Trophic ecology of young-of-the-year elasmobranchs in a critical habitat within the Río de la Plata outer estuarine waters
Abstract
Knowledge on the trophic ecology of elasmobranch species in all their size classes is important to determine complex trophic roles and relationships between members of the community, which ultimately promotes the development of more effective conservation measures. This study investigates the diet of young-of-the-year from two shark and one skate species that are common within the Southwest Atlantic Ocean. Identification and analysis of stomach contents indicated that the broadnose sevengill shark Notorynchus cepedianus fed mostly on fishes (96.08%IRI), whereas the angular angel shark Squatina guggenheim, and the smallnose fanskate Sympterygia bonapartii preyed mainly on crustaceans (68.73–99.96%IRI). In particular, Artemesia longinaris, a widely distributed small shrimp, was an important prey item for the two crustacean feeders. The high proportion of stomachs with food and the trophic levels suggest that the three species are active predators at a very young age. A high frequency of neonates was also observed for all species, suggesting that parturition events are probably occurring nearby. Overall, this study suggest that the nearshore waters of northern Argentina are functioning as an important feeding ground for co-occurring elasmobranch species within an important estuarine habitat.
Introduction
Many elasmobranch (i.e. sharks, rays and skates) populations have declined as a consequence of heavy exploitation (Dulvy et al. 2014), negatively impacting the structure and functioning of marine communities (Stevens et al. 2000). This is a result of the important role elasmobranchs play in the top–down control processes of marine food webs by directly feeding and/or inducing behavioural responses on lower trophic level taxa (Heithaus et al. 2008). Examples of this have been documented worldwide, including shifting baselines in the Gulf of Mexico (Baum & Myers 2004), cascading effects in the Northwest Atlantic and Mediterranean oceans (Myers et al. 2007; Ferretti et al. 2008), and disruptive effects on food webs in the Pacific Ocean (Schindler et al. 2002). Despite these effects are often disputed (e.g. Kuker & Barrett-Lennard 2010; Grubbs et al. 2016), it is important to obtain accurate biological and ecological information from these vulnerable species to contribute to the effective assessment and monitoring of their populations.
For threatened wide-ranging marine species such as many sharks and their relatives, conservation efforts usually focus on critical habitat for reproduction (Roberts 2000; van der Hoek et al. 2015; Marie et al. 2017; Moore 2018), where young-of-the-year classes (YOY, i.e. individuals <1 year old) are born and live for the first period of their lives. Effective fishery management of YOY individuals is usually sought in coastal areas (e.g. inshore bays, estuaries and mangroves) that function as nurseries and typically provide them with greater food resources and lower predation risk (Castro 1993; Simpfendorfer & Milward 1993; Heupel & Simpfendorfer 2011; Morgan et al. 2017). Despite more rigorous definitions (Heupel et al. 2007) and theoretical perspectives (Heithaus 2007) about shark nurseries that have been introduced in recent times, and the relevance of other key stages for population stability (Kinney & Simpfendorfer 2009), it is still important to identify critical areas utilized by YOY individuals in order to protect them. This is especially valuable in parts of the world where data collection protocols are hardly implemented and accurate information for effective management is lacking.
The outer estuarine waters of the Río de la Plata support high abundance and richness of shark, ray and skate species (Jaureguizar et al. 2004, 2006, 2016; García et al. 2010; Colonello et al. 2014). These waters play a role in the foraging (e.g. Vögler et al. 2003; Barbini et al. 2011; Belleggia et al. 2012) and reproductive ecology (e.g. Mabragaña et al. 2002; Colonello et al. 2007) of some elasmobranchs, and have been suggested as a nursery ground for at least one shark species (narrownose smoothhound Mustelus schmitti, Massa 1998; Cortés et al. 2011). Evidence from grey literature (e.g. Menni & García 1985; Lagos 2001), fishers’ knowledge and personal observations indicate that the inshore areas – where the boundary between estuarine and costal shelf waters occurs – could be critical in the life cycle of many other vulnerable elasmobranch species (e.g. Sphyrna zygaena, Mustelus fasciatus), but this needs further attention and study. In particular, the occurrence of YOY elasmobranchs is underreported in studies previously conducted in the region, and as a result no ecological baseline data exists for this stage, including its trophic ecology.
The purpose of this study was to investigate the trophic ecology of YOY individuals of three elasmobranch species commonly found in the Southwest Atlantic (SWA) – namely broadnose sevengill shark Notorynchus cepedianus (Péron, 1807), angular angel shark Squatina guggenheim Marini, 1936 and smallnose fanskate Sympterygia bonapartii Müller & Henle, 1841 – to determine diet overlap among species and trophic levels. We test the hypothesis that the co-occurring species under study feed in the area and compete for food resources. Although diet and trophodynamics of these species have been previously analysed in the region, data are limited and correspond mainly to the adult fraction of the population (e.g. Crespi-Abril et al. 2003; Vögler et al. 2003; Lucifora et al. 2005). To the best of our knowledge, this is the first study providing the diet of YOY individuals in the outer estuarine waters of northern Argentina based on identification and stomach content analysis.
Methods
Sample collection
All samples came from the nearshore waters along the southern boundary of the Río de la Plata, a stretch of coast extending approximately 45 km between San Antonio and Punta Médanos lighthouses (Fig. 1). This nearshore area undergoes seasonal influence of the Río de la Plata discharge and supports a small-scale fishery, named as ‘trans-ecosystem’ fishery because of the fluctuating estuarine or marine predominant conditions that define the inter-annual variation in its catch species composition (Jaureguizar et al. 2015) and CPUE of at least one small shark (Mustelus schmitti, De Wysiecki et al. 2017). Fishermen use demersal gillnets (nylon 0.6 mm, mesh size 90–120 mm) to catch a range of fish resources from which at least 12 chondrichthyan species are usually caught as target or bycatch (see full list in Jaureguizar et al. 2015). As described by Jaureguizar et al. (2015), an average monthly fishing trip by season showed the following characteristics: distance to the coast where the nets were soaked ranged from 3443.1 ± 2048 m (spring) to 5106.7 ± 2197 m (autumn), the soak time ranged from 8.7 ± 8.1 h (winter) to 21.2 ± 4.5 h (summer) and the net length ranged from 375 ± 181.1 m (summer) to 691.6 ± 322 m (autumn).
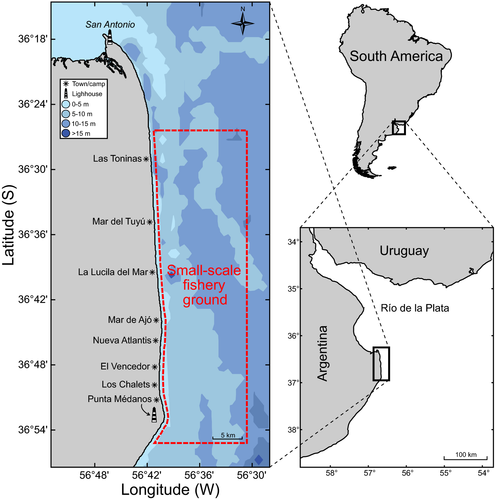
As a result of the lack of age and growth studies of elasmobranchs in the region, a visual criterion based on the presence of a visible yolk-sac wound – either in an open (neonate) or healed (non-neonate) condition – was used to identify young-of-the-year (YOY) elasmobranchs in the gillnet fishery landings. Specimens from different species were registered during three consecutive warm seasons (September–February, 2011–2013), among which N. cepedianus, S. guggenheim and S. bonapartii occurred in substantial numbers and were opportunistically collected, preserved (frozen at −20°C) and transported to the National Institute of Fisheries Research and Development (INIDEP, Argentina) for further analysis. Total length (LT, nearest mm), total mass (MT, nearest g) and sex were recorded. For diet estimates, stomach contents were identified to the lowest possible taxonomic level using taxonomic keys and specialist guidance. Prey items were counted and weighed to the nearest 0.1 g and trophic levels were assigned to each of them based on regional estimates (A.C. Milessi, unpubl. data, 2008).
Data analysis
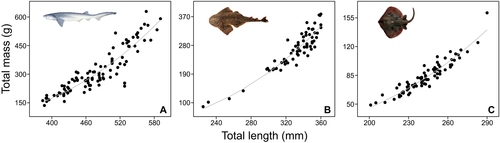
A conservative criterion was applied to avoid diet data from specimens that might be older than the YOY stage (i.e. individuals >1 year old), regardless of the presence of a visible yolk-sac scar. Specimens larger than half of the average between male and female LT50 (size at which 50% of individuals are mature) reported in the literature were discarded of our analysis. The LT50 values were 1800 mm LT for N. cepedianus (Irigoyen et al. 2018), 719 mm LT for S. guggenheim (Colonello et al. 2007) and 643 mm LT for S. bonapartii (Mabragaña et al. 2002). Therefore, based on our criterion, the final highest size threshold for a YOY stage was 900 mm LT for N. cepedianus, 359.5 mm LT for S. guggenheim and 321.5 mm LT for S. bonapartii. Once selected, data were first used to characterize growth of the YOY stage. The relationship between LT and MT for all species was estimated using a traditional non-linear regression model fitted by least squares following Kohler et al. (1995) as: 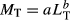 , where parameters a and b describe growth. Sexes were pooled as a preliminary analysis of covariance showed no differences in the non-linear regression parameters between males and females in all cases.
, where parameters a and b describe growth. Sexes were pooled as a preliminary analysis of covariance showed no differences in the non-linear regression parameters between males and females in all cases.
Diet of YOY individuals was described as percent by number (%N, the total number of each prey expressed as percentage of the total prey number), percent by mass (%M, the mass of each prey expressed as percentage of the total prey mass) and frequency of occurrence (%F, the number of stomachs containing each prey expressed as percentage of the total number of those containing any prey; Hyslop 1980). These three measures were then combined to calculate the Index of Relative Importance for each prey item/category i [IRIi = %Fi (%Ni + %Mi; Pinkas et al. 1971), expressed as a percentage of the total IRI for all prey or %IRI (Cortés 1997)], in order to determine the overall contribution of prey to the diet of each species. To prevent bias in stomach content measures, cases that regurgitated in situ were excluded from the analysis. Dietary composition between males and females was found to be similar in the three species (ANOSIM preliminary analysis, see below), hence data of the two sexes were pooled in each case. The questions of whether dietary composition was influenced by time (i.e. fishing season, year, month) or stage (neonate–non-neonate) were not addressed for any of the species because no substantial dietary data were available for any resulting time period or stage.
A one-way analysis of similarities (ANOSIM) was used to test for significant differences in dietary composition (based on broad prey categories) between species pooled across sampling periods. Similarity percentages (SIMPER) were then used to determine the dietary categories that contributed most to the dissimilarities found between groups (Clarke & Warwick 2001). For these analyses, dietary data (%M) were square-root transformed following White et al. (2004), and similarity matrices were constructed using the Bray–Curtis similarity coefficient. Finally, Schoener's index (SI) was used to evaluate the magnitude of interspecific food niche overlap between the species following Wallace (1981) as: 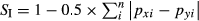 , where pxi and pyi are the proportion of prey category i in the diet of species x and y respectively. Diet overlap scoring greater than 0.6 indicates similar diet.
, where pxi and pyi are the proportion of prey category i in the diet of species x and y respectively. Diet overlap scoring greater than 0.6 indicates similar diet.
Further description of the trophic ecology of YOY included the mean trophic level (TL) for each species, calculated following Cortés (1999) as: 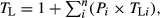 where Pi and TLi are the %M and trophic level of prey item/category i respectively. Margalef's index (RM) of species richness was also estimated for each species overall stomach contents following Margalef (1958) as: RM = (S − 1)−lnN, where S and N are the total number of prey items/categories and prey units respectively. All analyses were done with the statistical package R (R Core Team 2017).
where Pi and TLi are the %M and trophic level of prey item/category i respectively. Margalef's index (RM) of species richness was also estimated for each species overall stomach contents following Margalef (1958) as: RM = (S − 1)−lnN, where S and N are the total number of prey items/categories and prey units respectively. All analyses were done with the statistical package R (R Core Team 2017).
Results
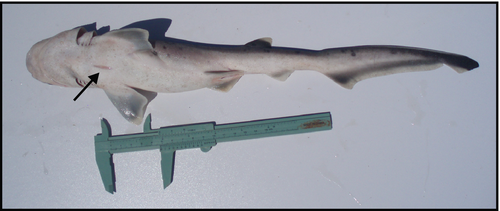
A total of 241 YOY individuals were sampled and analysed in this study. Number of specimens, length and mass measures, proportion of neonates and LT–MT regression coefficient estimates for the three species are shown in Table 1. Neonate specimens (i.e. present open yolk-sac wounds) occurred in more than 20% of the total in each species (Table 1). Length–mass regressions of YOY indicated positive (b > 3) allometric growth for N. cepedianus, S. guggenheim and S. bonapartii (Fig. 2 and Table 1). An example of an open yolk-sac wound observed during the study is presented in Figure 3. Other co-occurring species for which YOY individuals were also registered but in lower numbers included M. schmitti (n = 18), Galeorhinus galeus (n = 7), Myliobatis goodei (n = 4), Carcharhinus brachyurus (n = 2), Carcharias taurus (n = 2), Discopyge tschudii (n = 1), Pseudobatos horkelii (n = 1) and Sympterygia acuta (n = 1).
| Species | n (♀–♂) | LT range | MT range | Neonates | a (95% C.I.) | b (95% C.I.) | r |
|---|---|---|---|---|---|---|---|
| Notorynchus cepedianus | 99 (58–41) | 384–591 | 136–629 | 27.3 | 1 × 10−06 (−1 × 10−06–1 × 10−05) | 3.117 (2.825–3.409) | 0.915 |
| Squatina guggenheim | 64 (34–30) | 225–360 | 88–378 | 46.9 | 6 × 10−06 (−7 × 10−06–2 × 10−05) | 3.036 (2.646–3.426) | 0.957 |
| Sympterygia bonapartii | 78 (42–36) | 201–290 | 49–161 | 21.8 | 5 × 10−06 (−2 × 10−06–1 × 10−05) | 3.038 (2.788–3.289) | 0.939 |
- ♀ = females; ♂ = males; 95% C.I. = 2.5–97.5% confidence limits; r = Pearson’ coefficient of determination.
Diet analysis
Of the 240 stomachs examined, most contained food (93.3%), and only one case (N. cepedianus) was discarded because regurgitated contents in the field. The diet of the three species combined was comprised by 13 species, 9 genera, 10 higher taxa (five classes, one order, one suborder and three families) and unidentified remains, which defined five major prey categories: Fishes, Crustaceans, Molluscs, Polychaetes and Unidentified rest (Table 2).
| Prey category | Lowest taxonomic level | T L | Notorynchus cepedianus | Squatina guggenheim | Sympterygia bonapartii | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| %F | %N | %M | %IRI | %F | %N | %M | %IRI | %F | %N | %M | %IRI | |||
| Fishes | 83.51 | 82.57 | 95.17 | 96.08 | 23.29 | 22.22 | 33.13 | 20.42 | 0.62 | 0.10 | 2.17 | 0.02 | ||
| Carangidae | Trachurus lathami | 2.90 | 2.06 | 1.83 | 1.23 | 0.16 | – | – | – | – | – | – | – | – |
| Parona signata | 3.64 | 1.03 | 0.92 | 0.45 | 0.04 | – | – | – | – | – | – | – | – | |
| Clupeidae | Brevoortia aurea | 2.18 | 14.43 | 12.84 | 14.61 | 10.18 | – | – | – | – | – | – | – | – |
| Cynoglossidae | Symphurus spp. | 3.00 | – | – | – | – | 1.37 | 1.23 | 13.40 | 0.67 | – | – | – | – |
| Engraulidae | Engraulis anchoita | 2.73 | 1.03 | 0.92 | 0.42 | 0.04 | – | – | – | – | 0.62 | 0.10 | 2.17 | 0.02 |
| Gempylidae | Thyrsitops lepidopoides | 3.32 | 1.03 | 0.92 | 1.39 | 0.06 | – | – | – | – | – | – | – | – |
| Mugilidae | Mugil sp. | 2.61 | 1.03 | 0.92 | 3.71 | 0.12 | – | – | – | – | – | – | – | – |
| Paralichthyidae | Paralichthys spp. | 3.20 | – | – | – | – | 1.37 | 1.23 | 0.74 | 0.09 | – | – | – | – |
| Phycidae | Urophycis brasiliensis | 3.20 | 1.03 | 0.92 | 2.56 | 0.09 | – | – | – | – | – | – | – | – |
| Sciaenidae | Cynoscion guatucupa | 3.20 | 22.68 | 27.52 | 33.20 | 35.39 | 2.74 | 2.47 | 4.00 | 0.59 | – | – | – | – |
| Micropogonias furnieri | 3.19 | 1.03 | 0.92 | 0.71 | 0.04 | – | – | – | – | – | – | – | – | |
| Trichiuridae | Trichiurus lepturus | 3.83 | 1.03 | 0.92 | 1.09 | 0.05 | – | – | – | – | – | – | – | – |
| Remains | Actinopterygii | 3.00 | 31.96 | 29.36 | 30.23 | 48.93 | 17.81 | 17.28 | 15.00 | 19.07 | – | – | – | – |
| Chondrichthyes | 3.00 | 4.12 | 3.67 | 5.09 | 0.93 | – | – | – | – | – | – | – | – | |
| Rajidae | 3.00 | 1.03 | 0.92 | 0.47 | 0.04 | – | – | – | – | – | – | – | – | |
| Crustaceans | 4.12 | 3.68 | 1.80 | 0.15 | 57.53 | 60.49 | 47.00 | 68.73 | 98.15 | 99.71 | 96.32 | 99.96 | ||
| Diogenidae | Paguristes spp. | 2.57 | 1.03 | 0.92 | 0.08 | 0.03 | – | – | – | – | – | – | – | – |
| Loxopagurus loxochelis | 2.57 | – | – | – | – | 2.74 | 2.47 | 2.13 | 0.42 | – | – | – | – | |
| Penaeidae | Farfantepenaeus spp. | 2.30 | 1.03 | 0.92 | 0.67 | 0.04 | – | – | – | – | – | – | – | – |
| Artemesia longinaris | 2.30 | 1.03 | 0.92 | 0.08 | 0.03 | 20.55 | 24.69 | 14.49 | 26.71 | 40.74 | 39.40 | 48.91 | 58.15 | |
| Pinnotheridae | Pinnixa spp. | 2.50 | – | – | – | – | – | – | – | – | 15.43 | 3.11 | 3.55 | 1.66 |
| Sergestidae | Peisos petrunkevitchi | 2.30 | – | – | – | – | 26.03 | 25.93 | 19.33 | 39.08 | 1.24 | 0.20 | 0.40 | 0.01 |
| Serolidae | Serolis spp. | 2.30 | – | – | – | – | – | – | – | – | 1.24 | 0.20 | 0.06 | 0.01 |
| Solenoceridae | Pleoticus muelleri | 2.30 | – | – | – | – | 4.11 | 3.70 | 6.27 | 1.36 | 1.24 | 0.29 | 2.18 | 0.05 |
| Varunidae | Cyrtograpsus spp. | 2.57 | – | – | – | – | – | – | – | – | – | – | – | – |
| Remains | Malacostraca | 2.50 | 1.03 | 0.92 | 0.97 | 0.05 | 4.11 | 3.70 | 4.78 | 1.16 | 8.03 | 1.56 | 13.85 | 2.00 |
| Cumacea | 2.30 | – | – | – | – | – | – | – | – | 29.01 | 54.67 | 26.48 | 38.06 | |
| Gammaridea | 2.30 | – | – | – | – | – | – | – | – | 1.24 | 0.29 | 0.89 | 0.02 | |
| Molluscs | 10.31 | 11.01 | 2.62 | 3.61 | 1.37 | 1.23 | 3.08 | 0.20 | 0.62 | 0.10 | 0.20 | <0.0 | ||
| Nassariidae | Buccinanops spp. | 2.20 | 10.31 | 11.01 | 2.62 | 3.61 | 1.37 | 1.23 | 3.08 | 0.20 | – | – | – | – |
| Remains | Pelecypoda | 2.00 | – | – | – | – | – | – | – | – | 0.62 | 0.10 | 0.20 | <0.0 |
| Polychaetes | – | – | – | – | 6.85 | 6.17 | 3.24 | 2.14 | 0.62 | 0.10 | 1.32 | 0.01 | ||
| Glyceridae | Glyceridae | 2.00 | – | – | – | – | – | – | – | – | 0.62 | 0.10 | 1.32 | 0.01 |
| Maldanidae | Maldanidae | 2.00 | – | – | – | – | – | – | – | – | – | – | – | – |
| Remains | Polychaeta | 2.00 | – | – | – | – | 6.85 | 6.17 | 3.24 | 2.14 | – | – | – | – |
| Unidentified rest | – | – | 2.06 | 2.75 | 0.41 | 0.17 | 10.96 | 9.89 | 13.55 | 8.51 | – | – | – | – |
| Total number of stomachs (% containing food) | 98 (90.8) | 64 (85.9) | 78 (100.0) | |||||||||||
| Stomach mass range (g) | 1.4–67.7 | 1.7–44.8 | 0.7–9.1 | |||||||||||
| Margalef Richness Index (RM) | 3.65 | 2.33 | 1.44 | |||||||||||
| Trophic level (TL) | 3.92 | 3.58 | 3.31 | |||||||||||
- TL = trophic level.
Fishes were by far the most important components in N. cepedianus diet (96.08%IRI, Table 2). The primary prey species consumed included stripped weakfish Cynoscion guatucupa (Cuvier, 1830) and Brazilian menhaden Brevoortia aurea (Spix & Agassiz, 1829), although bony fish remains accounted for a greater importance value. Squatina guggenheim preyed most on crustaceans (68.73%IRI), and to a lesser extent, on fishes. In particular, shrimps (Peisos petrunkevitchi Burkenroad, 1945, and Artemesia longinaris Bate, 1888) comprised the bulk of the diet, accounting for nearly 34% of total mass. Sympterygia bonapartii fed almost exclusively on crustaceans (99.96%IRI), being A. longinaris and cumaceans the most important prey items. Unidentified rests were less important in all cases.
Dietary comparisons
The diet composition of the three sympatric species (all periods pooled) differed significantly (ANOSIM: R = 0.585, P = 0.001). The pairwise comparisons (SIMPER, percentage contribution to dissimilarity) showed that differences in diet between species were explained by a higher consumption of fishes by N. cepedianus compared to S. guggenheim (44.48%) and S. bonapartii (45.08%); by a higher consumption of crustaceans by S. guggenheim compared to N. cepedianus (36.48%) and fishes compared to S. bonapartii (25.97%); and a higher consumption of crustaceans by S. bonapartii compared to N. cepedianus (48.71%) and S. guggenheim (47.46%). Dietary overlap of broad prey categories was lower than 0.6 in the three cases: N. cepedianus–S. guggenheim (SI = 0.38), N. cepedianus–S. bonapartii (SI = 0.06) and S. guggenheim–S. bonapartii (SI = 0.52). Furthermore, stomach species richness was higher for N. cepedianus (3.65), intermediate S. guggenheim (2.33) and lower for S. bonapartii (1.44). Dietary information also indicated trophic levels corresponding to secondary consumption (all > 3.30), being N. cepedianus the top consumer (3.92) among the species analysed (Table 2).
Discussion
To the extent of our knowledge, we present for the first time quantitative diet information of YOY individuals of three elasmobranch species in the SWA. These species have been subjected to exploitation for decades and are currently classified as Endangered (S. guggenheim) or Data Deficient (N. cepedianus, S. bonapartii) by the International Union for Conservation of Nature. Hence, this study represents a substantial step towards increasing basic biological knowledge on the trophic roles and interactions of vulnerable elasmobranch fauna to improve management and conservation in the region.
Notorynchus cepedianus
The diet of YOY N. cepedianus was based on teleosts as previously observed in other regions of Argentina (Lucifora et al. 2005) and the world (Ebert 1991a, 2002; Braccini 2008) for this wide-ranging shark species. As part of a study in Anegada bay (40°S, ~650 km southwestwardly from the study area), Lucifora et al. (2005) analysed stomachs of a small number of young specimens (n = 12, <100 cm LT) identifying teleost remains as the main component of the diet. Here, based on a higher number of samples (n = 98), results add C. guatucupa and B. aurea as important prey items in northern areas (36–37°S). However, it is important to highlight that the choice of gillnets as sampling method may have played a part in overestimating diet results for these two prey, which are important components in the catch of the small-scale fishery sampled in this study (Jaureguizar et al. 2015). This is because sevengill sharks can feed upon entangled prey before getting caught, as observed in gillnet deployments conducted in California (Ebert 1991a) and Western Australia (Braccini 2008). Nonetheless, unidentified teleosts comprised most of the food contents as revealed in other studies. The accurate identification of prey consumed by N. cepedianus is particularly difficult as a result of the foraging mode adopted by the species that involves biting chunks of food (Ebert 1991b). No evidence of marine mammals was observed in the diet of YOY, which are important prey items for larger individuals (Crespi-Abril et al. 2003; Lucifora et al. 2005), but opportunistic scavenging (Ebert 1991b) on this prey category by YOY individuals is not discarded for the region.
Squatina guggenheim
The analysis of stomach contents of YOY S. guggenheim suggested that the diet is based on shrimp (Peisos petrunkevitchi and A. longinaris), and secondarily on teleosts. Previous qualitative (Vögler et al. 2003) and quantitative (Colonello 2005) studies in the region that included YOY specimens described the diet of this species as piscivorous, thus the results of this study are rather contrasting with the literature. Nonetheless, further work indicated that the distribution of angel sharks is highly subjected to environmental variation and hence the distribution of their prey (Vögler et al. 2008). This could explain the distinct diet composition obtained in our study because specimens come from different areas, seasons and years, meaning that availability of prey is likely to differ. Temporal changes in diet were also found for the Atlantic angel shark S. dumeril Lesueur, 1818 in the Gulf of Mexico (Baremore et al. 2010) and the Australian angel shark S. australis Regan, 1906 in southern Western Australia (Sommerville et al. 2011).
Sympterygia bonapartii
Diet analysis for YOY S. bonapartii indicated that it feeds on crustaceans, as was expected from previous studies in the region. In particular, A. longinaris and cumaceans dominated the diet of young individuals. This is consistent with a study conducted off Mar del Plata (~150 km southward from the study area), which documented a predominance of these components in the diet of young sizes (Barrera-Oro & Maranta 1996). However, crabs were important prey for young S. bonapartii in northern Patagonia, indicating spatial differences in diet probably due to prey availability (Estalles et al. 2016). Consumption of small crustaceans (e.g. shrimps, crabs, isopods and cumaceans) is also characteristic of young individuals of bignose fanskate S. acuta Garman, 1877 (Barbini & Lucifora 2016a) and from several other skates inhabiting the SWA coast. Some examples include zipper sand skate Psammobatis extenta (Garman, 1913; Braccini & Perez 2005; Barbini & Lucifora 2012a), Rio skate Rioraja agassizii (Müller & Henle, 1841; Barbini & Lucifora 2011), spotback skate Atlantoraja castelnaui (Miranda Ribeiro, 1907; Barbini & Lucifora 2012b) and eyespot skate A. cyclophora (Regan, 1903; Barbini & Lucifora 2016b). Therefore, preying on small crustaceans seems to be a wide-spread adaptation among early-life stages of these bottom-dwelling feeders in the region.
Further remarks
Our results indicate that the three elasmobranch species are active predators at a very young age in the nearshore waters along the southern boundary of the Río de la Plata. First, a very high proportion of stomachs with food content was found in this study, which is consistent with most YOY diet studies (e.g. Bethea et al. 2004; Elston et al. 2017). Young elasmobranchs have higher energy requirements for growth compared to larger individuals (Sims 1996), thus it is rather common to catch them with full stomach contents. Although, is important to mention that predation may have been stimulated during the study period (warm months, September–February) because warmer temperatures produce physiological increase in metabolism (Sims 2003; Bernal et al. 2012). Second, trophic levels indicate secondary consumer roles for all species based on different prey items, and significant differences in diet between the three species reflected contrasting habitat use from a bottom-dwelling to a more pelagic life.
Important proportions of neonates were observed in all species, suggesting that parturition events are probably occurring within the sampled area and in proximity to it. Since age information on YOY elasmobranchs is largely unknown in the SWA (only for M. schmitti, n = 9 individuals of age 0, Molina et al. 2017), a growth study of newborn scalloped hammerhead Sphyrna lewini (Griffith & Smith, 1834) conducted in captivity under stable conditions serves for comparison (Duncan & Holland 2006). In this study, the authors registered healed umbilical marks as soon as 14 days after birth, meaning that neonates identified by an open yolk-sac wound in our study are likely to be within a few weeks old. The occurrence of parturition events is probably related to the high productivity of the Río de la Plata estuarine ecosystem (Carreto et al. 2008), posing an advantage for the most energy-demanding stage of their life (Sims 1996). In the SWA, unlike for some teleost species, the association between highly productive areas and important egg-laying, parturition and nursing sites for elasmobranchs are only starting to be recognized. Recently, Vazquez et al. (2016) have shown that high densities of egg cases from at least four skate species occur within the Argentine shelf-break front, which coincide with chondrichthyans diversity hotspots in the region (Lucifora et al. 2012). Estuarine and marine front areas in the SWA are likely to sustain more food as opposed to other areas, offering similar services to YOY as those found in temperate estuaries of the world (e.g. Smale et al. 2015; Bangley et al. 2018). This is probably the main reason why these areas play a part during initial stages of many elasmobranch reproductive cycles. However, further research is necessary to address this hypothesis.
The utilization of a common area by YOY individuals from different elasmobranch species raise the question of whether they compete for food resources. Results indicated that the three species analysed in this study had limited overlap in major prey categories suggesting resource partitioning and limited interspecific competition for food in the area. Only the small shrimp A. longinaris was found to be largely consumed by both S. guggenheim and S. bonapartii for which the species may compete. However, this resource is highly abundant and widely distributed along the Río de la Plata area (Giberto et al. 2004), being more abundant in its outer waters where sampling of this study occurred. Therefore, limited competition for food may be a key for maximizing feeding and growth during the first year of life for co-occurring elasmobranch species within the Río de la Plata area.
We have discussed the trophic importance of the area for neonates and YOY of these species and also its relevance as a potential parturition zone in three consecutive warm periods. To the extent of our knowledge, only one study has identified the area as a shark nursery following the rigorous criteria introduced by Heupel et al. (2007). Based on research cruises data between 1993 and 2006, Cortés et al. (2011) demonstrated that immature M. schmitti are abundant in the Río de la Plata inshore area and persistently use it through seasons and years. Given this, this study represents a first step into considering further research in the area to potentially expand findings in Cortés et al. (2011) for N. cepedianus, S. guggenheim and S. bonapartii, and possibly other elasmobranch species. Conducting year-round surveys will allow us to address whether seasonal dietary patterns are occurring in the area which is a limitation of this study (i.e. no evidence of diet in autumn-winter period). Moreover, addressing multiple years surveys and exploring the occurrence of YOY individuals from other shark and ray species will help to define the area as a communal nursery if so. In addition to all species reported in this study, there is evidence of several other elasmobranch species that occur in small length sizes within the Río de la Plata estuarine area, including R. agassizii (minim. 240 mm LT, Barbini & Lucifora 2011), lesser guitarfish Zapteryx brevirostris (Müller & Henle, 1841; minim. 201 mm LT, Barbini et al. 2011), blotched sand skate P. bergi Marini, 1932 (minim. 110 mm LT, Barbini & Lucifora 2012a) and P. extenta (minim. 96 mm LT, Barbini & Lucifora 2012a). In any case, considering the interaction with local fisheries, it is important to promote co-management in the outer estuarine waters of the Río de la Plata to ensure effective conservation of endangered elasmobranch species that occur within the SWA.
Acknowledgements
We are grateful to the artisanal fishermen of Partido de La Costa that contributed towards the development of this study. We appreciate C. Silva, P. Casagrande and F. Cortés help during sampling. Federico Cortés kindly shared the photograph presented in Figure 3. Verónica García (Fundación Vida Silvestre) and two anonymous referees provided insightful comments that greatly improved an earlier version of this manuscript. This work was partially funded by Comisión de Investigaciones Científicas de la Provincia de Buenos Aires (CIC) and Instituto Nacional de Investigación y Desarrollo Pesquero (INIDEP), for whom we express our gratitude. This is an INIDEP contribution No 2139.




