphological features. Thanks to this agreement, we were able to identify our Type 1 as Ruffini corpuscle, Type 2 as Pacini corpuscle and Type 3 as Golgi corpuscle. Ruffini corpuscles are characterised by a loose axonal arborisation, Pacini corpuscles with only one axon inside are surrounded by an inner core, composed of concentric lamellae of Schwann cells, and Golgi corpuscles present the feature to have a very dense axonal arborisation and to be parallel to collagen fibres (Freeman and Wyke, 1967; Halata, 1977; Halata et al., 1985; Halata and Haus, 1989).
Contrary to earlier reports, where Type 1 or Ruffini corpuscles are described to be associated with clusters inside the joint capsule (Freeman and Wyke, 1967; Halata et al., 1985), we found them isolated in our equine menisci. This could probably be due to the anatomical region, due to variation between species or due to the experimental approach; indeed, stains like methylene blue or gold chloride used in these previous studies are not specific and may lead to misinterpretation (Freeman and Wyke, 1967; Lee et al., 2009; Rein et al., 2013a).
Regarding Pacini corpuscles, in agreement with several authors studying cat species, we usually found them in clusters of two (Freeman and Wyke, 1967; Halata, 1977). However, in our sections, the ‘inner core’ was not expanded as much as described in literature (Halata et al., 1985). Similar corpuscles with few lamellae, named Paciniform corpuscles (Barker, 1974; cited by Banks et al., 2009), have been described in the skeletal muscles of the hindlimb in cats. This terminology seemed to be more suited to our observation of Type 2 corpuscles in the anterior horn of equine medial meniscus, even if the distinction between these two corpuscles remains arbitrary (Barker, 1974; cited by Banks et al., 2009).
Finally, concerning Golgi corpuscles, their name is subject to discussion. Indeed, some authors have described ‘Golgi-like corpuscles’ in human knee joints, in contrast with the Golgi or Golgi tendon organs found in muscles. They have described these MCR in knee joints as corpuscles oriented in a direction parallel to collagen fibres and showing the same morphology as Golgi tendon organs (Halata et al., 1985; Rein et al., 2013a). This description is in agreement with our observations of the Type 3 corpuscles, and this terminology ‘Golgi-like’ will be therefore used in our future studies.
These encapsulated sensory end organs located in joints play a role in proprioception. Even if they are classified in most joints as acting as limit detectors in the range of movement, in comparison with the muscle spindles, their contribution is nevertheless required to have full proprioceptive acuity (Proske and Gandevia, 2012). By their proprioceptive functions, they might also play a role in protecting menisci and the joints from lesions. Indeed, the preservation of the areas rich in these sensory receptors during a partial meniscectomy at the posterior horn may prevent human menisci from being squeezed during movement of the knee joint (Saygi et al., 2005). Moreover, the osteoarthritic changes seen after a partial meniscectomy may be not only due to biomechanical disadvantages, but deterioration of the proprioceptive function of the knee may also contribute to it (Karahan et al., 2010). These studies have been performed in the posterior horn of the medial human meniscus, and subsequent studies will be necessary, of course, to confirm this hypothesis in the equine anterior horn of this meniscus.
Regarding the frequency of different types of MCR observed, we found that Ruffini corpuscles outnumbered Paciniform corpuscles and Golgi-like corpuscles. Although these results have a limitation in that they are based on a small sample size, the finding is interesting as these corpuscles perform different functions. Indeed, Ruffini corpuscles, classified as ‘slowly adapting receptors’, are sensitive to both static and dynamic situations, such as changes in intra-articular pressure, amplitude and direction of joint movement (Freeman and Wyke, 1967; Cavalcante et al., 2004). Joint position, coordinated by these corpuscles, could therefore have an enormous importance in proprioception at the level of the anterior horn of the equine medial meniscus. This is in agreement with previous studies on other regions like human ankle ligaments (Rein et al., 2013a).
The ligament of the cranial horn of the medial meniscus is also often implicated in equine meniscal tears, and grade 3 tears observed in arthroscopy (Walmsley, 2005) show very poor prognosis for future soundness (Cohen et al., 2009). Several studies of Fowlie et al. (2012) have shown the important forces during locomotion on the anterior horn of the meniscus and the meniscotibial ligament (Fowlie et al., 2012). The MCR may have therefore an important role to prevent lesions during equine locomotion. Further works are needed to examine these other regions of the meniscus.
It would be also interesting to identify and quantify nociceptive unmyelinated fibres, classified as Type 4 by Freeman and Wyke (1967), at the level of the regions of equine meniscus most frequently injured, namely the anterior horn of medial meniscus and its cranial ligament, for a better understanding of the origin of the pain sensation in equine meniscal tears (Mine et al., 2000).
In conclusion, from a purely fundamental point of view, our work highlights for the first time the presence of MCR at the level of the anterior horn of the equine medial meniscus and proposes a classification established by the use of specific immunocytochemical techniques. This morphological approach could serve as a basis for clinical studies on the relation between the location of lesions and possible proprioceptive deterioration and its impact on the appearance of osteoarthritis.
Acknowledgements
We would like to express our special thanks to Maitre D. and the staff of the autopsy room at the Faculty of Veterinary Medicine in Liège for the sample and to the technical assistance of Lemaitre O. and Weyckmans B.
Ethical Approval
This article does not contain any studies with human participants or alive animals performed by any of the authors.
Source of Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Conflict of Interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.




