Fire caused demographic attrition of the Tasmanian palaeoendemic conifer Athrotaxis cupressoides
Abstract
The temperate island of Tasmania is a global centre of plant endemism, with relictual lineages that persist in topographically rugged, wet and cool refugia. An iconic example of these palaeoendemic plants is the slow-growing conifer, Athrotaxis cupressoides D. Don (Cupressaceae). The geographic range of A. cupressoides has shrunk since European settlement because of destructive anthropogenic fires. Inscription of the Tasmanian Wilderness World Heritage Area in 1982 provided formal protection for Tasmania's palaeoendemic taxa, but they remain vulnerable to lightning-ignited landscape fires, which are becoming more frequent due to climate change. We surveyed stands across the species’ range and found that stands damaged by fires in the 20th century had higher grass cover and were more exposed to hot northerly winds than unburnt stands. A recruitment bottleneck was evident, with juveniles absent in 28% of unburnt and 47% of burnt transects. Transects on small islands in lakes had lower herbivore densities and less evidence of fire than comparable mainland transects. However, the island transects had lower densities of A. cupressoides seedlings and saplings, despite similar densities of adult trees, suggesting factors other than fire and herbivory contribute to the poor regeneration. We also studied the effects of a lightning fire in 2016, finding it killed 68% of stems overall, with stems less than 30 cm diameter and those scarred by previous fires more likely to die. These findings of high adult mortality and poor regeneration following fire suggest that the geographic range of A. cupressoides will contract due to the increasing frequency of lightning-ignited fires. Management responses to the increasing risk of landscape fires now include establishment of seed banks, restoration planting and use of irrigation to protect stands from active fires, in addition to rapid suppression of ignitions and targeted planned burning to reduce fuel loads in surrounding flammable vegetation.
Introduction
Extant ‘living fossil’ plant and animal species are of enormous scientific value because they illuminate evolutionary processes. Often these ancient lineages are geographically restricted and clustered together, a biogeographic pattern that has led to the recognition of ‘palaeoendemic’ taxa. Palaeoendemics typically occur in regions with stable environmental conditions and high topographic diversity, affording refugia in which to persist during adverse conditions (López-Pujol et al. 2011). The distributional patterns of endemic species have been used to infer the locations of important refugia in regions such as eastern China (López-Pujol et al. 2011), sub-Saharan Africa (Linder 2001), the eastern European Alps (Tribsch 2004) and Australia (Crisp et al. 2001). Typically, the habitats that sustain palaeoendemic taxa were once more widespread but have markedly contracted due to environmental change over geological timescales (Jordan et al. 2016). The biogeography of palaeoendemics provides a unique and irreplaceable insight into ecology and evolution of past biomes (López-Pujol et al. 2011; Jordan et al. 2016). Sites with concentrations of palaeoendemics are of high conservation value and are vulnerable to anthropogenic impacts.
One globally important hotspot for palaeoendemic conifer and angiosperm species is the temperate, rugged island of Tasmania, 200 km south of the Australian mainland (Kooyman et al. 2013). A striking, and globally unusual, feature of Tasmanian vegetation is the fine-scaled intermix of fire-adapted sclerophyll evergreen vegetation dominated by archetypic Australian genera, such as Eucalyptus, Acacia and Banksia, and fire-sensitive, palaeoendemic lineages that are readily killed by fire, and regenerate poorly following fire. Tasmania's palaeoendemic species are associated with environments with a globally rare combination of cool summers, low moisture deficits and hence infrequent fires, together with mild winters (Jordan et al. 2016). Such constantly moist temperate areas have become rare since the separation of Antarctica in the mid-Cainozoic following the Australian plate northward drift in the subtropical high-pressure zone and consequent aridification (Bowman 2000; Jordan et al. 2016). During the Quaternary Period, Tasmania experienced repeated glaciations and the associated periodic development of a small ice-cap on the low plateau (1500 m) in the centre of the island, as well as substantial oscillations of coastlines and treelines. Thus, refugial species had to track suitable niches through the Quaternary, which has shaped biogeographic patterns and species’ genetic diversity (Mokany et al. 2017). Lower sea levels during the ice-ages periodically connected Tasmania and mainland Australia, facilitating biotic exchanges, including the human colonisation before the height of the last glacial (35 000 years ago) (O'Connell & Allen 2015).
An iconic example of a Tasmanian palaeoendemic is the conifer Athrotaxis cupressoides D. Don (pencil pine), which is now restricted to subalpine areas of Tasmania, on its Central Plateau and western mountains (Cullen & Kirkpatrick 1988; Jordan et al. 2016). The Athrotaxis genus is estimated to be ~155 Myr old and is among the oldest of the similarly geographically restricted vascular plant groups in the world (Leslie et al. 2012; Jordan et al. 2016). Athrotaxis cupressoides is a slow-growing tree that forms large clonal populations (Worth et al. 2016). Individual stems can live for over 1000 years, while genets can be much older (Cullen & Kirkpatrick 1988; Worth et al. 2016). The species has low fecundity owing to mast seeding, poor seed dispersal and limited seedling establishment (Kirkpatrick & Dickinson 1984; Cullen & Kirkpatrick 1988). It is poorly adapted to fire, with high stem mortality and no long-lived aerial or soil seed bank (Cullen & Kirkpatrick 1988). Seedlings do not resprout after fire (Prior et al. 2018) although adults can resprout from root suckers (Cullen & Kirkpatrick 1988). The species’ biogeography and genetic architecture has been shaped by the interplay of fire and glacial cycles, resulting in populations with low genetic diversity, and populations that have experienced fire are less genetically diverse than populations that occur in fire-protected landscape settings (Dodson 2001; Worth et al. 2017). Palynological data suggest that the intensification of the El Niño climate phenomenon in the late-Holocene resulted in fires in montane areas where A. cupressoides established following post-glacial warming, causing widescale conversion of pyrophobic rainforest to pyrophytic, eucalypt-dominated vegetation (Fletcher et al. 2014; Stahle et al. 2017; Mariani et al. 2019). Current, fine-scale, fire refugia typically occur in either wet areas in the landscape, such as on shores of lakes or associated with sphagnum bogs, or south-facing slopes and rocky sites that provide topographic protection (Wood et al. 2011; Worth et al. 2016).
The role of Aboriginal ignitions in the decline of fire-sensitive taxa is unclear (Stahle et al. 2016, 2017; Mariani et al. 2017). The persistence of A. cupressoides through the late Pleistocene, including post-glacial re-expansion of the species, and the occurrence of Holocene archaeological sites in the species’ core refugia, suggests that Aboriginal burning was not damaging to this and other palaeoendemic species. By contrast, there were dramatic changes following European settlement at the beginning of the 1800s. A series of extensive wildfires set by the colonists led to an estimated 30% contraction in the range of A. cupressoides, including an estimated 10% loss from fires in the summer of 1960–1961 (Holz et al. 2015). While strategic fire management and prohibition of campfires in the Tasmanian Wilderness World Heritage Area have successfully reduced the incidence of human-caused fires, there has been an increase in large fires caused by dry lightning storms since the 1990s (Harris et al. 2018; Styger et al. 2018). Such fires threaten previously fire-free palaeoendemic refugia and are likely to become more frequent as the climate warms further and rainfall decreases, reducing the resilience of these communities (Grose et al. 2010; Love et al. 2016; Worth et al. 2016; Mariani et al. 2019).
In January 2016, dry lightning storms caused multiple fires across western Tasmania and burnt about 20 000 ha of the Tasmanian Wilderness World Heritage Area (TWWHA), representing 0.6% of the mapped distribution of the species (Senate 2016). The fires also affected wetland peats, cushion moors, sphagnum bogs and organic soils in the region. The damage to A. cupressoides was of international interest because of the threat to an aesthetically important component of the alpine landscapes of the TWWHA and to Tasmania's renowned Gondwanan legacy (Marris 2016; Senate 2016). These fires followed an exceptionally dry, warm spring which caused vegetation and usually damp organic soils to become combustible. Modelling suggested the extreme dryness and warmth was exacerbated by anthropogenic climate change (Black & Karoly 2016; Karoly et al. 2016).
Given the growing threat posed by climate change of fires to Tasmanian palaeoendemic species such as A. cupressoides, it is important to understand the effects of fire on the vegetation, and how these effects can be mitigated (Fox-Hughes et al. 2014; Holz et al. 2015; Styger et al. 2018). Despite the importance and known fire sensitivity of A. cupressoides, there have been few detailed studies of the impacts of wildfire, and the influence of fire severity, on populations of the species, or factors influencing the possible recovery of fire-affected populations. The 2016 fires therefore afforded an important opportunity to examine in detail the short-term effects on A. cupressoides populations. The persistence for over 100 years of fire-killed stands of A. cupressoides also presented the opportunity to understand the longer-term demographic effects of fires across the range of the species. Adding to concerns about loss of mature A. cupressoides trees, a marked lack of regeneration has been reported for A. cupressoides stands in many open montane communities on Tasmania's Central Plateau (Cullen & Kirkpatrick 1988; Holz et al. 2015). This recruitment bottleneck has been variously attributed to the legacy of summer domestic stock grazing, which was phased out in the 1970s and 1980s, and browsing by feral rabbits and native marsupials (Cullen & Kirkpatrick 1988; Holz et al. 2015). Native marsupial densities, and hence herbivore impacts, are likely to have increased over the last century because of the extinction of the top predator Thylacinus cynocephalus (the Tasmanian marsupial wolf), trapping of possums and macropods has ended (Holz et al. 2015), and populations of the native extant carnivore Sarcophilus harrisii (Tasmanian devil) have recently plummeted due to devil facial tumour disease (Hollings et al. 2015). This raises the question as to whether herbivore pressure is a potential barrier to regeneration of A. cupressoides. We tested this by comparing seedling and sapling densities on islands in freshwater lakes, which have low herbivore pressure, with environmentally similar areas on the surrounding Tasmanian mainland.
We used a multiscale field survey to describe fire and herbivore impacts on the demography of A. cupressoides. Specifically, we:
- Relate stem survival to minimum burnt twig diameter, a measure of fire severity, in three A. cupressoides populations affected by the 2016 wildfires.
- Describe the long-term legacy of wildfire on A. cupressoides populations by sampling stands with and without evidence of fire across much of its current range in Tasmania.
- Quantify the density of regeneration over most of the current range of A. cupressoides in relation to fire and herbivory by contrasting (i) burnt and unburnt areas, and (ii) transects on islands in tarns and lakes and surrounding mainland areas.
We discuss the findings in relation to processes that threaten the survival of A. cupressoides and briefly discuss potential restoration and management strategies.
Materials and methods
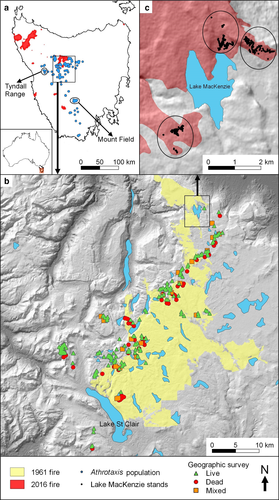
Lake Mackenzie post-fire survey
The 2016 wildfires burnt 13 822 ha at Lake Mackenzie, including about 141 ha of A. cupressoides (Senate 2016; Appendix S1). To quantify the short-term effects of wildfire on A. cupressoides populations, we conducted an intensive survey near Lake Mackenzie in the autumn of 2017, 12–13 months after the wildfire in January 2016. We recorded A. cupressoides tree survival, presence of seedlings and biophysical attributes of the sampled areas, as described below. Three sites near Lake Mackenzie were chosen as representative of the burnt A. cupressoides populations that were accessible on foot (Fig. 1). Two of these populations were on scree slopes, and the third was in a flat, boggy depression surrounded by rocky slopes covered in low Eucalyptus coccifera forest and subalpine shrubs. One site had been burnt by the 1960–1961 fires (Fig. 1). At each site, a systematic sweep of the entire population was conducted, using adjacent 10-m radius circular plots. Plots were centred on the largest A. cupressoides trees within 20 m of the population boundary. Targeting large trees ensured an adequate sample of all size-classes to detect an influence of tree size. Note that we operationally considered a ‘tree’ to be a group of stems with above-ground connections; however, it is likely that nearby ‘trees’ are, in fact, clones (Worth et al. 2016) and are possibly connected below-ground. Subsequent plots were then centred on the next large tree >10 m outside the previous plot boundary. In total, we established 300 plots that were burnt (wholly or partially) by the 2016 fires, including 81 plots that had been burnt by the 1960–1961 fires. We also established 20 plots in a nearby unburnt area, used to compare seedling densities between burnt and unburnt plots.
Although most plots had a radius of 10 m, where tree density was very high, the radius was reduced to capture only 10 trees. There was a maximum of 60 stems (and 57 live stems) within each plot. Individual stems were classified as ‘alive’ (green foliage present), ‘dead’ (killed by the 2016 fires, as indicated by dead foliage and bark present) or ‘previously dead’ (no foliage or bark). Diameter at breast height (DBH) and presence or absence of a fire scar on each stem were recorded.
A 2-m radius circular subplot around the base of each tree, divided into four quadrants, was used to measure ground cover (sphagnum, soil, rock, burnt and unburnt soil, and water), percentage shrub cover and presence or absence of A. cupressoides ‘seedlings’. Our term ‘seedlings’ included both true, sexually produced seedlings and clonal root suckers, because it was impractical to differentiate between seedling types without damaging the plants. As a proxy for fire severity, minimum twig diameter was also estimated for each quarter, from the average diameter of the five smallest twigs on burnt shrubs (measured with calipers). This measure is based on the assumption that hotter fires consume more twig biomass, and hence, the more severe the fire, the greater mean minimum twig diameter (Pérez & Moreno 1998; Whight and Bradstock 1999). For our tree-scale analyses, we averaged data over the four quadrants. Burnt shrub cover and minimum twig diameter in unburnt quadrants were both recorded as zero.
Geographical survey
To investigate demographic legacy effects of wildfire, in the summer of 2013–2014 (before the 2016 wildfire), we sampled A. cupressoides populations over the core of their geographic range, which is centred on the Central Plateau (Fig. 1). Most A. cupressoides populations are found in remote, rugged areas and not accessible by road. We used the Atlas of Living Australia (http://www.ala.org.au), in conjunction with a GIS, to select areas where A. cupressoides populations could be efficiently sampled on foot. We strategically located wilderness base camps in positions from which we could safely access multiple sampling sites within a few hours’ walk. Walking routes were planned to a target lake or other feature associated with A. cupressoides, and if A. cupressoides stands were encountered on the way, or on walks between base camps, these were also sampled. As far as possible, we placed transects to capture the full range of landscape settings in which A. cupressoides occurs. Islands on lakes were especially targeted, because herbivory is considered to constrain A. cupressoides regeneration (Cullen & Kirkpatrick 1988), and we hypothesised that islands could provide some protection. Islands with A. cupressoides stands were accessed using light weight inflatable kayaks.
On the eastern side of the Central Plateau, there was widespread, deliberate use of fire between 1820s and the early 1960s to promote native pasture for livestock grazing. It is known that major fires occurred in the region in the 1890s, 1930s and the summer of 1960–1961, but there were few fires after 1970 (Johnson & Marsden-Smedley 2002). However, high-resolution spatial mapping of fires is available only for recent decades, so it was not possible to obtain long-term fire histories for individual study sites. We therefore used field assessments of dead stems with charring or live trees with fire scars to infer whether the sampled A. cupressoides populations had been exposed to fire since the late 1800s. This is possible because the wood is very durable, and dead stems persist in the landscape for 50–100 years or more (Holz et al. 2015).
We recorded the following attributes of the area within a 20 m radius of the middle of each of the 256 targeted stands: spatial coordinates (using a GPS), aspect and slope, topographic position (valley, flat, upper slope or ridge), insularity (whether or not on an island), sphagnum and rock cover, drainage (‘boggy’ if there was standing water, sphagnum or wet mud present, otherwise ‘dry’) and evidence of fire (charring or fire scars on trees). We refer to stands where evidence of fire was recorded as present or absent as ‘burnt’ and ‘unburnt’, respectively. Evidence of fire was not noted for our first 45 transects; these transects were excluded from our analyses, except for the insularity study, where fire history was inferred from stand structures to boost replication (see below). Vegetation can be influenced by both slope and aspect, so for each transect we calculated the Northness index of French et al. (2016), which was calculated as sin (°slope)*cos (°aspect). The index therefore potentially ranges from −1 (perpendicular, south facing) to +1 (perpendicular, north facing).
At each targeted stand, we laid out one 10 × 6 m transect and measured the diameter at breast height (1.3 m; DBH) of all the A. cupressoides stems within it. Stems were classified as ‘mature’ (>4 cm DBH) or ‘sapling’ (≤4 cm DBH and >30 cm tall), and as alive or dead. Mature Eucalyptus stems (>4 cm DBH), both live and dead, were also counted. Five circular plots, each with an area of 2 m2 (0.8 m radius), were placed along the midline of each transect, with centres 0, 2.5, 5, 7.5 and 10 m from the start. Within each plot, we counted A. cupressoides ‘seedlings’ (defined as ≤30 cm tall), scats of wallabies, wombats, possums, rabbits (scats of other species were not observed), and measured grass cover. We visually estimated the percentage volume occupied by shrubs (other than A. cupressoides juveniles) in the 3 m above each 2-m2 circular plot (i.e. a 6 m3 cylinder), according to Holz et al. (2015).
Insularity
To compare island versus ‘mainland’ transects, we used the subset of data collected in the geographical survey from areas with islands containing stands of A. cupressoides (namely the Walls of Jerusalem, southern Walls of Jerusalem and Travellers Range areas). Transects outside these areas were not included. In total, there were 36 transects on islands and 86 on nearby mainland areas. Evidence of fire had not been searched for on 29 of these transects, which were therefore not used in the main geographical analysis, but were used in the insularity analysis to boost replication (this study originally focussed on herbivory). The fire history of these 29 transects was therefore inferred from the percentage of A. cupressoides basal area that was alive, based on data from transects where evidence of fire was searched for (Fig. 2f shows there was little overlap between transects with and without evidence of fire). Transects with values above 80% were considered unburnt (29 transects, which included 21 transects with 100% unburnt), and those below 75% were considered burnt (11 transects, including ten with no live stems). We considered only two transects indeterminate, so these were not used in our analyses: one had an intermediate per cent live basal area (77%), and the other had no mature A. cupressoides stems, live or dead (but 43 live saplings).
Statistical analyses
Our statistical approach was to use generalised linear models (glm) and model selection based on AICc, a robust second-order form of Akaike's information criterion that balances model fit and simplicity (Burnham & Anderson 2002). Complete subset regression was used for variable selection (Elliott et al. 2013), where we constructed candidate sets of generalised linear models containing all possible additive combinations of explanatory variables and calculated Akaike weights (wi). The importance value of each variable was evaluated by calculating w+, the sum of wi for all models in which that variable occurs. We considered that w+ values >0.73 indicated support for an effect of a variable (Murphy et al. 2010). Linear glm was used for the continuous response variables, binomial glm for presence/absence and survival data, and zero-inflated Poisson glm (with a regressor for the zero component, if this improved model fit) for the count data. The R statistical software was used for all analyses, with the ‘lm’ and ‘glm’ functions of the base package used for linear and binomial glm, and the ‘chart. Correlation’ function of the package Performance Analytics (Peterson & Carl 2014) used for correlations, the ‘zeroinfl’ function of the package pscl (Jackman 2015) for zero-inflated Poisson glm and the ‘glmer’ function of the package lme4 (Bates et al. 2015) for mixed effects glm.
Lake Mackenzie post-fire survey
Athrotaxis cupressoides stem survival in burnt transects was first investigated in relation to fire severity, tree size and presence of fire scars. Medium and large trees are generally more fire resistant than small trees (e.g. Williams et al. 1999; Prior et al. 2009), and we considered it possible that the presence of fire scars, with loss of protective bark, could make trees more vulnerable to subsequent fire. The first analysis used complete subset regression, with minimum burnt twig diameter (a proxy for fire severity; Pérez & Moreno 1998) and DBH as explanatory variables, and stem status (alive or dead) as the response variable in binomial glm. Inspection of the data indicated a quadratic response was likely for DBH, so AIC was used to test whether adding the term ‘DBH2’ improved the model. The term ‘Fire scar’ was then added to test whether this further improved the model. Finally, we tested whether there were residual effects of ‘Site’ that were not already captured by the terms in our best model, by adding the term ‘Site’ to it.
We also analysed the environmental correlates of both fire severity and stem survival, using three environmental variables as explanatory variables, based on a priori knowledge of fire effects and preliminary data exploration: rock cover (rock is not flammable, so rocky areas potentially provide micro-refugia); burnt shrub cover (shrubs are a fuel source, and could increase local fire intensity and severity); and unburnt soil cover. Complete subset regression was used to calculate w+ for each term in both the fire severity and stem survival analyses. For the survival analysis, we then used AIC to test whether adding the terms ‘DBH’, ‘DBH2’ and ‘(rock cover)2 improved the models, as inspection of the data indicated a quadratic response was likely for DBH and rock cover. Finally, we tested whether adding the terms ‘Fire scar’ and ‘Site’ would further improve the model.
Seedling presence or absence was compared around trees in burnt vs. unburnt plots at Lake Mackenzie using binomial mixed effects models, with fire as an explanatory variable and plot as a random effect. We then tested the following as potential explanatory variables of seedling presence around trees in burnt plots, using complete subset regression: cover of burnt shrubs, cover of unburnt soil, burnt twig diameter and effective diameter of the tree (calculated from the sum of the basal area of stems joined above-ground).
Geographic survey
In the geographically extensive survey of stands, we tested for differences between biophysical and vegetation variables, with fire status (evidence of fire present or absent) as a categorical explanatory variable. We tested whether burnt and unburnt transects differed using glm and comparing the AIC weight of the model containing the term ‘fire’ with that of the intercept-only (null) model. Variables and model type are specified in Table 1. We then tested whether live A. cupressoides stand structures in burnt and unburnt transects conformed to a negative exponential distribution, which suggests reasonably constant rates of recruitment, growth and survival (Prior et al. 2011). We binned stems into 10-cm DBH classes and fitted a Poisson glm of log(count of stems) versus mid-point of the DBH class. We considered stands conformed to this distribution if the slope of the relationship was negative, and the Poisson glm with DBH was superior (AICc at least 2 lower) to the intercept-only model (Prior et al. 2011).
| Units | Unburnt | Burnt | w+ | Model type | |
|---|---|---|---|---|---|
| Biophysical attributes | |||||
| Altitude | m | 1166 | 1183 | 0.43 | Linear |
| Slope | ° | 6.58 | 6.63 | 0.26 | Linear |
| Northness | sin(°slope)*cos(°aspect) | −0.042 | −0.042 | 0.26 | Linear |
| Distance to lake | m | 190 | 299 | 1.00 | Linear (with log-transform) |
| Direction to lake | ° | 98 | 112 | 0.76 | Linear (with cosine-transform) |
| Rock cover | % | 13.4 | 19.8 | 0.73 | Linear |
| Sphagnum cover | % | 6.6 | 12.3 | 0.78 | Linear |
| Grass cover | % | 5.2 | 11.8 | 0.99 | Linear |
| Shrub volume | % | 5.5 | 5.7 | 0.26 | Linear |
| Wallaby scats | per 10 m2 | 22 | 57 | 1.00 | ZIP, regressor for zeroes |
| Wombat scats | per 10 m2 | 0.85 | 1.14 | 1.00 | ZIP, regressor for zeroes |
| Possum scats | per 10 m2 | 2.7 | 6.5 | 1.00 | ZIP, regressor for zeroes |
| A. cupressoides | |||||
| Mature stems, live | per 60 m2 | 12.8 | 5.1 | 1.00 | ZIP, regressor for zeroes |
| % zeroes | 4 | 55 | |||
| Mature stems, dead | per 60 m2 | 1.90 | 10.1 | 1.00 | ZIP, regressor for zeroes |
| Basal area contributed by live stems | % | 90 | 31 | 1.00 | Linear |
| Saplings, live | per 60 m2 | 8.3 | 1.5 | 1.00 | ZIP, regressor for zeroes |
| % zeroes | 38 | 79 | |||
| Seedlings, live | per 10 m2 | 15.0 | 6.25 | 1.00 | ZIP, regressor for zeroes |
| % zeroes | 70 | 82 | |||
| Eucalyptus species | |||||
| Mature stems, live | per 60 m2 | 0.33 | 0.54 | 0.69 | ZIP, regressor for zeroes |
| Mature stems, dead | per 60 m2 | 0.10 | 0.25 | 0.48 | ZIP, regressor for zeroes |
| Basal area contributed by live stems | % | 81 | 71 | 0.32 | Linear |
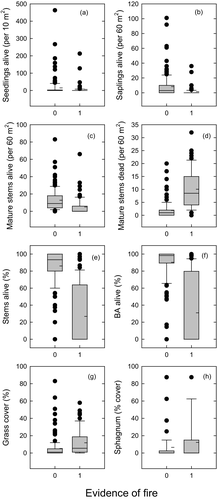
- Distribution of data for A. cupressoides is shown as boxplots in Fig. 2. Only plots with recorded fire status are included here. w+ indicates the statistical support for a difference between unburnt and unburnt plots, with values >0.73 considered to show support, and shown in bold (Murphy et al. 2010). Much of the count data was zero-inflated, so zero-inflated Poisson (ZIP) models were fitted, with a regressor for zeroes. ‘Mature’ stems were defined as >4 cm diameter at breast height. ‘Saplings’ were defined as >30 cm tall and ≤4 cm diameter. ‘Seedlings’ were defined as <30 cm tall.
To identify the environmental correlates of A. cupressoides survival in burnt transects, we tested which of the explanatory variables altitude, distance to lake, Northness, rock cover, drainage and topography were supported as predictors of survival, using complete subset regression. Transect level analysis was most appropriate for this analysis because environmental variables were assessed at a transect level.
Inspection of the raw data showed little relationship of sapling or seedling counts with any physical variable we measured, or with scat counts, either in burnt or unburnt transects (Appendix S2), so no further analysis was conducted on these correlates of regeneration. Similarly, there was no significant correlation of juvenile density with sphagnum, grass or shrub cover, and these were not tested further. Hypothesising that regeneration would depend on the number of live mature A. cupressoides stems present, we tested whether sapling and seedling counts were correlated with the number of live mature A. cupressoides stems present, using zero-inflated Poisson glm with a regressor for the zero. Analyses were done separately for burnt and unburnt transects.
To investigate effects of insularity, we first compared presence/absence of fire on islands and the mainland using binomial glm, with insularity as an explanatory variable. There were only two burnt transects on islands, so we could compare vegetation between islands and mainland only in unburnt areas. Using unburnt transects only, with insularity as a categorical explanatory variable, we tested for differences in physical and vegetation variables (as response variables) using glm. These response variables and model types are listed in Table 2.
| Response variable | Units | Island | Mainland | w+ | Model type |
|---|---|---|---|---|---|
| Number of observations | 36 | 85 | |||
| Altitude | m | 1144 | 1210 | 1.00 | Linear |
| Northness | Index | 0.001 | −0.060 | 0.94 | Linear |
| Distance to lake | m | 6.3 | 73 | 1.00 | Linear (with log-transform) |
| Rock cover | % | 9.4 | 15.2 | 0.53 | Linear |
| Sphagnum cover | % | 1.0 | 8.6 | 0.90 | Linear |
| Grass cover | % | 1.6 | 4.9 | 0.68 | Linear |
| Shrub volume | % | 9.8 | 4.2 | 1.00 | Linear |
| Live mature A. cupressoides stems | per 60 m2 | 12.6 | 14.1 | 0.31 | Negative binomial |
| % zeroes | 0 | 3.5 | |||
| Dead mature A. cupressoides stems | per 60 m2 | 2.1 | 2.2 | 0.26 | ZIP |
| Live A. cupressoides saplings | per 60 m2 | 7.1 | 11.7 | 1.00 | ZIP |
| % zeroes | 28 | 36 | |||
| Live A. cupressoides seedlings | per 10 m2 | 0.39 | 14.64 | 1.00 | ZIP, regressor for zeroes |
| % zeroes | 94 | 73 | |||
| Wallaby scats | per 10 m2 | 0.2 | 34.0 | 1.00 | ZIP, regressor for zeroes |
| Wombat scats | per 10 m2 | 0 | 1.5 | 0.99 | ZIP |
| Possum scats | per 10 m2 | 0 | 4.2 | 1.00 | ZIP |
- Unburnt transects were identified using ‘inferred fire’, which also includes transects where evidence of fire was not searched for, and fire was inferred from stand structures. The explanatory variable in these analyses was insularity (island or mainland). Average values and the statistical support (importance values, w+, based on Akaike weights) for an effect of insularity are shown. Much of the count data was zero-inflated, as shown below, so zero-inflated Poisson (ZIP) models were fitted, with a regressor for zeroes where this improved fit. Values of w+ ≥ 0.73, indicative of statistical support, are shown in bold.
Results
Lake Mackenzie post-fire survey
We measured 3816 stems in total in the burnt and unburnt plots, of which 544 (14%) were ‘previously dead’, that is already dead at the time of the 2016 wildfire. Of the 3118 stems that were alive in the burnt plots at the time of this fire, 2149 (68%) were killed by it; values for the three surveyed populations individually were 59%, 69% and 73%.
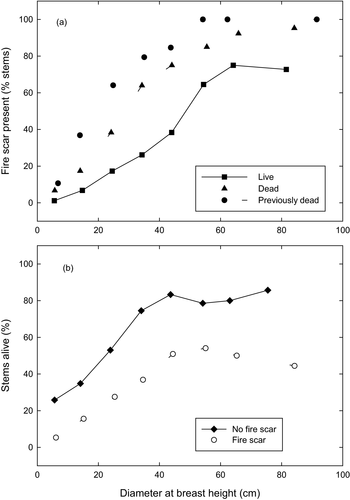
There was greater survival of A. cupressoides stems associated with lower minimum twig diameter (which alone explained 22% of the deviance in the data) and larger DBH (Fig. 3). Together, these two factors explained 32% of the deviance in the data. The relationship with DBH was quadratic, with highest survival for stems around 45 cm DBH (Fig. 3b; Appendix S3). Pre-existing fire scars had a strong, negative effect on stem survival after the 2016 fires; adding the term ‘Fire scar’ lowered the AIC of the best environmental model by 21.6 (Fig. 4b; Appendix S3a). Of all stems alive at the time of the fire, 17% had fire scars, but for stems larger than 50 cm DBH this value was 79% (Fig. 4a). The prevalence of fire scars in large trees appeared to contribute to the overall decrease in survival as stems grew large. Overall survival of stems 20–30 cm DBH was 53% for stems without fire scars, compared with 28% for stems with fire scars. Likewise, dead stems, especially those already dead in 2016, were more likely to have fire scars than live stems of the same size (Fig. 4a). Adding site to this model led to only a marginal improvement (delta AIC = 2.0), suggesting there was little residual effect of site that was not already explained by burnt minimum twig diameter, A. cupressoides stem diameter and fire scars.
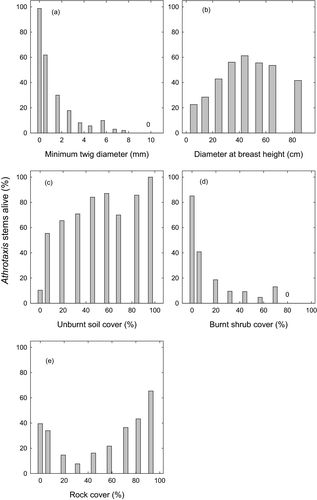
Burnt minimum twig diameter was itself weakly associated with the environmental factors unburnt soil cover (negative relationship, 4.0% deviance explained), rock cover (negative relationship, 0.2% deviance explained) and burnt shrub cover (positive relationship, 1.1% deviance explained), with the model containing these terms receiving all the support in the complete subset regression and explaining 5.2% of the deviance in burnt twig diameter (model not presented). These influences on fire severity translated to stem survival being positively correlated with unburnt soil cover and rock cover, and negatively correlated with burnt shrub cover (Fig. 3). For example, where there was no unburnt soil, survival was only 10% (Fig. 3c), and conversely, where there were no burnt shrubs, survival was 85%, demonstrating the importance of fire conditions in the immediate environment of each stem in these burnt plots. The model containing the environmental variables burnt shrub cover, rock cover and unburnt soil cover received all the support in the complete subset regression and explained 13.3% of the deviance in the survival data. Adding the terms DBH, DBH2, (rock cover)2 and Fire scars improved the model, which explained in total 26.1% of the deviance in stem survival (Appendix S3c,d).
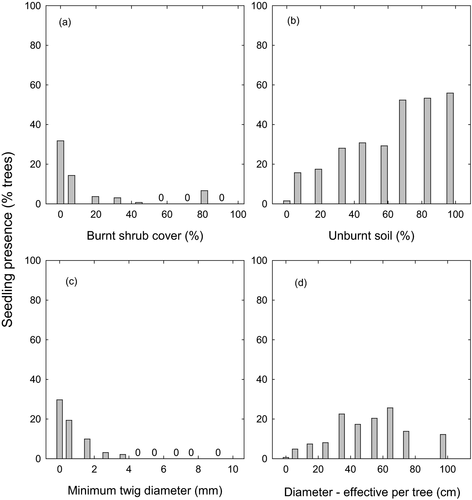
There were no differences in A. cupressoides seedling presence between burnt and unburnt plots in the Lake Mackenzie survey (w+ = 0.27). Athrotaxis cupressoides seedlings were present in 14% of all burnt plots, and 9% of all trees within burnt plots had seedlings within 2 m of their base. Within burnt plots, there were negative relationships between seedling presence and both minimum twig diameter (27% deviance explained) and burnt shrub cover (6% deviance explained), and positive relationships with both cover of unburnt soil (9% deviance explained) and stem diameter (8% deviance explained; Fig. 5; Appendix S4).
Geographic survey
Comparison of burnt versus unburnt transects
The stand structures of A. cupressoides populations, aggregated for all unburnt transects and all burnt transects, both approximately conformed to a negative exponential distribution, as shown by the log-linear, negative relationship between stem counts and diameter class for both burnt and unburnt transects (Appendix S5). This suggests that at a regional scale, frequent recruitment has occurred, and that fire causes attrition from burning across all size-classes. The effect of past fire on demographics is apparent when comparing transects with and without evidence of past fires: relative to unburnt transects, burnt transects had fewer live mature A. cupressoides stems and more dead ones, and therefore a lower proportion of A. cupressoides stems and basal area alive (Table 1; Fig. 2c–f). There was an average of 0.21 live trees m−2 in the unburnt transects, compared with 0.08 live stems m−2 in the burnt transects. Live seedling and sapling densities were higher in unburnt than burnt transects (Table 1; Fig. 2a,b). Average seedling density in unburnt transects was 1.50 m−2 compared with 0.62 m−2 in burnt transects, with corresponding values for saplings of 0.138 and 0.024 m−2. Burnt transects were also more likely to have no live mature stems, no live saplings or no live seedlings (Table 1; Fig. 2a–c). Juveniles were absent in 28% of unburnt transects and 47% of burnt ones (Table 3). Presence and counts of juveniles were correlated with counts of mature stems, although this was not statistically important for seedlings in unburnt transects: there were no juveniles (either seedlings or saplings) recorded in 86% of burnt transects with no live mature stems, compared with 50% of transects with live mature stems (Table 3; Appendix S6).
| Live mature stems | Present | Absent | Total |
|---|---|---|---|
| Unburnt transects | |||
| Number of transects with no juveniles | 36 | 2 | 38 |
| Total number of transects | 129 | 6 | 135 |
| % Transects with no juveniles | 27.9 | 33.3 | 28.1 |
| Burnt transects | |||
| Number of transects with no juveniles | 17 | 36 | 53 |
| Total number of transects | 34 | 42 | 112 |
| % Transects with no juveniles | 50.0 | 85.7 | 47.3 |
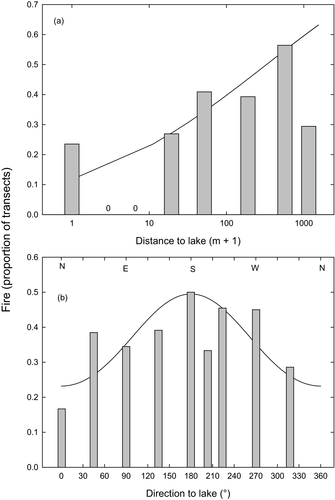
Comparisons of unburnt and burnt transects showed that fires were more likely further from lakes and when the nearest lake was to the south (Fig. 6), and that had a higher cover of grass and sphagnum (Table 1). There were more marsupial herbivore scats in the burnt plots, and these were strongly associated with increased grass cover (r = 0.65 for wallaby scats vs. grass cover; Appendix S2b). Shrubs were present in 98% of the circular plots, and shrub volume was similar in both burnt and unburnt transects. Burnt transects were slightly rockier than unburnt ones (Table 1), although the proportion of living A. cupressoides trees in burnt transects showed a weak, positive relationship with high levels (~80%) of rockiness (w+ = 0.74) (Appendix S2b). The other variables that discriminated burnt and unburnt transects (Northness, topography and distance to lake) were not important predictors of live A. cupressoides trees on burnt transects. Athrotaxis cupressoides regeneration in burnt transects was not correlated with any of our physical variables (Appendix S2b), but was positively related to the density of live, mature A. cupressoides stems.
Juvenile density in relation to herbivory
Wallabies appeared to be the most abundant herbivore in our transects; there were on average 31 ± 3 (SE) wallaby scats per transect, compared with 0.9 ± 0.3 wombat scats and 3.9 ± 0.7 possum scats. However, there was no significant relationship between wallaby scat counts and counts of seedlings or saplings in either burnt or unburnt transects, with the largest correlation coefficient being 0.078 (Appendix S2). Correlation coefficients for wombat and possum scats were also low (Appendix S2).
Insularity
Islands experienced far less fire than mainland areas: only two of 38 island transects appeared to have been burnt, compared with 73 of 158 mainland ones (Table 4). Because there were so few burnt transects on islands, it is not possible to test for interactions between fire and herbivory, and our analysis of insularity is based on unburnt transects only. Islands appeared to have far fewer herbivores than comparable mainland areas, as shown by the much smaller number of scats detected (Table 2). On average, we found only 0.2 scats in each island transect, compared with 34 in mainland transects. There was evidence of only a small number of wallabies on islands, whereas mainland transects also had wombats and possums present. There were also physical and vegetation differences between the islands and mainland (Table 2). Islands transects were on average much closer to lakes, 66 m lower in altitude, less likely to be south-facing and had less sphagnum cover. Densities of live and dead mature A. cupressoides stems were similar for both island and mainland transects. However, compared with the mainland, islands had 39% fewer A. cupressoides saplings, and 97% fewer A. cupressoides seedlings, and more transects with zero counts of saplings or seedlings (Table 2).
| Island | Mainland | w+ | |
|---|---|---|---|
| Evidence of fire | |||
| Number of transects | 31 | 125 | |
| Number burnt | 2 | 62 | |
| % Burnt | 6.5 | 49.6 | 1.00 |
| Inferred fire | |||
| Number of transects | 38 | 158 | |
| Number burnt | 2 | 73 | |
| % Burnt | 5.3 | 46.2 | 1.00 |
- Binomial glm, with fire as the response variable, was used to test the statistical support for an effect of insularity (importance values, w+, based on Akaike weights). Results are presented for both evidence of fire and ‘inferred fire’ (which also includes transects where evidence of fire was not searched for, and fire was inferred from stand structures). Values of w+ ≥ 0.73, indicative of statistical support, are shown in bold.
Discussion
Landscape fire has a pronounced and lasting demographic impact on the palaeoendemic conifer A. cupressoides. However, these deleterious effects are influenced by environmental settings, past fires, herbivores and recruitment barriers. The predominance of fire in shaping the population viability of the species presents substantial challenges in both managing the increasing frequency of lightning-ignited fires and predicting the future range of the species in response to climate change. Below, we discuss these issues by reference to our findings, previous studies and currently evolving management responses.
Our study highlights the vulnerability of A. cupressoides to landscape fire. The 2016 fires killed most mature A. cupressoides trees, with only 22–41% of mature trees surviving in the three populations we surveyed at Lake Mackenzie. We assessed survival 1 year after the fire, and it is very likely that some severely damaged trees have subsequently died; this warrants a resurvey. The fire intensity that is lethal for A. cupressoides trees is unknown but likely to be at the lower end of the spectrum of vegetation sensitivity, given the contraction of its populations following earlier fires (Holz et al. 2015). We found a steep decline in stem survival as fire severity (measured by burnt twig diameter) increased, indicating the species is vulnerable to high severity fires. The burnt twig diameter at Lake Mackenzie was similar (2.9 mm) to that in Myrtaceaeous-dominated shrub vegetation in south-western Tasmania (2.1–3.3 mm) following a wildfire with an average fire radiative power of 360 MW, according to MODIS hotspot data (French et al. 2016). This is more intense than 90% of fires detected by MODIS, but well below maximum values of more than 1500 MW (Ichoku et al. 2008). Athrotaxis cupressoides seedlings are known to be very sensitive to fire: in an experiment using a gas burner, 95% of A. cupressoides seedlings were killed by brief exposure (<60 s) to simulated, very low fireline intensities of 33 kW m−1 and none resprouted (Prior et al. 2018).
Stem size strongly influenced survivorship, with medium-sized trees the least vulnerable. This pattern is explicable because smaller stems have thinner bark and foliage closer to the ground and are thus more affected by surface fires, whereas larger stems are more likely to have pre-existing fire scars, which increases their vulnerability to fire. Overall, 17% of stems near Lake Mackenzie had fire scars, a similar value to the 20% reported by Holz et al. (2015), but the frequency of fire scars increased with stem size, with most stems >40 cm DBH displaying scars. Stems of 40 cm DBH are probably about 285 years old, given average diameter growth rates of 1.4 mm year−1 (Cullen & Kirkpatrick 1988). Only live stems form fire scars, so scarred trees killed by the 1960–1961 fire had been exposed to and survived fires before that fire event, a conclusion also reached by Holz et al. (2015). We are uncertain why fire-scarred trees are more vulnerable to fire, but the loss of bark over the scar exposes the dead wood to flames; the wood is dry and the stem could therefore be more likely to burn than stems protected by living bark. We note that in Australian savannas, trees hollowed by termites are more likely to be killed by fire because the dry centre of the trees readily burns (Werner & Prior 2007). Our finding of elevated risk of stem mortality associated with fire scars suggests any increases in fire frequency could have a potent effect on A. cupressoides populations, because survivors of one fire will be more vulnerable to subsequent fires. Importantly, given the long lifespan of this species, with individual stems 1000 years old (Ogden 1978) arising from clones that potentially established soon after deglaciation, a very low fire frequency could be sufficient to drive population collapse. For instance, sediment cores showed four spikes in microscopic charcoal between 6000 and 2500 years ago were each associated with a sharp decline of Cupressaceae pollen, considered a proxy for Athrotaxis (Fletcher et al. 2014).
Fire severity and patchiness are known to be affected by fire weather, landscape setting and microsite characteristics, including fuel load and continuity (Bradstock et al. 2010; Wood et al. 2011; Leonard et al. 2014). Accordingly, stems that survived the 2016 fire at Lake Mackenzie were typically associated with patches of unburnt soil, no burnt shrubs and high rock cover (Fig. 3). Across this survey area, landscape setting was shown to be important: compared with unburnt stands, burnt stands were more distant from lake edges, more likely to be to the north of the nearest lake and thus exposed to northerly fire bearing winds, and seldom found on islands. Both burnt and unburnt stands had diverse understoreys (shrub, grass, sphagnum and rock), but burnt stands had more grass cover, and thus higher herbivore activity. We found no difference in shrub volume between burnt and unburnt stands, possibly because shrubs were ubiquitous in our geographic survey plots. However, Holz et al. (2015) showed that within a localised area, stands of A. cupressoides burnt in the 1961 fire had more understorey shrubs, with a greater proportion of resprouters, compared with stands unburnt by the 1961 fire. They postulated a feedback, whereby fire leads to more shrub cover, which leads to more severe fire. We were unable to test whether this feedback occurred at Lake Mackenzie as there were insufficient transects affected by the 1961 fire. While our broad-scale geographical survey showed general patterns of fire occurrence within the subalpine range of A. cupressoides, numerous interacting influences make it difficult to reliably identify fire-protected microsites on the open undulating terrain where it often occurs. A particularly important confounder of the geographical survey is the unknown variability in fire weather conditions and associated fire behaviour. It is possible that under mild fire weather, intact stands are fire resistant, but under severe conditions, this resistance is overwhelmed, as has been shown for the north Australian conifer Callitris intratropica (Trauernicht et al. 2012).
Unlike in the fire-adapted vegetation which now dominates most Tasmanian landscapes, such as eucalypt forest, buttongrass sedgeland and coastal heath (French et al. 2016; Prior et al. 2016; Nicholson et al. 2017), there was very limited post-fire regeneration of A. cupressoides within a year of the Lake Mackenzie fire. Our results suggest that A. cupressoides regeneration is not fire-stimulated and that post-fire regeneration can, at best, just offset fire-induced seedling mortality. There was some seedling establishment or suckering evident in some burnt populations, but densities were similar to, rather than higher than, unburnt plots. Furthermore, the geographic survey found that stands burnt about 50 years ago contain fewer seedlings and saplings than do long unburnt stands. This is consistent with previous studies, which have identified a post-fire recruitment bottleneck (Kirkpatrick & Dickinson 1984; Cullen & Kirkpatrick 1988). For example, Holz et al. (2015) found no seedlings or saplings in A. cupressoides stands killed during the 1960–1961 fire and also sampled 50 years later. Regeneration failure has been attributed to the lack of a long-lived seed bank, episodic seed supplies dues to masting, susceptibility of seeds to fire, poor seed dispersal, inability to recover vegetatively after intense fire and intense post-fire herbivory (Kirkpatrick & Dickinson 1984; Cullen & Kirkpatrick 1988; Holz et al. 2015). Fire-induced mortality of adults also contributes to the recruitment bottleneck because live, mature trees are needed as a source of seeds or suckers. Indeed, the geographic survey showed a strong relationship exists between live mature trees and seedling and sapling density.
Holz et al. (2015) suggested that fire and herbivory exerted negative, synergistic effects on A. cupressoides regeneration. Browsing pressure is likely to be higher in burnt areas because herbivores are attracted to nutritious resprouting vegetation, and under more open conditions juveniles are easier to find than in unburnt areas. Our geographic survey found there were more marsupial herbivore scats in the burnt plots burnt in the last century, probably reflecting the increased grass cover. A previous study showed grazing exclosures increased survival of A. cupressoides germinants (87% vs. 41% for unprotected germinants) when there were still low numbers of sheep present, as well as rabbits and native marsupials (Cullen & Kirkpatrick 1988). A more recent study, after pastoralism had ceased, found many seedlings following a mast seeding event (an average of 50 000 ha−1 in unburnt plots, compared with 15 000 ha−1 in unburnt plots in our geographical study), but that very few saplings had been recruited during the last century, suggesting large-scale attrition from grazing animals during that time (Holz et al. 2015). Yet, factors other than fire and herbivory also appear to contribute to the A. cupressoides recruitment bottleneck: we found no relationship between scat counts and densities of A. cupressoides juveniles. In addition, the small islands surveyed showed almost no evidence of herbivores, yet there were 97% fewer seedlings and 39% fewer saplings, in unburnt stands on islands than in similar nearby unburnt mainland stands. It is possible that competition from greater shrub cover inhibits regeneration on islands, or that low severity disturbance from animal digging or consumption or trampling of vegetation could stimulate regeneration on the mainland. Alternatively, island stands could have had subtle differences in microhabitat not captured by our measurements. More research is required to understand the factors affecting the establishment of A. cupressoides germinants on burnt and unburnt sites, including the effects of herbivory.
Collectively, our demographic surveys combined with previous studies suggest a series of factors that interact to drive the attrition and eventual collapse of A. cupressoides populations. Single fires kill and damage stems, and surviving damaged stems are more likely to be killed in subsequent fires. Loss of mature individuals reduces the reproductive output of burnt populations, given the relationship between regeneration and adult trees. Burnt stands may have more combustible understoreys of grass (this study) or flammable shrubs (Holz et al. 2015), and be more likely to burn again, killing any regeneration. These interactions are poorly captured in species distribution models, which have highlighted the vulnerability of A. cupressoides and other palaeoendemics to anthropogenic climate change (Porfirio et al. 2014; Keppel et al. 2015; Mariani et al. 2019). The palaeoendemic refugia are contracting because of warmer and drier climates. Ogden (1978) observed that oldest, largest A. cupressoides trees were all found towards the upper limit of the species distribution, suggesting these are the most secure parts of its range. However, there is little capacity for the species to migrate upslope, because there are only very small areas higher than its existing range on Tasmania's Central Plateau. Additionally, the trend of increased lightning ignitions across western Tasmania (Styger et al. 2018) combined with predicted hotter, drier weather and worsening fire danger (Fox-Hughes et al. 2014) means previous fire-free or rarely burnt refugia are being penetrated by lightning fires, as was the case in 2016 (Appendix S1). It is concerning that a trade-off could exist between topographic fire protection and moisture availability for drought-sensitive species, because the rocky areas that provide the best fire protection are also the driest (Landesmann et al. 2015). Other southern hemisphere relictual conifers are being threatened by the combined ‘press’ of climate change and the ‘pulse’ of landscape fires (Harris et al. 2018), such as the critically endangered conifer Widdringtonia cedarbergensis in South Africa (White et al. 2016), Callitris sulcata in New Caledonia (Haverkamp et al. 2015) and Austrocedrus chilensis in Patagonia (Landesmann et al. 2015).
The increasing threat to Tasmanian palaeoendemics from the interactive, threatening processes of climate change and fire raises philosophical and practical questions about the conservation and management approaches in a ‘wilderness area’ (Gill 2016; Rickards 2016). Increased surveillance and rapid attack of uncontrolled fires, combined with carefully targeted, planned burning to reduce fuel loads in open sedgeland vegetation, are widely acknowledged as being essential management responses to conserve palaeoendemics (Senate 2016; Kirkpatrick et al. 2018). Sedgelands are able to vegetatively recover from fire, and planned burning can reduce fire intensity and severity, thereby increasing the capacity for managers to control fires (French et al. 2016) and provide fire breaks to protect palaeoendemic refugia (Senate 2016; Kirkpatrick et al. 2018). Management agencies have established seed banks and are trialling planting seedlings on fire-affected areas at Lake Mackenzie, and in 2019, spray irrigators were used near a wilderness lake to protect remnant A. cupressoides stands from a lightning fire. Fire retardants and suppressants are now being used to contain uncontrolled fires that threaten the values of the Tasmanian Wilderness World Heritage Area, as proposed by Styger (2018).
In conclusion, increased fire activity associated with climate change is likely to cause rapid contraction of the palaeoendemic conifer A. cupressoides. The increased fire frequencies driven by climate change and associated increased lightning ignitions are likely to trigger a series of feedbacks that exceed the species’ capacity to persist in formerly fire-protected refugia. The demographic attrition of A. cupressoides is thus an extreme example of the demographic fire-climate ‘interval squeeze’ model proposed by Enright et al. (2015), in which fire intervals are shortening, while fecundity and growth rates are declining, as a result of climate change. There is increasing acceptance that the survival of slow-growing, fire-sensitive palaeoendemics such as A. cupressoides requires intensive fire protection, and targeted restoration interventions if they are burnt.
Acknowledgements
This research was supported by the Australian Research Council (DP110101950) and the Bushfires and Natural Hazards Cooperative Research Centre. We thank Sam Wood and Andréas Holz for initial discussions about the geographical survey, Karl Rann for field assistance and Dario Rodriguez-Cubillo for preparing the map.
Open Research
Data accessibility
The data used in this study are available at figshare (https://doi.org/10.6084/m9.figshare.7622081).




