Acoustic stimuli from predators trigger behavioural responses in aggregate caterpillars
Abstract
Prey detect their predators through predator signals and cues and, consequently, respond with anti-predatory behaviours to inhibit the action of their aggressors. Lepidopterans can intercept signals emitted by predators and may defend themselves through chemical, morphological or behavioural responses. In this study, we investigated the effect of acoustic stimuli of different predators on defensive behaviour of gregarious caterpillars. Our results demonstrated that Hylesia nigricans (Lepidoptera, Saturniidae) caterpillars alter their behaviour (i.e. abruptly raising the head) in response to the acoustic stimulus of the predators (i.e. predation risk signals from birds and wasps). The magnitude of this response depended on predator identity and caterpillar body size. Larger caterpillars responded more strongly to predatory stimuli than smaller caterpillars. However, regardless of the size of the caterpillars, they responded more strongly to the stimuli of wasps. In addition, we identified that H. nigricans caterpillars emit ultrasonic noise after detecting the stimuli of the predators – this noise seems to function as an alert about the risk of predation during the early stages of development (second and fifth instars). The duration of ultrasonic emission (i.e. milliseconds) increases with the number of repetitions of the stimuli (i.e. wing-beat sounds of the wasps and insectivorous birds). These results provide novel information about predation risk in interactions among caterpillars and their predators, and indicate possible communication among invertebrates mediated by the risk of predation.
Introduction
Organisms often detect stimuli that announce the presence of predators, who, in turn, seek to develop strategies to remain imperceptible (Romero et al. 2011; Breviglieri & Romero 2016). However, during foraging, subtle signals emitted by predators (i.e. chemical, visual or acoustic) may reveal their presence and often their identity (Gonçalves-Souza et al. 2008; Breviglieri et al. 2013). These signs trigger different anti-predatory strategies (Bernot & Turner 2001) that can vary depending on the identity of prey (Lima 1998; Zuberbühler et al. 1999; Breviglieri et al. 2013, 2017). Several mechanisms allow prey to recognise their predators, such as innate behaviour, learned behaviour patterns developed through observing the behaviour of congeners (Griffin 2009) or heterospecifics (Fallow et al. 2013), and learned behaviour patterns developed through direct interactions with predators (Chivers & Ferrari 2013). Effective identification of a possible predator can maximise the chance of escape and more efficient energy use (e.g. foraging, breeding, territorial defence).
Invertebrates can intercept and identify acoustic signals emitted by predators (e.g. Tautz & Markl 1978; Fullard 1988; Jacobs et al. 2008; Fournier et al. 2013) and may defend themselves through chemical, morphological or behavioural responses (Murphy et al. 2010). Chemical defences are especially important for specialist caterpillars (Lepidoptera) and have been shown to function against a variety of predators (Dyer & Bowers 1996; Trigo 2000). For instance, caterpillars sequester pyrrolizidine and tropane alkaloids or develop chemical camouflage based on the similarity of the cuticular lipids that match with chemistry of its host plants (Trigo 2000). Physical defences, such as cryptic coloration patterns, enable larvae to blend in with their natural environment and may minimise detection by predators (Stamp & Wilkens 1993; Grant 2006). In addition, some species may mimic snakes (i.e. morphologically and behaviourally) to inhibit their predators (Janzen et al. 2010). Behavioural defences include escape holes, head or tail wagging, suspension on silken threads, aggregation, frass ejection, frass chains, construction of leaf shelters, among others (Castellanos & Barbosa 2006; McClure & Despland 2011).
Another interesting defence mechanism in Lepidoptera is a sound production, which might inhibit the predator attack (Bura et al. 2016) or attract natural bodyguards (i.e. ants; DeVries et al. 1999). Many insects produce sound when attacked by a predator (Bura et al. 2016; Dookie et al. 2017). Recent studies suggest that these acoustic signals are directed primarily to vertebrate predators (Bura et al. 2016), although caterpillar sounds may also intimidate invertebrate predators such as wasps, ants, beetles, true bugs and spiders (Greeney et al. 2012). Several caterpillar species (e.g. hawkmoth caterpillars; see details in Bura et al. 2016) produce sonic displays that are the result of direct attacks by insectivorous birds (Brown et al. 2007; Dookie et al. 2017). Despite the diversity of defensive sounds, their functions and the significance of their varied characteristics are poorly understood (Dookie et al. 2017).
In this study, we investigated the effect of acoustic stimuli of different predators (i.e. birds and wasps) on defensive behaviours of gregarious caterpillars (Hylesia nigricans, Saturniidae, Lepidoptera). We predicted that (i) gregarious caterpillars can identify, through acoustic cues (i.e. wing beat), the approximation of predators and then respond by adopting anti-predator behaviour. In addition, we predicted that (ii) the intensity of the anti-predatory response of the gregarious caterpillars (e.g. number of individuals responding to the stimuli) will be associated with the predator's identity (i.e. wasps or birds). Like guppies (Botham et al. 2006), birds (Zuberbühler et al. 1999) and monkeys (Seyfarth et al. 1980), the gregarious caterpillars can discriminate between different predators (i.e. likely because of very different modes of attack) and respond appropriately. McClure and Despland (2011) demonstrated that the defensive responses (e.g. synchronised reaction) of gregarious caterpillars differ between predators (e.g. wasps, parasitoids, spiders and stinkbugs) and depend on larval instar and group size and, in general, led to increased survival. Thus, considering that larger caterpillars (i.e. latter instars) are more aggressive against predator attacks (McClure & Despland 2011), we predicted that (iii) groups of caterpillars constituted by larger individuals will respond more intensely to all stimuli regardless of predator identity. In addition, animals living in groups may reduce their risk of predation in several ways. With more ‘eyes’ present, groups might detect clues associated with the approximation of predators more efficiently (see ‘many-eyes’ hypothesis, Lima 1990). However, since caterpillars have a poor visual system (Warrant et al. 2003), they often rely on acoustic communication during various stages of their life cycles (e.g. foraging, defence and aggregation, Cocroft 2001). Alternatively, they could respond in different ways to the stimuli of different predators (Castellanos & Barbosa 2006). There is evidence that acoustic signalling in some insects is an alert reaction against possible aggressors (Heinrich 1993; Brown et al. 2007). Thus, we predicted that and (iv) after detecting clues of a possible aggressor that arrives by flying, the gregarious caterpillars respond with synchronised anti-predatory behaviours stimulated by the movement of neighbouring caterpillars and the intra-specific acoustic communication.
Methods
Study area and organisms
The present study was conducted in the Serra do Japi Biological Reserve located in the municipality of Jundiaí, state of São Paulo, south-eastern Brazil (23º11′S, 46º52′W). The reserve has a mountain landscape, with altitudes ranging from 700 to 1300 m asl. Two climate types occur in the area: humid temperate with warm summer ‘Cfa’ and humid temperate with mild summer ‘Cfb’. The average annual temperature varies from 15 to 19°C, depending on the altitude. The vegetation of this region is classified as semi-deciduous seasonal forest (see details about the biological reserve in Morellato 1992).
More than 800 butterfly species have been found in this biological reserve (Brown 1992), but the diversity of moths is still unknown (M. Duarte, Pers. comm.). Hylesia nigricans (Berg, 1875; Lepidoptera: Saturniidae) is a common moth species in the reserve (Fig. 1a,b). The American genus Hylesia contains about 110 species, but the biology of most of them is poorly studied (Pereira et al. 2009). The genus has achieved notoriety in parts of South America because of urticating hairs (Lamy & Lemaire 1983), structures that are present at all stages of development (i.e. eggs, all larval instars and the adult stage; see Specht et al. 2006). Hylesia nigricans occurs from south-east Brazil to Argentina and Uruguay (Specht et al. 2006). The caterpillars present gregarious behaviour (see Fig. 1a,b). This experiment used 11 aggregations composed of 87.63 individuals on average (SD ± 63.11), found on leaves of Alchornea glandulosa Poepp. & Endl. (Euphorbiaceae), up to two metres above the ground.

In addition, more than 200 bird species have been recorded in this biological reserve (Silva 1992). Myiothlypis leucoblephara (Vieillot, 1817; Passeriformes: Parulidae) is strictly insectivorous (Sick 1997; Sigrist 2006) and is one of the most commonly observed species (Fig. 1c). In Brazil, its distribution is from southern Minas Gerais and Rio de Janeiro to Rio Grande do Sul (Sick 1997). This species flies at low heights, shifting among the bushes and branches with long jumps (Sick 1997; Sigrist 2006), and emits a faint flap of wings as it moves between vertical perches (Sigrist 2006). This species of bird is considered an efficient predator of caterpillars (Jordani et al. 2015). We observed M. leucoblephara attacking the aggregation of other species of stinging caterpillars in the field (C.P.B. Breviglieri, pers. comm.), but as far as we know, there is no such information in the literature. However, we did not observe any event of attacking H. nigricans.
This biological reserve is inhabited by many social wasp species. Many wasp species (e.g. Mischocyttarus sp., Polistinae: Vespidae; Fig. 1d) forage upon the vegetation in search of small spiders or other invertebrates (Richter 2000), but a great part of their diet also includes caterpillars (Prezoto et al. 2006). Mischocyttarus wasps are able to remove hairs of the stinging caterpillars during attack (Bowers 1993). We witnessed approximation of these genera of wasps to aggregations of H. nigricans and events of predation to other stinging caterpillar species in the field (C.P.B. Breviglieri, pers. comm.).
Flight sound recording for each treatment
We recorded the acoustic frequencies produced by the wing beats of two predatory species (i.e. a social wasp (Mischocyttarus sp.) and an insectivorous bird (Myiothlypis leucoblephara), Appendix S1) and a non-predator (blood-sucking mosquito; control treatment). The recordings were made while these organisms moved among the branches of A. glandulosa, the host plant used by H. nigricans. The sounds were recorded one metre away from the organisms using a directional microphone (Shotgun Yoga ht-81, frequency of response: 100–16 000 Hz and sensitivity: −44 ± 2 dB) and a digital record (Sony PCM D100, LPCM A 44.1 kHz, 16 bits). Sounds were analysed using Raven (Raven Pro v.1.5).
The wing-beat sound frequency of wasps was measured from a 2.5-s segment of the acoustic file. Spectral analysis was performed on 300 wing-beat cycles (512-pt dFFT with Hanning Window; frequency resolution of 202 Hz) soon before the wasp landed on the leaves, that is during its movement from one leaf to another. Sounds (Appendix S1: Fig. S1a) were pulsed owing to cyclic wing beats, and the fundamental frequency had a peak of 187.5 Hz (SD ± 1.5) and a peak power of 90.59 dB (SD ± 7.77).
The beating frequency of the bird wings was measured from a 400-ms segment of the acoustic file. Spectral analysis was performed on 10 wing-beat cycles (512 pt dFFT with Hanning Window; frequency resolution of 188 Hz) soon before the bird landed on the branches, during its movement from one branch to another. Sounds (Appendix S1: Fig. S1b) were pulsed owing to cyclic wing beats, and the fundamental frequency had a peak of 225 Hz (SD ± 90) and peak power of 94.35 dB (SD ± 9.73).
Finally, for the control treatment, the wing-beat frequency of blood-sucking mosquito was measured from a 2.2-s segment of the acoustic file. Spectral analysis was performed on 371 wing-beat cycles (512 pt dFFT with Hanning Window; frequency resolution of 152 Hz). Sounds (Appendix S1, Fig. S1c) were pulsed owing to cyclic wing beats, and the fundamental frequency had a peak of 613.63 Hz (SD ± 141.04) and peak power of 83.50 dB (SD ± 5.28).
Experimental design
To test whether predation risk perception of aggregated caterpillars (H. nigricans, Fig. 1a,b) is based on acoustic signals, and whether these signals induce behavioural changes in response to predator identity, we developed a randomised block experimental design. Each block (n = 11) consisted of an aggregation of caterpillars on an A. glandulosa leaf. Each block had the following treatments: (i) playback of the sound of wing beating of the insectivorous birds (M. leucoblephara, Fig. 1c), 20 cm from the aggregation of caterpillars on an A. glandulosa leaf. (ii) Playback of the wing beating sound of the social wasps (Mischocyttarus sp., Fig. 1d), emitted in the same conditions as the previous treatment. (iii) Playback of the blood-sucking mosquito, emitted in the same conditions as the former treatments. The order of each treatment application within each block was randomly assigned. Each playback was repeated every six seconds (i.e. time points; eleven times for each treatment). One-minute intervals were used between the treatments. Our playbacks simulate the behaviour adopted by each predatory species in the natural environment while foraging among the branches of the tree. The birds jumped among the branches of A. glandulosa, flapping their wings shortly. The wasps hovered in the air in front of the leaves and then headed to the nearest leaf. The mosquitoes flew from leaf to leaf landing briefly on each of them.
We filmed the caterpillar aggregations for two min with a camcorder (Sony DCR-DVD 610). We also recorded the sounds emitted by the caterpillar aggregations during the experiments with the Pettersson D100 (Pettersson Elektronik) ultrasonic detector (frequency range: 10–120 kHz and bandwidth: 8 kHz (±4 kHz), −6 dB). This equipment can detect and record ultrasound (e.g. issued by bats, rodents, crickets) by connecting to a digital recorder; however, it has some limitations. For example, the sounds recorded cannot be analysed using sound analysis software to generate sonograms. Thus, we were limited to identifying only when and for how long the caterpillars emitted ultrasonic noises at the time of recording and to what frequency it occurs. Thus, comparing the video recordings with the acoustic information obtained through the Raven software (Raven Pro 1.5, Charif et al. 2008) allowed us to estimate the average duration (ms) of the sounds emitted by the caterpillar aggregations among the time points, since the recordings were obtained with the same device. The average frequency of the noises (kHz) was obtained by the tuning of the ultrasonic detector. For this, before the experiments, we set the tuning of the device (i.e. between 0 and 120 kHz) to the frequency range of the noises emitted by the caterpillar groupings stimulated by the playbacks. The camera and recorder were positioned 30 cm from the aggregation.
We considered as variable response the proportion of caterpillars (i.e. number of responsive individuals/total number of individuals in the aggregation) that were influenced by the different treatments in each time points. The quantified anti-predatory behaviour of the caterpillars was the fast movement of the head back immediately after the playbacks. The average proportion of caterpillars that responded to the stimuli was calculated for each time points, considering the number of caterpillar aggregations as replicate (N = 11). This species exhibit six to seven instars (Specht et al. 2006). In this research, we studied groupings formed by individuals in second (smalls n = 7) and fifth (large n = 4) instars. Aggregations formed by small caterpillars (second instar, Fig. 1a) were composed of individuals with yellowish colour, with the black cephalic capsule (Specht et al. 2006), with small urticating structures along the body and with the average body length of ca. 7.14 mm (SD ± 0.87). Aggregations formed by large caterpillars (fifth instar, Fig. 1b) were composed of individuals with dark greenish colour, black and white cephalic capsule (Specht et al. 2006), well-developed urticating structures distributed throughout the body, and mean body length of ca. 34.78 mm (SD ± 1.78). We used the blood-sucking mosquitoes as the control treatment because these insects are not predators and are extremely common in the environment where caterpillars naturally occur. In addition, we know that the frequency of invertebrate wing beating varies among species (Chen et al. 2014) and, therefore, would not resemble those of a parasitoid wasp or other species of invertebrate aggressor. Playbacks of the sound of wing beatings of birds, wasps and blood-sucking mosquitoes were broadcasted using portable speakers (model: Mini Beat Box Bluetooth, frequency band: 2.402–2.480 GHz and >80 dB).
Statistical analyses
To test for the effects of treatments (i.e. playbacks of wing beat of birds, wasps and blood-sucking mosquitoes) on the caterpillar behaviours, we performed a linear mixed-effects model coded in lme function of the nlme package of R (Pinheiro & Bates 2000). The treatments (predator identity; three levels), size of the caterpillars (large and small; two levels) and interaction between these variables were considered as fixed effects. We control the number of times sounds were played in each treatment (N = 11). However, the time in milliseconds of the playbacks differed between of the treatments (i.e. because of the underlying features of stimuli of each model organism used) and abiotic factors (e.g. air humidity, shading of the branches and movement of branches due to sudden gusts of wind) along of the reproductions also may have influenced the proportion of caterpillars that responded to each stimuli. Therefore, the caterpillar aggregations (blocks) and each playback over the two minutes of recording (time points) were treated as random effects; time was nested within blocks through the function random = ~1|Blocks/Time. Paired comparisons among different predator identities (birds, wasps and blood-sucking mosquitoes) on caterpillar behaviour were run using Tukey's HSD post hoc tests coded using the glht function of the multcomp package in R (Hothorn et al. 2016). We emphasise that only one researcher quantified the number of caterpillars that responded to the stimuli in each recording.
The correlation between the average proportion of caterpillars (i.e. regardless of body size-class and, individually, for small and large caterpillars) that responded to the acoustic stimuli and the increase over the time points of the stimulus repetitions (i.e. eleven per treatment) was determined by the Spearman test (i.e. coded as the cor.test function by the R stats package). The correlation between ultrasonic emission (average duration in milliseconds) and the average proportion of caterpillars that responded to the acoustic stimuli was determined by the Spearman correlation coefficients. In both cases, the data did not meet the assumptions of normality and homoscedasticity (Best & Roberts 1975).
All the analyses were performed in R 3.1.2 (R Development Core Team, 2016). The significance level was α = 0.05. We graphically inspected our data for variance heterogeneity, homogeneity, normality and outliers (e.g. qq plots, Cook's distance and influence) and used statistical tests of homogeneity of variance to inspect our data (e.g. Levene's test and Fligner–Killeen test). Data were log-transformed whenever necessary for analyses, but raw data were used to draw the plots.
Results
Anti-predatory behaviours of the caterpillars
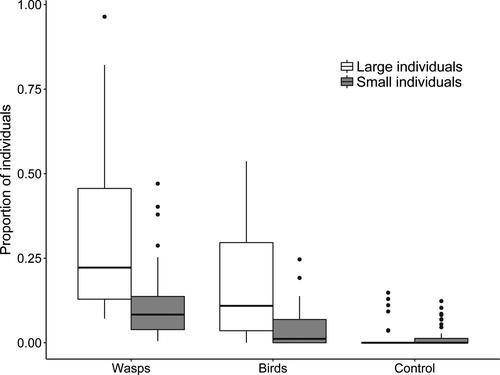
Our linear model detected a strong effect of the treatments on the anti-predatory behaviour of gregarious caterpillars (Table 1; Fig. 2), effects of caterpillar body size, and a significant interaction term between treatments and body size (Table 1). The proportion of larger caterpillars that responded to the wasp stimulus was on average two times higher than to the stimuli of the insectivorous birds, and on average 24 times higher than to the stimuli from blood-sucking mosquito (Fig. 2). The proportion of small caterpillars that responded to the wasp stimulus was on average 2.86 times higher than to the stimuli of the insectivorous birds, and on average 8 times higher than to the stimuli from blood-sucking mosquito (Fig. 2). The proportion of larger caterpillars that responded to the playbacks of wasp (i.e. 32%) and insectivorous bird (i.e. 16%) was 3.26 and 4 times higher compared to the proportion of small caterpillars (i.e. 10% and 4%), respectively (Fig. 2). However, the proportion of large and small caterpillars that responded to the control treatment did not differ (i.e. ca. 1%, Fig. 2).
| Sources of variation | numDF | denDF | F | P |
|---|---|---|---|---|
| Treatment | 2 | 238 | 122.73 | <0.001 |
| Body size | 1 | 9 | 5.3 | 0.0468 |
| Treatment:body size | 2 | 238 | 41.09 | <0.001 |
Ultrasonic response of caterpillar aggregation
When stimulated by playbacks, the caterpillars responded with ultrasound emission. Due to the limitation of the device used, we cannot present the sonograms and their parameters. However, the frequency of ultrasound emitted by the caterpillars was 21 kHz; this was obtained directly by tuning the detector. Therefore, by tuning in this frequency we could compare the video record and identify when and for how long the caterpillars were emitting the ultrasonic noises. Regardless of body size-class, the proportion of caterpillars that responded to the playbacks from the predator stimuli increased over time points (Spearman correlation; wasps ρ = 0.96, P < 0.001 and birds ρ = 0.98, P < 0.001). On the other hand, the proportion of caterpillars that responded to the control treatment did not increase over time points (Spearman correlation; mosquito ρ = −0.38, P = 0.243). Consequently, the duration of ultrasonic emission (in milliseconds) manifested by the caterpillars (i.e. regardless of body size-class), in response to predators (wasps and birds), was prolonged. This resulted in a positive correlation (Spearman's rank) between the proportion of caterpillars that responded to wasp (Fig. 3a) and bird stimuli (Fig. 3b), and the duration of the ultrasonic emissions. However, this trend was not observed when stimuli by mosquito were emitted (Fig. 3c). The potential outlier observed in Fig. 3a was not influential (i.e. wasps ρ = 0.99, P < 0.001, Appendix S2: Fig. S2a).
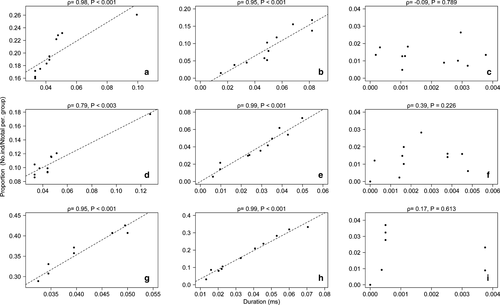
The proportion of small caterpillars that responded to the playbacks from the predator stimuli increased over time (Spearman correlation; wasps ρ = 0.82, P < 0.001 and birds ρ = 0.99, P < 0.001). Likewise, the proportion of large caterpillars also responded to the playbacks over time points (Spearman correlation; wasps ρ = 0.94, P < 0.001 and birds ρ = 0.99, P < 0.001). However, the proportion of small and larger caterpillars that responded to the control treatment did not increase over time points (Spearman correlation; ρ = −0.56, P = 0.075 and ρ = −0.046, P = 0.892, respectively). As expected, the duration of ultrasonic emission (in milliseconds) manifested by small and large caterpillars, in response to predators, was prolonged. This also resulted in a positive correlation between the proportion of small and larger caterpillars that responded to wasp (Fig. 3d,g) and bird stimuli (Fig. 3e,h), and the duration of the ultrasonic emissions, respectively. However, the proportion of moving caterpillars (collective head flicking) was mediated by the stage of development (i.e. instars) and identity of predators (see, Fig. 3). In contrast, this trend was not observed when stimuli by mosquito were emitted to small (Fig. 3f) and large (Fig. 3i) caterpillars. Again, the potential outlier observed in Fig. 3d was not influential (i.e. wasps ρ = 0.78, P = 0.02, see details in Appendix S2, Fig. S2b).
We present in Appendix S3 the behaviour of caterpillars in response to the stimulation of the wing beat of the birds and the period of emission of the ultrasonic noises emitted by the caterpillars increasing along the time points. In the Appendix S4, we present the ultrasonic noises emitted by the caterpillars aggregated at two time points after the reproduction of the beating of the bird's wings. We highlight that this recording was obtained using the heterodyne detector (i.e. Pettersson D100) and therefore cannot be analysed using sound analysis software to generate sonograms.
Discussion
Our results demonstrated that gregarious caterpillars alter their behaviour in response to acoustic stimuli of predators. However, the intensity of this response depended on the predator's identity and caterpillar's body size. Larger caterpillars responded more strongly to the stimuli of predators than smaller ones and were more responsive to stimuli from wasps than from birds. In addition, we identified that H. nigricans caterpillars emit ultrasonic signals after detecting the stimuli of the predators. This signal, which seems to function as an alert to stimulate other members of the aggregation, resulted in different behavioural responses (i.e. collective head flicking or immobility). The time of ultrasonic emission (i.e. milliseconds) and the proportion of the caterpillars that respond to the stimuli increase with the number of repetitions of the stimuli. These results provide novel information about predation risk in interactions among caterpillars and their predators, and in communication among gregarious caterpillars.
The H. nigricans caterpillars responded to the acoustic cues that announced the approximation of the predators, confirming our first prediction. As far as we know, this is the first study to demonstrate that acoustic stimuli from predators (i.e. sound of wing beats) influence the behaviour of gregarious caterpillars. Several species of invertebrates can intercept acoustic signals emitted by predators (Fullard 1988; Jacobs et al. 2008; Fournier et al. 2013). It is known that airflows produced by the wing beats of approximating predators (Greeney et al. 2012) and substrate-borne vibrations produced by vertebrate and invertebrate predators walking on a leaf can trigger anti-predatory responses in caterpillars (Castellanos & Barbosa 2006; Castellanos et al. 2011). Although anti-predatory responses to different stimuli emitted by predators have been previously suggested, little experimental work has been done to empirically test the behavioural ecology of caterpillar defences (Botham et al. 2006; Castellanos & Barbosa 2006; McClure & Despland 2011). Thus, our findings suggest that caterpillars can identify predators through acoustic stimuli and respond to the risk of predation by adopting anti-predatory behaviour.
The caterpillars were able to identify different species of predators through acoustic stimuli, thus corroborating our second prediction. However, a significant correlation between treatment and body size indicates that body size is an important factor mediating anti-predatory behaviour (i.e. intensity of this behaviour), which was our third prediction. Caterpillars can identify their predators through a combination of different stimuli and modes of attack (McClure & Despland 2011), but the anti-predatory responses may vary among different Lepidoptera species or larval instars (McClure & Despland 2011). For example, some species of caterpillars showed anti-predatory behaviour only after a wasp landed and began walking on a leaf (Castellanos & Barbosa 2006), and others reacted only when they were touched by predators (Castellanos et al. 2011). We suggest that small larvae of H. nigricans could detect acoustic cues associated with the approximation of the predators, but most of them remain immobile. This behaviour can be explained by the fact that they are less conspicuous, and synchronised movement may not represent an efficient defence against visually oriented predators (e.g. birds, wasps). Even if this species exhibits stinging structures at all instars, it could still be preyed on by young and naive birds (Bowers 1993). On the other hand, as individuals grow, their vulnerability (i.e. parasitoids, Hawkins et al. 1997) and detectability to predators (i.e. birds Mänd et al. 2007) increase; thus, they must develop more efficient anti-predatory mechanisms. We can note that stinging structures are more developed in the older than younger caterpillars (see Fig. 1a,b). In addition, it is known that larger caterpillars are more likely to defend themselves with aggressive behaviour against wasps, parasitoids, spiders and stinkbugs (Gentry & Dyer 2002; McClure & Despland 2011). Therefore, the presence of well-developed stinging structures along with the synchronised aggressive behaviour (i.e. head flicking) can inhibit the attack from predators that approach by flying. In addition, it is known that young and naive predatory species (e.g. birds, social wasps, spiders) may not be able to cope with large prey, or the costs of subjugating them may be very high (Iwao & Wellington 1970; Bowers 1993; Cohen et al. 1993; Gaston et al. 1997; Romero et al. 2011). Thus, we suggest that H. nigricans caterpillars can identify their aggressors and that their body size-classes (i.e. developmental stage) may influence the intensity of the anti-predatory response. Large caterpillars equipped with stinging structures can inhibit predator attacks by adopting synchronised aggressive behaviour, and, on the other hand, most small caterpillars remain immobile, to avoid attracting the attention of their predators.
We found that predator identity and stage of development (i.e. instars) can also predict the intensity of anti-predatory behaviour. In fact, we found that caterpillars are more responsive to stimuli from wasps. On the other hand, the proportion of small caterpillars that responded to the stimuli was smaller in relation to the large ones, independently of the predator's identity. Although stinging structures or body size may reduce predation by some natural enemies (Stamp 1982; Heinrich & Collins 1983), they may be ineffective against others (Furuta 1983; Stamp & Bowers 1988). For example, social wasps can surpass most of these defences. When wasps attack stinging caterpillars, they bite off the spines before cutting the caterpillar up into pieces of a size that they can carry (Bowers 1993). In contrast, though birds are also responsible for substantial mortality in other hairy caterpillar species (Mason & Torgersen 1983), several Neotropical birds avoid stinging caterpillars (Heinrich & Collins 1983; Barber & Conner 2007). Thus, wasps may present a greater risk to stinging caterpillars than birds, a fact that would explain the more intense reaction of the aggregation to loud and strident sounds similar to those of the wasps (see Hodge 1972). Therefore, the interpretation of several signals (e.g. acoustic stimuli and substrate-borne pulses) seems to be important throughout the development of caterpillars, since they may avoid energy expenditure by expressing defensive behaviour in response to non-risk animals (Lima & Dill 1990). Considered that top-down pressure on caterpillars is stronger in the tropics (where the present study was conducted) and that this relationship is mostly driven by invertebrates (wasps and ants; Van Bael et al. 2003; Tvardikova & Novotny 2012; Sam et al. 2014; Roslin et al. 2017), we predict that the results found here might be slightly different for caterpillars from temperate regions. This represents a topic for further investigations.
Our results demonstrated that the caterpillars emit ultrasonic signals after detecting the sound stimuli that simulate predators. In addition, we identify a correlation between the proportion of caterpillars that adopted aggressive behaviour (i.e. head flicking) and the time of noise emission (see Fig. 3 and Appendices S2–S4), along of time points. Hylesia nigricans emit ultrasonic signals (above 21 kHz), and this frequency is higher than the acoustic capacity of its main natural enemies, such as birds (20 kHz, Beason 2004) and wasps (15 kHz, Danci et al. 2010), or other natural bodyguards (i.e. ants; 2 kHz, DeVries et al. 1993). Thus, we suggest that both behaviours (i.e. head flicking and ultrasonic noise) may stimulate aggregation to adopt synchronised defensive behaviours, corroborating our fourth prediction. Therefore, the noise could (i) alert their neighbours to a potential risk without attracting the attention of predators that approach by flying (i.e. wasps and birds) and then; (ii) organise synchronised anti-predatory responses to inhibit the attack of aggressors (see Stamp & Casey 1993; Bura et al. 2009, 2016). In addition, the defensive behaviours seem to be mediated by the stage of development of the larvae and identity of the predators. When they are touched, large caterpillars (i.e. between the five and seventh instars) elevate the anterior part of the body, regurgitating, moving rapidly, twisting and finally throwing themselves on the ground – this behaviour aims to intimidate or inject the stinging structures into the aggressor (Specht et al. 2006). In this study, when touched, small caterpillars (i.e. second instar) remain motionless (C.P.B. Breviglieri, pers. comm.). On the other hand, when motivated by acoustic stimuli, small caterpillars remained motionless and the large manifested aggressive and synchronised behaviour. The acoustic signalling is a very widespread form of communication among Lepidoptera (Yack et al. 2001), being considered as anti-predatory mechanism or intra-specific communication (Hodge 1972; Stamp & Casey 1993, 1993; Brown et al. 2007; Bura et al. 2009, 2016; Greeney et al. 2012). Therefore, our results provide new insights into future studies that could investigate the role of these ultrasonic emissions as an anti-predatory mechanism.
In summary, our results suggest that caterpillars of the species H. nigricans can identify acoustic cues that announce the approximation of their predators and, therefore, can respond aptly to each type of predator. The predatory response depends not only on the predator identity, but also on the size of the prey. In addition, our findings suggest that gregarious caterpillars communicate with each other through ultrasonic emissions and that this communication seems to function as an alert to stimulate other members of the aggregation against possible predators. These results reveal novel information about predation risk in interactions among caterpillars and their predators, and in communication among gregarious caterpillars.
Acknowledgements
We thank the Graduate Program in Ecology of the University of Campinas (UNICAMP) for the logistic support. C.P.B.B. received a postdoctoral scholarship from the National Postdoctoral Program/Brazilian Federal Agency for the Support and Evaluation of Graduate Education (Coordination for the Improvement of Higher Education Personnel [PNPD/CAPES]). G.Q.R. received research grants from CNPq and FAPESP. We thank the staff of the Biological Reserve of Serra do Japi for hosting and for allowing the data collection. We also thank Thiago Pires, Junia Carreira, Amalia M. S. Palacios, Tháles A. Pereira, Luis Salles and Júlia C. Teresa for help on the field during the experiment. We also thank Dr. N. Andrew, Associate Editor and anonymous reviewers for their valuable comments on the manuscript.




