Habitat but not group size or recent predator activity affect corvid collective vigilance at carcasses
Abstract
Vigilance is an important anti-predator behaviour that can be an indicator of the predation risk faced by potential prey animals. Here, we assess the collective vigilance, or the vigilance level of an entire group, of corvids (Family: Corvidae) at experimentally placed carcasses in a desert environment in Australia. Specifically, we explore the relationship between collective vigilance levels and the habitat in which the carcass was placed, the time since a potential predator (dingo Canis dingo, wedge-tailed eagle Aquila audax or red fox Vulpes vulpes) was present at a carcass, and the group size of corvids around the carcass. We found that corvids are more vigilant in open habitat, but that group size and the recent presence of a potential predator does not affect the collective vigilance behaviour of corvids. The results demonstrate the important link between habitat and vigilance, and that animals may adopt anti-predator behaviours irrespective of the size of the group in which they occur or the recent presence of a potential predator.
INTRODUCTION
Predators influence prey populations directly through consumptive effects, but they can also impact prey non-lethally by shaping how individuals perceive their risk of being attacked or killed at any one time or place, that is, “the landscape of fear” (Langerhans & Elewa, 2007; Lima & Dill, 1990). Both short-term and long-term predation risk contribute to the landscape of fear, with short-term risk often linked with the presence of predators in the landscape and long-term risk associated with differences in habitat structure (Cresswell & Quinn, 2011; Dröge et al., 2017; Périquet et al., 2010; Ward & Webster, 2016; Whittingham & Evans, 2004). Both types of predation risk typically play out across landscapes at the same time, and prey species must weigh these risks against the availability of potential opportunities like foraging resources (Lima & Dill, 1990). For example, after the reintroduction of wolves (Canis lupus) to Yellowstone National Park, USA, elk (Cervus elaphus) began to avoid open areas in preference for the more sheltered forest edges, where there was reduced foraging opportunities but also lower risk of predation (Laundré et al., 2001, 2010).
Prey animals often detect the presence of a predator directly by sight or sound, or through cues such as faecal and urinary odours (Eccard et al., 2017; Ekner & Tryjanowski, 2008; Mpemba, 2019; Verdolin, 2006; Yiu et al., 2021). The influence of olfactory cues on the behaviour of many mammalian species is well-known but has long been overlooked for other animals like birds. There is, however, growing evidence that like mammals, many bird species rely on odour cues to assess predation risk (as well as for finding food), and adjust their behaviour accordingly (Amo et al., 2015; Amo & Saavedra, 2021; Cunningham et al., 2009; Eichholz et al., 2012; Grigg et al., 2017; Roth et al., 2008). For example, blue tits (Cyanistes caeruleus) increase anti-predator behaviours around their nests in response to ferret (Mustela furo) scent (Amo et al., 2008) and carrion crows (Corvus corone) avoid the scent of stressed conspecifics (Wascher et al., 2015). Studies also suggest that animals tend to respond less to older, or smaller amounts of predator odours, with, for instance, rats exhibiting less fearful behaviour in the vicinity of small compared to large quantities of cat urine (Takahashi et al., 2005). This means that temporal changes in odour strength, for example following the natural decay of odour associated with a urination or defecation event, could also trigger altered behavioural or fear responses in certain species over time.
Vigilance is a behavioural response to the changing landscape of fear, and prey animals may be more vigilant in response to different odour cues, or in some habitat types compared with others (Gigliotti et al., 2021; Laundré & Hernández, 2003; Marino & Baldi, 2008). There are different types of vigilance. Social vigilance is used to gather social information such as the location of resources or the risk of competition with conspecifics (Dannock et al., 2019; Monclús & Rödel, 2008). Anti-predator vigilance on the other hand entails monitoring the environment for predators (Périquet et al., 2010; Wolff & Horn, 2003). Anti-predator vigilance changes with fluctuating levels of predation risk and can therefore be used as a measure of how different animals perceive risk (Laundré et al., 2010; Welp et al., 2004). For example, a study examining behavioural responses in lesser Rhea (Rhea pennata pennata) showed increased vigilance by this animal in closed habitats and in other areas where they were at greater risk of being hunted by humans (Barri et al., 2012).
Vigilance can also be influenced by group size, with one benefit of forming a group being that animals can reduce their individual vigilance without suffering a loss in overall, or collective, levels of vigilance (Wang et al., 2021). The ‘many eyes hypothesis’ predicts that as group size increases, collective vigilance (i.e., the vigilance level of the entire group) should also increase (Fernández et al., 2003; Li et al., 2016). The relationship between group size and individual vigilance levels remains a subject of debate but recent meta-analyses show some support for the ‘many eyes’ hypothesis (Beauchamp, 2008; Beauchamp et al., 2021).
Animal carcasses present an ideal model with which to study anti-predator behaviour. They often become a focal point for animals that function both as scavengers and predators (DeVault et al., 2003) and may therefore affect predation risk in the local environment. For example, carcasses have been shown to influence the behaviour of smaller, co-occurring species like rodents, with individuals showing greater fear responses in the vicinity of carcass resources (Frank et al., 2020; Steinbeiser et al., 2018). Carcasses, and particularly large carcasses (>10 kg), often also attract a high density of animals that are all competing for a shared, valuable resource, which can influence competitive species interactions. For example, predators are likely to gain greater nutritional benefit from a carcass than from pursuing smaller prey (DeVault et al., 2003), meaning that larger (apex) scavengers may competitively exclude smaller (meso) scavengers from carcasses. Increased competition and greater encounter rates around carcasses could also result in higher levels of intraguild predation, for example, when apex scavengers defend the resource from meso-scavengers (Prugh & Sivy, 2020). The landscape of fear around carcasses may therefore be based on competitive exclusion as well as direct predation risk.
Corvids (Corvus spp.) are common meso-scavengers globally, especially in areas where vultures are absent. Corvids are often the first species to arrive at carcasses (Gomo et al., 2017; Lafferty et al., 2016) and can exploit carcasses in large groups, sometimes leading them to dominate and drive off other scavengers (Heinrich, 1988; Kendall, 2013). As meso-scavengers, corvids are at risk of being preyed upon or attacked by larger scavengers, potentially when competing over the shared resource of a carcass. In Australia, larger scavengers that may attack or exclude corvids from carcasses include dingoes (Canis dingo), wedge-tailed eagles (Aquila audax), red foxes (Vulpes vulpes) and feral cats (Felis catus) (Doherty et al., 2019, 2015; Olsen, 2005; Rowley, 1973b). Understanding the influence of these larger scavengers, and other factors, on corvid vigilance behaviour will provide novel insight into the scavenging dynamics of corvids and deepen our understanding of predation effects around carcasses. To date, there has been little work examining the vigilance behaviour of meso-scavengers or species interactions around carcasses generally, limiting our understanding of how animals use this important food resource.
Here we explore the vigilance behaviour of corvids around experimentally-placed carcasses by measuring levels of collective vigilance. Predation risk is likely to change with habitat (long-term risk; Dröge et al., 2017; Périquet et al., 2010), and thus we predict that (A) collective vigilance of corvids will be higher in closed than open habitat, as more structurally complex habitat may reduce corvids' ability to spot potential predators and escape, requiring more vigilance for the same level of safety. Predation risk may also be elevated when predators have recently been present at a carcass (short-term risk; Creel et al., 2014; Pays et al., 2021), thus we predict that (B) the level of collective vigilance will decrease with time since a predator was last present at a carcass. Previous work has found that collective vigilance increases as group size increases (Beauchamp, 2008; Beauchamp et al., 2021), including in some mixed species groups (Bshary & Noë, 1997; Cords, 1990; van Langevelde et al., 2022) and we predict that (C) the vigilance levels in corvids will follow this pattern across habitat and varying levels of predator presence.
METHODS
We assessed the vigilance behaviour of corvids (Corvus spp.) around experimentally placed red kangaroo (Osphranter rufus) carcasses in June 2019. The study was conducted on Bush Heritage Australia's Ethabuka Reserve in the Simpson Desert, Queensland, Australia. In the Simpson Desert, three corvid species are present: the Australian raven (Corvus coronoides), the little crow (Corvus bennetti) and the Torresian crow (Corvus orru). The Australian raven and the little crow are relatively common across this region, however the Torresian crow is considered an occasional visitor, generally increasing in number following periods of high rainfall (A. Tulloch, unpublished data). The scavenging and vigilance behaviours of these species likely differ. Australian ravens tend to maintain territories as breeding pairs whereas both species of crow form large transient flocks that move through the desert in search of food (Rowley, 1973a). The habitat preference of these species is probably also different, with little crows more closely associated with open habitat compared with Australian ravens (Rowley, 1973a). These three species are best differentiated by their calls, which were not recorded during this study, and they can be difficult to identify to species level using only photographs (Campbell et al., 2015). We therefore decided to group all corvids into the single classification of Corvus spp., whilst acknowledging that by doing so we could be overlooking possible behavioural differences between the species.
The Simpson Desert is characterized by long parallel sand dunes. The vegetation on the dune crests is sparse, with a patchy cover of ephemeral and perennial plants including Goodenia cycloptera, Euphorbia drummondii, Grevillea stenobotrya, Sida spp., Acacia spp. and spinifex (Triodia basedowii). In the interdunal swales are stands of gidgee trees (Acacia georginae), mallee eucalypts and other Acacia shrubs (Wardle et al., 2015). These two habitats were designated as open (dune crests) and closed (gidgee trees) and were used to compare levels of corvid vigilance.
Carrion is observed frequently in arid Australia due to both climate-induced animal mass mortality (e.g. during droughts) and widespread lethal control (animal culls) of both native and introduced species for management purposes, so the behaviours observed around experimentally placed carcasses are likely to be natural for local scavengers. For this study, ten red kangaroo carcasses were placed at sites at least 1 km away from each other, with five positioned in open habitat and five in closed habitat. The carcasses were sourced fresh from a commercial harvester working on nearby properties. Each carcass was tied to a stake to ensure it was not removed from the field of view of the camera. A stake was then hammered into the ground ~3 m from the carcass and a Reconyx PC800 Hyperfire™ camera (Professional Reconyx Inc.) was attached 1 m above the ground. These cameras take photos by day and night using infrared flash triggered by a heat and movement signature (rapid fire, with 10 consecutive triggers and no period) and then store information in each image (EXIF data; temperature, date and time). Each carcass was monitored for 3 months, and the first 30 days of monitoring data were used to test our predictions. This encapsulated the period when vertebrate scavengers, especially corvids, were most active on carcasses (Bragato et al., 2022); red kangaroo carcasses in the study area take an average of 14 days to decompose (Spencer & Newsome, 2021).
Some studies have used the proportion of time where at least one animal is vigilant as a measure of collective vigilance (Fernández et al., 2003; Li et al., 2016; Pays et al., 2012; Vitet et al., 2020). However, collective vigilance can also be measured as the proportion of vigilant animals in a group (Childress & Lung, 2003; Creel et al., 2014; Dröge et al., 2017; Iranzo et al., 2018). This measure of collective vigilance provides insight into the level of predation risk faced by the whole group. We therefore decided to quantify the collective vigilance of corvids around carcasses as the proportion of individuals vigilant during independent carcass visitation events. An event was assumed independent if there was no corvid activity (i.e., no photos with corvids present) at a carcass for 10 min (Bragato et al., 2022; O'Brien et al., 2010). Only events where corvids remained at the carcass for more than 10 min were analysed, consistent with the time used in previous studies and ensuring that most events had an adequate number of photos (Marino & Baldi, 2008). Although this may exclude times when corvids were most at risk, i.e., when they fled from the carcass quickly, it was necessary to ensure that an adequate number of photos was available for sampling.
During each event 10 photos were selected for analysis. The photos were selected to be as evenly spaced throughout the event as possible, whilst also ensuring a non-biased sample (Marino & Baldi, 2008). Other methods, such as scanning samples at designated intervals could not be employed as the camera trap was only triggered by motion and did not continuously photograph entire events. To ensure that an adequate number of photos in each event was sampled but that the maximum number of events possible were analysed, we discarded events with fewer than 10 photos from analysis (mean number of photos per event ± SE = 88.4 ± 10.1). In each selected photo the number of corvids in the frame, and the number of vigilant individuals were recorded. Vigilance was defined as corvids having their head above the horizontal plane and looking away from the carcass (to differentiate from social vigilance; Dannock et al., 2019; van Deventer & Shrader, 2021) (Figure 1). The proportion of corvids vigilant in each frame was calculated. This per-frame vigilance proportion was then averaged for each event, resulting in an average vigilance value (Childress & Lung, 2003; Dröge et al., 2017).

To test our three predictions, the proportion of corvids vigilant was analysed in relation to habitat (open and closed; long-term predation risk) (A), the time since a predator (dingo, eagle or fox) was present at the carcass (short-term predation risk) (B) and the corvid group size (C). Feral cats were excluded from analysis as there were too few photos (only six visitation events across three sites). Group size was measured as the maximum number of corvids present in a single frame during each event. If corvids visited a carcass after a predator, we calculated the time from predator visit to corvid arrival using timestamps on photos, truncating this at 24 h as we assumed that any effects of a predator would be negligible after 1 day.
Multiple linear mixed models with normal distribution were fitted to assess the impact of habitat, predator presence and species and group size on corvid vigilance. All models included site as a random factor to account for multiple measures of vigilance at each carcass. Interactions between habitat and time since a predator visit, as estimates of long-term and short-term risk, were included in the models following Dröge et al. (2017). The interactions of predator species and predator presence and habitat were also included to assess the species-specific effects of each predator. The models were ranked using Akaike Information Criteria, correcting for small sample size (AICc; Table 1). Models with Δi < 2 units relative to the model with the lowest AICc were considered to have equal support (Burnham & Anderson, 2002). All analysis was performed in R Version 4.1.0 (R Core Team, 2020) and using the package nlme for modelling (Pinheiro et al., 2017). Mean values are presented with plus or minus standard error (±SE).
| Model (proportion of corvids vigilant ~ …) | AICc | Delta | Weight |
|---|---|---|---|
| Habitat | −51.71 | 0 | 9.63e−1 |
| Group size | −43.22 | 8.49 | 1.38e−2 |
| Group size + Habitat | −42.96 | 8.75 | 1.21e−2 |
| Species + Habitat | −42.12 | 9.59 | 7.97e−3 |
| Time since predator + Habitat | −39.10 | 12.60 | 1.76e−3 |
| Time since predator | −37.91 | 13.79 | 9.73e−4 |
| Habitat × Species | −37.17 | 14.54 | 6.71e−4 |
| Time since predator + Group size + Habitat | −30.57 | 21.14 | 2.47e−5 |
| Time since predator + Group size | −29.24 | 22.47 | 1.27 e−5 |
| Habitat × Species + Group size | −27.42 | 24.28 | 5.13 e−6 |
| Species + Time since predator | −27.03 | 24.67 | 4.23 e−6 |
| Time since predator × Habitat | −22.30 | 29.40 | 3.97 e−7 |
| Habitat × Species | −21.50 | 30.20 | 2.66 e−7 |
| Time since predator: Habitat | −18.96 | 32.74 | 7.47 e−8 |
| Time since predator + Group size + Habitat + Species | −18.30 | 33.40 | 5.38 e−8 |
| Time since predator × Habitat + Group size | −13.93 | 37.77 | 6.05 e−9 |
| Time since predator: Habitat + Group size | −10.23 | 41.47 | 9.49 e−10 |
| Time since predator: Species | −0.85 | 50.86 | 8.72 e−12 |
| Time since predator × Species | 7.38 | 59.08 | 1.42 e−13 |
| Time since predator × Species + Habitat | 7.94 | 59.65 | 1.07 e−13 |
| Time since predator × Species + Habitat + Group size | 16.69 | 68.39 | 1.35 e−15 |
- Note: The models are ranked by AICc. Only one model was supported.
RESULTS
Corvids discovered all carcasses within 67.5 h (mean ± SE = 32.7 ± 6.7 h) of placement in the field. Overall, 77 590 photos of corvids were assessed, and these comprised 878 independent carcass visitation events. Corvids spent on average 8.1 ± 0.6 min at carcasses during each event.
We assessed 51 375 photos of predators, comprising 167 independent carcass visitation events. Predators spent on average 14.0 ± 1.9 min at carcasses during each event. Predators were present at every carcass site, with dingoes present at all 10 (84 total visitation events across all sites), eagles at 7 (45 total visits) and foxes at 4 (29 total visits) carcass sites.
In total, corvids were recorded in 108 independent events that lasted longer than 10 min, at carcasses that were visited in the last 24 h by a predator. Of these 108 events, 56 were recorded at carcasses in open habitat while 52 were recorded at carcasses in closed habitat, and the events were distributed across all 10 carcass sites. The group size of corvids varied from 1 to 13 and on average 50 ± 2% of corvids were vigilant during each event.
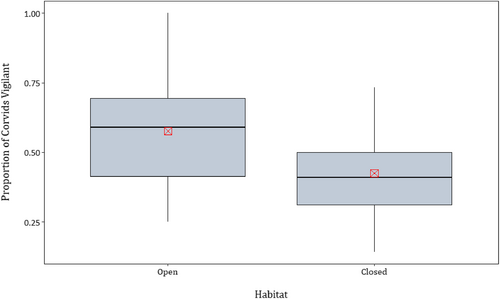
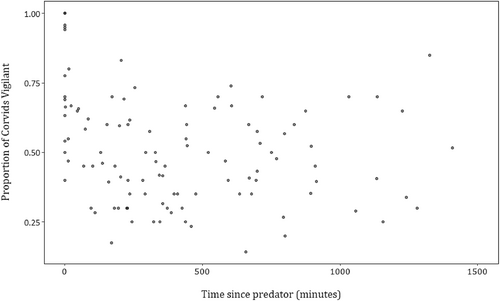
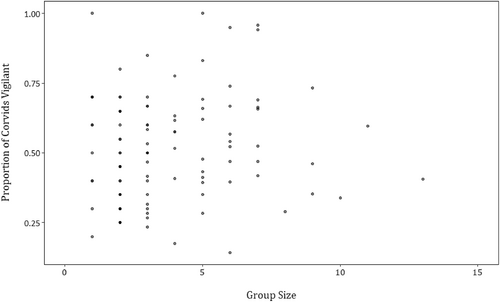
Following the ranking of the candidate models by AICc, only one model was supported (Table 1). This model included the habitat variable, and showed that the proportion of corvids vigilant was higher in open compared with closed habitats (Table 2, Figure 2). There was no effect of either the recent presence of predators (Figure 3) or the group size of corvids (Figure 4) on corvid vigilance levels.
| Variables | Estimate | SE | t-Value | p |
|---|---|---|---|---|
| Random effects | ||||
| Site | 0.009 | 0.175 | ||
| Fixed effects | ||||
| Intercept | 0.577 | 0.024 | 23.942 | <0.001 |
| Habitat | −0.151 | 0.035 | −4.329 | 0.003 |
- Note: The model is assessing the effect of habitat on the vigilance behaviour of corvids at carcasses in the Simpson Desert, Australia in June 2019 (n = 108).
- Bold p values indicate significance at α = 0.05.
DISCUSSION
This study shows that corvids change their vigilance levels in response to habitat but not group size or recent predator activity. Specifically, we found that open habitat was potentially perceived by corvids as riskier than closed habitat (against prediction A). We then showed that the collective vigilance level of corvids was not affected by the recent presence of predators at the carcass (against prediction B). Similarly, the species of predator was also not included in the supported model, although this was possibly due to insufficient data that prevented us from assessing each species independently. Finally, in contrast with other past research investigating the relationship between vigilance levels and group size (Beauchamp et al., 2021; Bshary & Noë, 1997; Cords, 1990; Li et al., 2016; Marino & Baldi, 2008; van Langevelde et al., 2022), we showed that group size did not affect the collective vigilance of corvids (against prediction C).
Habitat (long-term risk)
Habitat has a major influence on predation risk (Brown & Kotler, 2004; Creel et al., 2014; Laundré et al., 2010; Whittingham & Evans, 2004). Here, and in other studies focusing on vigilance, habitat has been used as a proxy for long-term risk (Dröge et al., 2017; Li et al., 2016; Marino & Baldi, 2008; van Deventer & Shrader, 2021). In this study we predicted that closed habitat would provide a riskier option for scavenging corvids, as the more vegetatively complex gidgee forest could limit their ability to detect predators and escape compared to the more open dune crests (Dellinger et al., 2019; Heithaus et al., 2009; Wirsing et al., 2010).
The increase in vigilance levels of corvids in open compared with closed habitat, although unexpected, could be due to several factors. First, during the study period predator activity and corvid activity were both greater in open habitat, probably because carcasses are found more quickly in open than in closed habitat (Bragato et al., 2022; Spencer & Newsome, 2021). Elevated activity in open habitat could potentially lead to more frequent encounters between predators and prey, and larger scavengers are likely to exclude corvids from carcasses to monopolize the valuable resource. Corvids may perceive the increase in predator activity in open habitat as an increase in risk of attack at carcasses by predators either attempting to depredate corvids or defend the resource.
Second, in open habitats corvids may be more vulnerable to certain predators. Wedge-tailed eagles hunt most efficiently in the open and may represent a higher risk to scavenging corvids in this habitat type (Olsen, 2005). Although their effects were not investigated here, feral cats are also more efficient at hunting in open habitats (McGregor et al., 2015).
Third, in the Simpson Desert the ‘closed habitat’ of gidgee trees provides relatively sparse cover compared with closed habitats in other ecosystems, for example old growth forests. This may mean that the corvids' sight and flight responses are not impeded by the vegetation in our closed habitat. Gidgee trees also offer perches from which corvids can scan large areas for predators, and can provide refuges from predators; distance from refuge can be an important factor influencing vigilance levels (Dear et al., 2015; Marino & Baldi, 2008). On the other hand, in open habitat, corvids are restricted to the ground and their view is more limited. This could reduce their ability to detect predators and so they must maintain a higher level of vigilance to remain safe.
These possibilities show that further research is needed to fully understand corvids' perception of increased predation risk in open habitat. Investigating the occurrence of corvids in each predator's diet would help to determine the relative risk that each predator species presents, although predators are also likely to present non-consumptive risks, particularly around carcasses. Therefore, it would be prudent in future to investigate the responses of corvids to the presence of individual predator species and attempt to determine whether corvids remain vigilant at carcasses due to risk of direct predation or from competitive exclusion by larger scavengers. Measuring more detailed habitat characteristics, such as ground cover and field of view, may also provide insight into why corvids perceive open habitat as risky.
Presence of predators (short-term risk)
The presence of a predator usually increases vigilance activity in prey as the landscape of fear is elevated (Creel et al., 2014; Han, 2020; Pays et al., 2021; Wolff & Horn, 2003). Thus, as a predator moves further away from prey animals, in time or space vigilance activity should decrease (Creel et al., 2014) provided that prey species are able to detect the decline in predation risk (Gaynor et al., 2019). Our results here indicate that the recent presence of predators at the carcass sites had no effect on the vigilance levels of corvids. We will explore possible explanations of this result below.
We proposed that corvids may be able to detect predators through olfactory traces, of which there is increasing evidence of their importance to birds (Amo et al., 2008; Wascher et al., 2015; Zidar & Løvlie, 2012). Yet our results indicate that corvids may not be able to detect the cues left behind by predators (Ekanayake et al., 2015), or ignore them if they are detected. Corvids have excellent memories, for example they may remember researchers' vehicles and follow them to resource sites (Ekanayake et al., 2015; Emery & Clayton, 2004). While travelling around our field site, we often spotted corvids circling carcasses in the air, providing them with an opportunity to assess the activity levels of other scavengers including predators before visiting a carcass. Avian responses to visual cues of predators has been shown in other contexts, with great tits (Parus major) avoiding areas with clear signs of predation (fur and mangled feathers) (Ekner & Tryjanowski, 2008). The lack of an effect of recent predator presence on corvid vigilance levels may therefore indicate that corvids only increase vigilance when they have seen a predator near or at a carcass site. However, when both predators and corvids were present at carcasses at the same time, no attempts at predation were observed; the larger predators (scavengers) simply attempted to exclude corvids from the valuable carcass resource. This is likely because monopolizing the carcass resource is far more valuable than attempting to capture and eat a corvid. It is therefore possible that corvids may not have been at risk of predation from these scavengers at the carcass, meaning that the presence of these predators is unlikely to change corvids' vigilance levels.
It is also important to consider the species-specific interactions between corvids and their predators and the important influence on the landscape of fear (Creel et al., 2014, 2019; Makin et al., 2018). For example, eagles hunt more effectively in open habitat (Olsen, 2005) and thus corvids would likely be more vigilant when eagles are present in these areas. However, dingoes may be more effective predators in closed habitats, with corvids likely more vigilant there when dingoes are present. As predators were pooled across all species in our analyses this may have obscured the response of corvids to the presence of predators. The exclusion of species from the supported models does not eliminate the possibility of species-specific interactions, rather it indicates the need to further investigate these relationships particularly given the different levels of activity of each predator species. Such investigations would illuminate interactions between short and long-term risk and provide further insight into the mechanisms involved in predator–prey interactions.
Group size
The many eyes hypothesis, which proposes that as group size increases so should collective vigilance, was not supported by our study. Previous studies have found mixed support for this hypothesis, including in multi-species groups (Beauchamp, 2008; Beauchamp et al., 2021; Bshary & Noë, 1997; Cords, 1990; van Langevelde et al., 2022). We found that as group size increased, corvid vigilance behaviours stayed relatively consistent. This result could provide evidence for the ‘dilution effect’ (Cresswell & Quinn, 2011), wherein individual vigilance is reduced as group sizes increase, neutralizing any effects on collective vigilance levels. Corvids may also form groups around carcasses for reasons other than vigilance benefits, such as to exploit the carrion quickly and reduce the energetic benefit conferred to other individuals.
Carcasses are unique resources in the landscape, and this distinctiveness may also affect vigilance levels in relation to group size. Large groups are more likely to attract predators via the ‘detection effect’ (Fernández et al., 2003). However, as carcasses are already highly attractive to predators (Cortés-Avizanda et al., 2009), and have possibly already been visited by predators, the detection effect is likely to have little additional influence on predation risk. In some studies, scavenging groups of corvids have mobbed and driven off larger scavengers in order to monopolize carrion (Kendall, 2013; Schlacher et al., 2013). This has also been observed in the Simpson Desert, with corvids chasing off wedge-tail eagles (E. Spencer, unpublished data). This behaviour may result in predation risk remaining relatively constant even as group size changed around carcasses, leading to the observed consistency in collective vigilance levels.
Limitations and future directions
Factors other than perceived predation risk may affect collective vigilance levels in corvids, such as the possibility of synchronizing vigilance, leading to elevated levels of collective vigilance (Favreau et al., 2010; Pays et al., 2007). Synchronization can occur in response to a stimulus; for example, if a stick breaks all animals look towards the sound (Pays, Dubot, et al., 2009). One animal being vigilant may also lead another animal to become vigilant (Beauchamp et al., 2012; Pays, Goulard, et al., 2009). This may be due partly to the fact that this reduces an animal's risk of predation as vigilant animals are less likely to be attacked and more likely to escape (FitzGibbon, 1989; Krause & Godin, 1996); therefore, to ensure they are not more vulnerable than their group members, animals copy their vigilance behaviour. From our observations, corvid vigilance behaviour can be interpreted as primarily anti-predator in focus. However, there is a possibility that some corvids were socially vigilant. The effect of social vigilance and synchronization of vigilance would need greater consideration if vigilance increased with group size, because as group size increases the levels of social vigilance and vigilance synchronization are also likely to increase.
As mentioned previously the species of corvid present at the carcasses could not be identified, and this therefore limits the conclusions that can be drawn around vigilance patterns in corvids. The different species of corvid possibly display different scavenging behaviour and also vary in group size, for example Australian ravens normally forage as pairs whereas little crows and Torresian crows typically forage in larger groups (Rowley, 1973a). Australian ravens are also thought to prefer closed habitats, whereas crows may typically be associated with open habitat (Rowley, 1973a). These differences in species scavenging strategies may have driven some of the results in this study. However, this study still provides insight into the varying landscape of fear around carcasses and can be used as a basis for further work on the species-specific interactions that occur around carcasses.
The scavenging dynamics around carcasses in the Simpson Desert varies with season (Spencer & Newsome, 2021). In winter, carcasses persist for far longer than those in summer, as insects dominate carcasses in the warmer months. The scavenging pattern of larger vertebrates is also altered in summer, for example in one summer dingoes were not detected at any of 20 carcasses placed out for observation in the study site (Spencer & Newsome, 2021). The changes in predator presence and carcass persistence may alter the landscape of fear, resulting in changes in corvid vigilance behaviour. Corvids also vary their scavenging levels in response to changes in resources levels (i.e., during drought and following periods of high rainfall) and with seasons in the desert (Bragato et al., 2022). It is therefore important to investigate any seasonal effects on vigilance behaviour in the future.
Overall, our results demonstrate the effects of short and long-term risk on collective vigilance behaviour in corvids and reiterate the importance of habitat in relation to predation risk. In doing so, we also support the notion that carcasses are a hub for species interactions and provide an excellent opportunity to study not just vigilance, but many aspects of wild animal behaviour.
AUTHOR CONTRIBUTIONS
Patrick J. Bragato: Conceptualization (lead); formal analysis (lead); investigation (supporting); methodology (equal); software (lead); visualization (lead); writing – original draft (lead). Emma E. Spencer: Conceptualization (supporting); investigation (lead); methodology (equal); software (supporting); writing – review and editing (equal). Chris R. Dickman: Conceptualization (supporting); methodology (supporting); supervision (equal); writing – review and editing (equal). Mathew S. Crowther: Formal analysis (supporting); methodology (supporting); writing – review and editing (equal). Ayesha Tulloch: Formal analysis (supporting); investigation (supporting); methodology (supporting); writing – review and editing (equal). Thomas M. Newsome: Conceptualization (supporting); investigation (supporting); methodology (supporting); supervision (equal); writing – review and editing (equal).
ACKNOWLEDGEMENTS
We are indebted to Bush Heritage Australia for providing access and accommodation during field studies, Ethabuka reserve managers Helene Aubault and Kyle Barton for their advice and support throughout this study, and Wangkamadla Traditional Owners for permission to work on country. We acknowledge the Wangkamadla people as the Traditional Owners of Ethabuka Reserve. We recognize and respect the enduring relationship they have with their lands and waters, and we pay our respects to Elders past, present and future. Invaluable assistance was provided in and out of the field by members of the Desert Ecology Research Group, including Glenda Wardle, Bobby Tamayo and members of the Global Ecology Lab including James Vandersteen and Chris Fust. Thanks to landholders in Boulia Shire, western Queensland, who provided materials for this project. We are also very thankful to the many co-workers and volunteers who assisted, particularly Guillamue Tutton, Joon Kim, James MacDiarmid and Hayden Griffith. Open access publishing facilitated by The University of Sydney, as part of the Wiley - The University of Sydney agreement via the Council of Australian University Librarians.
FUNDING INFORMATION
This work was supported by the Australian Government's National Environmental Science Program through the Threatened Species Recovery Hub [Theme 1, Subproject 1.1.11 Cat suppression to conserve the night parrot]; and the Margaret Middleton Fund for Endangered Species.
CONFLICT OF INTEREST STATEMENT
The authors have determined that there are no conflicts of interests.
ETHICAL APPROVAL
Scientific licences were obtained to relocate and monitor the carcasses (SL WA0006737), and all research was approved by the University of Sydney Animal Ethics Committee (Project number 2017/1173).
Open Research
DATA AVAILABILITY STATEMENT
The data and code used in the analyses for this paper are available in a GitHub repository at: https://github.com/PatBragato/Corvid_vigilance_paper.




