Characteristics of hollows and hollow-bearing trees in semi-arid river red gum woodland and potential limitations for hollow-dependent wildlife
Abstract
Up to 37 species of the birds and microbats inhabiting inland Australia are dependent on tree cavities for breeding or roosting. The river red gum (Eucalyptus camaldulensis), a well-known hollow-bearing tree species, occurs in linear semi-arid woodland along thousands of kilometres of ephemeral river channels and is the only tree species that provides widespread, aggregated hollow resources across a landscape otherwise dominated by shrublands. Here we assess the type and quantity of hollows available along ephemeral rivers of the MacDonnell Ranges bioregion in central Australia and determine which characteristics of river red gums best predict the abundance and characteristics of different tree hollows, as first steps towards assessing the current availability of hollows in the region. Approximately a third of all river red gums sampled were hollow-bearing, but individual trees with abundant hollows were rare. Further, 36% of hollows had an entrance ≤ 5 cm, and 37% had entrances which were 6–10 cm in diameter, whereas only 13% of hollows had an entrance diameter > 20 cm suitable for larger hollow-using species. Large and high hollows only occurred on trees that did not display post-disturbance resprouting. Trees with multiple and diverse hollows were rare and tended to be in advanced stages of senescence and had larger stems (82.3 ± 3.33 cm) and were taller (14.4 ± 0.53 m) compared to non-hollow-bearing trees (23.44 ± 1.68 cm, 8.0 ± 0.34 m). Further research is required to establish whether the current abundance of hollows and diversity of hollow types are limiting to cavity-dependent wildlife, and to identify any threats to availability of hollows.
Introduction
Tree hollows are a key structural component for arboreal wildlife in forests and woodlands globally (Goldingay 2009; Seymour & Dean 2010; Voigt et al. 2014). In Australia, it is estimated that 15% of birds, 31% of mammals, 10% of reptiles and 13% of amphibians use tree hollows (Gibbons & Lindenmayer 2002). In recognition of the importance of tree hollows to many Australian animals, research has focussed on quantifying the availability of hollow-bearing trees at different spatial scales (Smith & Lindenmayer 1988; Lindenmayer et al. 2000; Gibbons & Lindenmayer 2002; Taylor & Chisholm 2005; Koch & Baker 2011; Davis et al. 2014; Rayner et al. 2014; Ellis et al. 2015). However, most research has occurred in temperate climates, and knowledge of the characteristics and abundance of hollow-bearing trees in arid and semi-arid Australia (which represents 70% of the continent) remain poor (but see Rayner et al. 2014; Ellis et al. 2015).
In the arid interior of the Australian continent, the majority of hollows suitable for wildlife are confined to Eucalyptus woodlands along river channels and waterways. River red gum (Eucalyptus camaldulensis subsp. arida) woodlands occupy an estimated 7000 km of river channel and floodplain habitat (Geoscience Australia Spatial Data 2016) in central Australia. The river red gum is the only tree in the region that grows in sufficient density, and with a distribution broad enough to provide widespread, predictable, aggregated hollow resources. Other hollow-bearing trees (e.g. ghost gum, Corymbia aparrerinja; coolabahs, Eucalyptus spp.; bloodwoods, Corymbia spp.; and desert oak, Allocasuarina decaisneana) have a scattered distribution. In central Australia, therefore, we propose that river red gum woodlands are critical for animals that use hollows.
Most obligate hollow-users in inland Australia are birds and microbats. Tree hollows are used by as many as 37 bird species (Aumann 2001; Gibbons & Lindenmayer 2002; Doucette et al. 2011; Pavey et al. 2014), including parrots, owls, kingfishers, pardalotes, tree creepers, tree martins and waterfowl. Of the 12 microbat species that occur in central Australia, 10 use tree hollows for roosting and maternal colonies (Milne & Pavey 2011). Of these species, the princess parrot is federally listed as vulnerable; three bat species – the bristle-faced free-tailed bat (Mormopterus eleryi), yellow-bellied sheath-tailed bat (Saccolaimus flaviventris) and inland forest bat (Vespadelus baverstocki) are listed as threatened species in New South Wales primarily due to the loss of hollow-bearing trees in that region. To date, there have been no studies in central Australia which have investigated hollow use by any species, except the princess parrot (Polytelis alexandrae) (Pavey et al. 2014) and nankeen kestrel (Falco cenchroides) (Aumann 2001). Additionally, records of hollow use from other regions in Australia are limited for most of the central Australian hollow-dwelling species.
The availability of suitable hollows often determines whether hollow-dependent species will occupy a site (McElhinny et al. 2006). Many species select for hollows with specific characteristics, such as the height and diameter of entrances (Saunders et al. 1982; Lumsden et al. 2002); whether the wood surrounding the hollow is alive or dead (Saunders et al. 1982); the number of hollows on a single tree (Koch et al. 2008); and location of the hollow within the stem or on branches (Goldingay 2009). Many of these hollow characteristics correlate with the size and form of the tree (Gibbons et al. 2000) and, if these relationships are strong, tree characteristics can potentially be used to estimate the availability of hollows within a stand.
In temperate and tropical Australia, maturity and form of Eucalyptus trees are complementary indicators of the presence and abundance of hollows (Smith & Lindenmayer 1988; Gibbons & Lindenmayer 2002; Rayner et al. 2014). Characteristics such as stem diameter and tree height are established and useful predictors of the presence and abundance of hollows in many Eucalyptus species (Smith & Lindenmayer 1988; Gibbons & Lindenmayer 2002; Rayner et al. 2014; Stojanovic et al. 2014; Ellis et al. 2015). However, the subspecies arida, which occurs across much of inland Australia, does not possess the linear growth and senescence pattern of many other eucalypts and does not fit easily within pre-defined forestry-derived categories of canopy form (Westerhuis 2015). Individuals of this subspecies regularly shed branches, regrow lost stems and resprout vertically from stems or branches felled by winds or floods and hence may possess a single upright stem or several stems (Colloff 2014). The subspecies is also relatively fire-tolerant, resprouting from epicormic buds or lignotubers following burning (Nicolle 2006). Fires may result in either partial or complete destruction of above-ground biomass, such that the form of regrowth often differs substantially from the original canopy (Westerhuis 2015) which can influence the abundance of hollows. Knowledge of relationships between availability of hollows and the morphology of trees could enable efficient monitoring of the hollow resource.
- What are the typical densities of hollow-bearing river red gums in central Australian riparian woodlands?
- What types of hollows are available in river red gum woodlands and do they provide a diverse range of roosting and nesting sites for hollow-dependent fauna?
- Can the abundance and types of hollows be predicted from morphology of trees?
Methods
Study site
We studied river red gums in the MacDonnell Range bioregion of central Australia in 2017. The mountain ranges cover approximately 40 000 km2, creating a catchment for a large network of ephemeral creeks and rivers (Duguid 2015). All creeks and rivers in the region are part of the Lake Eyre Basin, but flood out on the northern and eastern margins of the Simpsons Desert (Duguid 2015). Thirteen lowland channel sites (Duguid 2015) were selected as a representative sample of the river red gum woodlands of the bioregion. Eleven river sites were randomly selected within a 200 km section of the ranges aiming to encompass variation in local density and canopy cover. At these sites, every tree was sampled within a single 25 m × 100 m plot. Two additional sites were sampled by undergraduate students as part of an ecology field course. At these latter sites, trees along a 500 m transect were randomly selected for measurement using the point-quarter method. Based on knowledge of regional-scale variability in river red gum woodlands (Westerhuis 2015), we are confident that our sample is representative of populations in the bioregion.
Field measurements
Across the 13 sites, we quantified the morphological attributes of 325 individual river red gums using five variables: DBH (diameter at breast height), tree height, canopy form, number of stems and resprouting. The first two variables represented the characteristics known to be associated with hollow abundance in other Eucalyptus species (Smith & Lindenmayer 1988; Gibbons & Lindenmayer 2002; Rayner et al. 2014); and canopy form, number of stems and resprouting type were included to capture some of the unique characteristics of river red gums that we predicted would be associated with abundance of hollows.
Tree circumference was measured at chest height, and if the tree was multi-stemmed, the circumferences of the three largest stems were measured. Circumferences were converted to DBH, and for multi-stemmed trees, the equivalent DBH per tree was calculated using the square root of the sum of squared diameter for each stem. Equivalent DBH was used as a measure of tree size because it enabled comparison of sizes between single- and multi-stemmed trees. Tree height was measured with a Haglöf ECII-D Electronic Clino / Height Meter. Canopy form was classified according to the percentage of live original canopy: >75% (form 1); 25–75% (form 2); <25% canopy (form 3); or dead (form 4); this scheme was modified from Smith and Lindenmayer (1988) and Rayner et al. (2014). Stems which had developed following post-disturbance resprouting were not included in this assessment of canopy form; however, the type (from lignotuber or epicormic buds) and length of resprouting were recorded separately.
Each tree was systematically examined with binoculars from the ground for at least two minutes and from a variety of aspects. Hollows were defined as any opening > 1 cm where the diameter was less than the depth of the hollow (Rayner et al. 2014). If estimation of hollow depth was precluded (or not possible) because of the orientation of the hollow, it was excluded. Because of the general limitations of assessing hollows from the ground (Koch 2008; Rayner et al. 2011), we may have under- or overestimated the abundance of small hollows; however, it is unlikely that we missed large hollows. For each hollow identified, the height of the entrance was measured, the entrance diameter estimated, wood condition assessed (dead or alive), and location of the hollow recorded (stem or branch). We assessed hollows only on cloud-free days, such that discrimination was maximised between hollows suitable for wildlife and ‘blind’ hollows not deep enough for use. Further, assessments were made or directly supervised by one observer only (EW).
Data analyses
A range of data exploration tools was used to inspect the data visually prior to the analysis (Zuur et al. 2010). Data pertaining to our three questions (Table 1) were analysed using mixed effect models (Bates et al. 2015; R Core Team 2018) and multivariate analysis (Anderson et al. 2008).
| Question | Attributes measured | Analysis type |
|---|---|---|
|
|
|
|
|
|
|
|
|
When using generalised mixed linear modelling of the relationship between hollow abundance and tree characteristics (Table 1; question 3 (i), we nested tree within site as a random effect to model dependencies among hollows located on the same tree and trees co-located within sites. The response variable was number of hollows; because many trees had a zero value, a negative binomial model was used. Differences in Akaike information criteria value (AIC) from the best model were reported, along with model weights. We validated the model fit and performance by checking for over-dispersion and overly influential observations (Zuur et al. 2010). Equivalent DBH had a skewed distribution and was log-transformed prior to the analysis, and equivalent DBH and tree height were strongly correlated so only DBH was used in the model. For multivariate analysis of the relationship between type of hollow and tree characteristic (Table 1; question 3, (ii), we used PERMANOVA (instead of distance-based linear modelling) because hollows were nested in trees within sites, and because we were most interested in relationships between characteristics of the trees and the clusters of hollows identified by the analyses under question 2. Data on characteristics of hollows and trees were normalised prior to the analysis and Euclidian distance was calculated between samples, as the attributes were a mix of continuous and ordinal values measured on different scales.
Results
Density of hollow-bearing trees
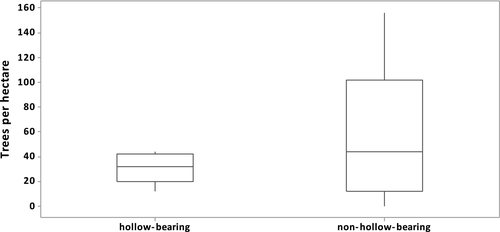
Between 9 and 73 trees were sampled at each of the 13 sites (n = 325 trees in total). The density of river red gums differed significantly among sites (two Factor PERMANOVA – site: d.f. = 12, Pseudo-F = 2.07, P = 0.02), ranging from 36 to 308 trees ha−1, and there were significant differences between densities of hollow-bearing and non-hollow-bearing trees (two Factor PERMANOVA – hollow presence: d.f. = 1, Pseudo-F = 3.10, P = 0.047). Thirty-five per cent (n = 113) of river red gums sampled were hollow-bearing, and the density of hollow-bearing river red gums was between 16 and 48 trees ha−1 (mean = 31 trees ha−1). By comparison, the density of river red gums without hollows ranged from 4 to 272 trees ha−1 (mean = 67 trees ha−1). There was a significant difference in dispersion between hollow-bearing and non-hollow-bearing trees (PERMDISP – difference between groups: Hollow-bearing, Non-hollow-bearing, t = 3.3542, P = 0.016) with hollow-bearing trees occurring at less variable densities than non-hollow-bearing trees (Fig. 1).
Characteristics of hollows
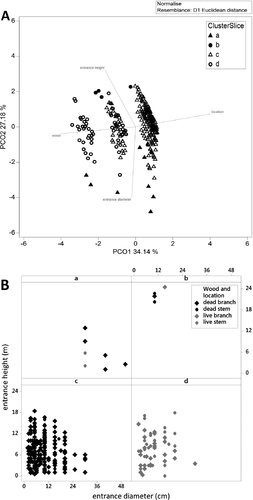
External characteristics of 328 hollows were recorded from the 113 hollow-bearing river red gums. Most hollows were located on tree branches (79%, n = 258) rather than on the main stems (21%, n = 70). The abundance of hollows on dead branches (69%, n = 225) was much higher than on live branches (10%, n = 33), hollows in live stems (10%, n = 34) and hollows in dead stems (11%, n = 36). Only four of the hollows occurring on stems were located at the base of tree, with 66 located between 2 and 23 metres above the ground. Four clusters of hollow types with distinctive combinations of attributes were discerned a posteriori from SIMPROF analysis (π = 0.111, P = 0.01; Fig. 2). Most hollows (77%, n = 252) were placed in a single category (cluster c) within which hollows were homogeneous in entrance height, entrance diameter, wood condition and location. A Principal Co-ordinate (PCO) biplot of the matrix of hollow characteristics indicated that the total variation explained by the first two axes was 61% (Fig. 2a). Wood condition and location of the hollow had a strong relationship with the first axis, which also corresponded to separation of the two largest groups, c and d; wood condition was primarily responsible for the clustering (Fig. 2b). Entrance diameter and height had a strong relationship with the second axis which also corresponded with separation of groups a and b from groups c and d. The two most abundant groups (cluster c and d) were characterised by entrances which were small and close to the ground (Fig. 2b), differing only in wood condition. The remaining two uncommon groups of hollows were defined by above-average entrance diameter (cluster a) or entrance height (cluster 2b).
Relationships between tree morphology and abundance and type of hollows
The best model for predicting the number of hollows on river red gums used a combination of equivalent DBH, stem number and canopy form. There were significant and positive relationships between the number of hollows and equivalent DBH, and the number of hollows and trees of form 2 (with 25–75% canopy) (Table 2). The model predicted that equivalent DBH was the best indicator of hollow abundance with canopy form held constant. Multi-stemmed river red gums with 25–75% canopy had the most hollows per tree; compared to this reference level, single-stemmed trees, dead trees, trees with < 25% canopy and trees with nearly full canopy had fewer hollows.
| Model | LogLik | AIC | Weight | Delta | Deviance | d.f. residual |
|---|---|---|---|---|---|---|
| Hollows ~ stems + form + log dbh + (site) | −380.00 | 773.99 | 1.00 | 0.00 | 759.99 | 318.00 |
| Hollow ~ log dbh + form + (site) | −383.45 | 778.90 | 0.10 | 4.91 | 766.90 | 319.00 |
| Hollows ~ log dbh + stems + (site) | −408.54 | 825.09 | 0.00 | 51.10 | 817.09 | 321.00 |
| Hollows + log dbh + (site) | −412.55 | 831.09 | 0.00 | 57.10 | 825.09 | 322.00 |
| Hollows ~ form + (site) | −646.74 | 1303.49 | 0.00 | 529.50 | 1293.49 | 320.00 |
| Hollows ~ stems + (site) | −703.41 | 1412.82 | 0.00 | 638.83 | 1406.82 | 322.00 |
| Modelled variables | Estimate | SE | z value | 2.50% | 97.50% | Pr(>|z|) |
|---|---|---|---|---|---|---|
| Intercept | 0.001 | 0.518 | −12.925 | 0.000 | 0.003 | <0.001 |
| Equivalent DBH (log) | 6.458 | 0.106 | 17.537 | 5.242 | 7.954 | <0.001 |
| Single Stemmed | 0.767 | 0.101 | −2.638 | 0.629 | 0.934 | 0.008 |
| Form 1 | 0.636 | 0.123 | −3.674 | 0.500 | 0.810 | <0.001 |
| Form 3 | 0.677 | 0.151 | −2.575 | 0.503 | 0.911 | 0.010 |
| Form 4 | 0.310 | 0.166 | −7.041 | 0.224 | 0.430 | <0.001 |
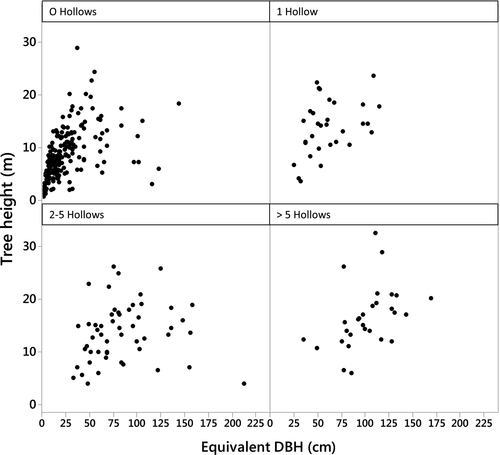
Hollow-bearing trees had larger stems (82.3 ± 3.33 cm) and were taller (14.4 ± 0.53 m) than non-hollow-bearing trees (23.44 ± 1.68 cm, 8.0 ± 0.34 m). Although height was not used in the best statistical model of hollow abundance, trees with the most hollows were usually more than 10 m tall (Fig. 3). Trees with more than five hollows were likely to have a DBH > 80 cm for single-stemmed trees (5%, n = 15) and > 50 cm for multi-stemmed trees (4%, n = 14). Smaller trees either had no hollows or one small hollow (70%, n = 226).
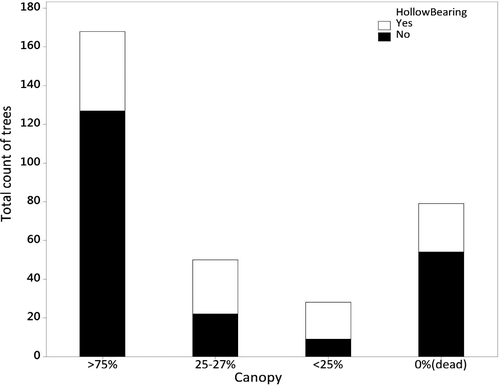
Of trees with hollows, 78% were live (n = 88) while 22% were dead (n = 25). Live trees mostly had > 75% canopy cover, a result likely reflecting the predominance of this tree type (Fig. 4). Trees with intermediate and low canopy cover mainly had hollows, but such trees were much less abundant overall.
There was a significant relationship between tree characteristics and the type of hollows occurring on a tree (three Factor PERMANOVA – hollow type: d.f. = 3, Pseudo-F = 27.179, P = 0.001), and tree characteristics also varied significantly among sites (three Factor PERMANOVA – tree (site): d.f. = 74, Pseudo-F = 2.045, P = 0.001). In contrast to the relationship between hollow abundance and tree characteristics, which indicated that equivalent DBH and canopy form were most significant in explaining abundance of hollows, SIMPER analysis showed that tree height and basal resprouting best explained the characteristics of hollows (Table 3). Trees with large hollows or hollow entrances at greater heights were taller on average than other trees and lacked basal resprouting. Tree height contributed most to dissimilarity in four-group comparisons, while basal resprouting contributed most to dissimilarity between the remaining two groups. SIMPER analysis also showed that basal resprouting contributed most to differences between group c (hollows on dead wood) and group d (hollows on live wood), and that group c had greater basal resprouting than trees with hollows in living wood.
| Average values for each hollow type | |||||
|---|---|---|---|---|---|
| Hollow type | Tree height (m) | Equivalent DBH | Tree form | Length of basal resprouting (m) | Length of epicormic resprouting (m) |
| A | 15.7 | 95.55 | 3 | 0 | 0 |
| B | 27.8 | 117.83 | 3 | 0 | 0.1 |
| C | 15.3 | 94.40 | 2 | 0.66 | 0.62 |
| D | 16.1 | 92.67 | 2 | 1.56 | 0.35 |
| Dissimilarity between hollow types based on tree characteristics | |||||
|---|---|---|---|---|---|
| Hollow type | Tree height | Equivalent DBH | Tree health | Basal resprouting | Epicormic resprouting |
| A & B | 81.1% | 7.4% | 10.95% | – | 0.5% |
| A & C | 30.5% | 15.9% | 28.09% | 10.25% | 15.24% |
| A & D | 26.9% | 10.1% | 23.90% | 30.52% | 8.61% |
| B & C | 56.5% | 13.6% | 11.90% | 7.22% | 10.84% |
| B & D | 49.8% | 10.4% | 10.34% | 23.08% | 6.39% |
| C & D | 17.5% | 17.4% | 18.33% | 29.06% | 17.68% |
Discussion
Our research has focussed on river red gum woodlands associated with ephemeral river channels in central Australia. These woodlands occupy thousands of kilometres of linear river channel but their distribution overall represents only a small fraction of the landscape. We found that hollow-bearing trees were relatively common in the river red gum woodlands of central Australia, but individual hollows tended to be small and most trees had few hollows. This confirms our view that river red gum woodlands provide a concentrated abundance of hollows and important habitat for hollow-dependent fauna in this region given the low abundance of hollow-bearing trees across the broader landscape.
The mean density of hollow-bearing river red gums recorded at our sites in the MacDonnell Ranges (39 trees ha−1) was higher than the density of hollow-bearing trees in western NSW (16 trees ha−1, Rayner et al. 2014), northern Victoria (18 trees ha−1, Bennett et al. 1994), eastern Victoria (22 trees ha−1, Gibbons et al. 2000) and south-east Queensland (38 trees ha−1, Treby & Castley 2015). Despite the relatively high density of hollow-bearing trees, our ground surveys only provide an index of availability of hollows and not a direct measure of their utility for wildlife.
It is likely that only a fraction of available hollows is suitable for hollow-using animals. Based on signs of the previous occupancy, research in temperate forest indicates that many existing hollows are never used. Where attempts have been made to quantify hollow use, previous signs of occupancy have been detected in as few as 5% (Stojanovic et al. 2014) or as many as 43% of available hollows (Gibbons et al. 2000). Additionally, the characteristics of hollows (Saunders et al. 1982; Gibbons & Lindenmayer 2002; Lumsden et al. 2002; Stojanovic et al. 2014) and the spatial arrangement of hollows, whether at the scale of a site or an individual tree, (Brigham et al. 1998; Sedgeley & O'Donnell 1999; Goldingay 2009) are important determinants of occupancy. It is noteworthy that most hollows observed in our study had small entrances, with 36% of hollows having an entrance ≤ 5 cm and a further 37% having entrances which were 6–10 cm in diameter. Only 13% of hollows sampled had an entrance diameter > 20 cm suitable for larger hollow-using species.
The hollow requirements of the 37 species of hollow-dependent birds and microbats inhabiting central Australia are not well known (see Pavey et al. 2014), but are undoubtedly diverse, as these species range from the 4 g inland forest bat (V. baverstocki) to the 7 kg red-tailed black cockatoo (Calyptorhynchus banksii). The many hollows with narrow entrances that we recorded are likely to be useable by only a subset of the fauna, precluding access by large birds such as the red-tailed black cockatoo, Major Mitchell's cockatoo (Lophochroa leadbeateri), little corella (Cacatua sanguinea), southern boobook (Ninox novaesellandiae), barn owl (Tyto alba) and nankeen kestral (F. cenchroides). The relative paucity of hollows with high entrances suggested by our study may also be of importance, as some evidence suggests that some species such as striated pardalote (Pardalotus striatus) (Haseler & Taylor 1993), yellow-bellied sheath-tailed bat (S. flaviventris) (Clews 2017) and white striped free-tailed bat (Austronomus australis) (Rhodes and Wardell-Johnson 2006) select hollows higher than 10 m when they are available. Alternatively, Goldingay (2009) noted that many parrot species selected hollows with entrances that were < 10 m high. It is unclear to what extent hollow entrance height is of importance in central Australia because hollow height tends to be determined by tree height and river red gums in the region rarely reach heights above 20 m. Although we found no evidence of a difference in abundance between hollows on dead vs. live branches and stems, the relative availability of these hollow types is potentially important in a highly variable climate where hollows with different thermal properties may be required depending on the time of year (Bondarenco et al. 2016). The microclimate within hollows provides a buffer against daily and seasonal temperature fluctuations (Gibbons & Lindenmayer 2002; Goldingay & Stevens 2009; Doucette et al. 2011) but this effect is lessened in dead wood (Coombs et al. 2010), and hollows on branches may have different thermal properties to those occurring on stems. The limited information about use of hollows in the arid zone prevents firm conclusions, as yet, about the utility of hollows for fauna in river red gum woodlands.
Our results confirm that stem size is an important indicator of hollow abundance per tree, and the abundance of large river red gums can be used to estimate hollow abundance. In particular, we found that river red gum trees that are tall (> 14 m) and with a DBH > 50 cm (multi-stemmed) or > 80 cm (single stemmed) were most likely to be hollow-bearing. These results are consistent with previously documented patterns in river red gums and other Eucalyptus species (Bennett et al. 1994; Gibbons & Lindenmayer 2002; Goldingay 2009; Rayner et al. 2014). Bennett et al. (1994) found that river red gum stems with abundant (> 5) hollows developed multiple hollows at a size 25–40% wider than other Eucalyptus species.
Canopy form was also an important indicator of hollow abundance per tree, with living trees in advanced stages of senescence more likely to have hollows than very young healthy trees. Living trees with hollows are preferred over dead trees with hollows by species such as inland free-tailed bats (Mormopterus petersi) (Bondarenco et al. 2014), Gould's wattled bats (Chalinolobus gouldii) (Lumsden and Bennet 2002) and southern boobooks (Ninox novaeseelandiae) (Higgins & Peter 2002). Microbats and owlet-nightjars have also been found to use hollows in dead trees (which have less insulation from external temperature than live wood) to passively arouse from daily torpor and reduce physiological demands in extreme temperature (Doucette et al. 2011; Bondarenco et al. 2016). Dead standing river red gums were relatively abundant, but few were hollow-bearing. Dead trees bearing hollows tended to be tall ‘stags’ with a large diameter. Such trees are known to be important as maternal roosts for several species of microbats (Lumsden et al. 2002; Stawski & Currie 2016; Rueegger et al. 2018), as they allow females to congregate during parturition and the post-partum period (Pennay 2006; Clews 2017). Considering the rarity of large, hollow-bearing trees, whether dead or alive, and their likely importance for certain animals, we attest that a priority for management of riverine habitats should be to retain and protect river red gums with large stems, including dead standing trees, to maximise the availability of hollows with diverse characteristics.
Changing fire regimes in river and flood plain environments in central Australia is likely to be the most important emerging threat to the ongoing persistence of hollow-bearing river red gums in central Australia. Cenchrus ciliaris, an introduced invasive grass, has become widespread in riverine habitats of the MacDonnell Ranges and elsewhere throughout arid Australia, substantially increasing fuel loads and promoting fire (Schlesinger et al. 2013; Friedel et al. 2014). Large hollow-bearing trees are particularly vulnerable to wildfire damage (Haslem et al. 2011, 2012), and changing. We observed a negative association with fire impacts and the abundance of some hollows. Epicormic or basal resprouting was rare in the small subset of river red gums with large hollows (> 30 cm) and high hollows (> 20 m), suggesting that these trees have been protected from intense fire for long periods.
Although fire damage can be associated with hollow development in trees (McLean et al. 2015), it also destroys existing hollows, with the net effect being dependent on the intensity and frequency of fire and the health of the tree (Haslem et al. 2011; Flanagan-Moodie et al. 2018). Based on the increasing frequency of fire and the destruction of large trees that can be observed in areas invaded by buffel and other introduced grasses, it is possible that the population structure of river red gums – and associated hollow abundance – is being simplified in central Australia.
Conclusion
Although we recorded numerous hollow-bearing trees in the river red gum woodlands of central Australia, these woodlands are limited in their distribution, occurring only in river channels. Trees with more than five hollows (< 9% of all trees sampled) and hollows with entrances > 20 cm in diameter or > 10 m high were rare, which may limit hollow-using species requiring large or high hollows. Trees with the greatest variety and abundance of hollows tended to be trees in advanced stages of senescence. Due to lack of knowledge of hollow selection and occupancy for cavity-dependent fauna in central Australia, we cannot yet determine whether hollows are a limiting resource or to what extent altered fire regimes affect hollows. Further research is required into the relationship between fire, the types and availability of hollows, and the use of hollows by wildlife, to assist appropriate management of the resource.
Acknowledgements
We thank Nigel Westerhuis, Carly Humphrys and the students enrolled in the 2016 CDU Desert Field Ecology course for assistance with data collection; Dr Mirjam Kaestli for advice on analyses; and Andy and Jane Hayes from The Gardens Station, and Sheridan Martin, Mark Anderson and Shannon Carne from the Parks and Wildlife Commission of the Northern Territory for support while in the field. We acknowledge the Traditional Owners of Tjoritja National Park, Owen Springs Reserve and Trephina Gorge Nature Park. Comments from Ross Goldingay and an anonymous reviewer greatly improved the manuscript. The research was funded by Charles Darwin University, and EW was supported by an Australian Postgraduate Award.




