Decomposition and nutrient release of grass and tree fine roots along an environmental gradient in southern Patagonia
Abstract
Decomposition of fine roots is a fundamental ecosystem process that relates to carbon (C) and nutrient cycling in terrestrial ecosystems. However, this important ecosystem process has been hardly studied in Patagonian ecosystems. The aim of this work was to study root decomposition and nutrient release from fine roots of grasses and trees (Nothofagus antarctica) across a range of Patagonian ecosystems that included steppe, primary forest and silvopastoral forests. After 2.2 years of decomposition in the field all roots retained 70–90% of their original mass, and decomposition rates were 0.09 and 0.15 year−1 for grass roots in steppe and primary forest, respectively. For N. antarctica roots, no significant differences were found in rates of decay between primary and silvopastoral forests (k = 0.07 year−1). Possibly low temperatures of these southern sites restricted decomposition by microorganisms. Nutrient release differed between sites and root types. Across all ecosystem categories, nitrogen (N) retention in decomposing biomass followed the order: tree roots > roots of forest grasses > roots of steppe grasses. Phosphorus (P) was retained in grass roots in forest plots but was released during decomposition of tree and steppe grass roots. Calcium (Ca) dynamics also was different between root types, since trees showed retention during the initial phase, whereas grass roots showed a slow and consistent Ca release during decomposition. Potassium (K) was the only nutrient that was rapidly released from both grass and tree roots in both grasslands and woodlands. We found that silvopastoral use of N. antarctica forests does not affect grass or tree root decomposition and/or nutrient release, since no significant differences were found for any nutrient according to ecosystem type. Information about tree and grass root decomposition found in this work could be useful to understand C and nutrient cycling in these southern ecosystems, which are characterized by extreme climatic conditions.
Introduction
Decomposition of fine roots is a fundamental process that relates to carbon and nutrient cycling in terrestrial ecosystems (Rao et al. 2001; Son & Hwang 2003). Fine root turnover may be faster than aerial components, and therefore fine root decay may provide one of the major inputs of organic matter and nutrients to soil (Fernandez & Caldwell 1975; Jackson et al. 1997; Rao et al. 2001; Son & Hwang 2003; Larreguy et al. 2012). Analysis of global data concluded that root decomposition can be fairly well predicted by a combination of litter quality and environmental parameters (Silver & Miya 2001). Moreover, it was reported that latitude may explain 45% of the variability in fine root decomposition in graminoids and broadleaf species (Silver & Miya 2001). However, to date the relative importance of litter quality and environmental factor determinants of root decomposition in austral Patagonian ecosystems is unknown and information to solve this question is missing or scarce (Silver & Miya 2001; Bontti et al. 2009; Hobbie et al. 2010).
In Patagonia, Argentina, Nothofagus antarctica (ñire) native forest distributes in a narrow strip with an extensive area from 36° 30′ to 56° 00′SL. In general, these forests are occasionally used for timber production, and more often as silvopastoral systems, where the understory vegetation is grazed by cattle and sheep (Peri & Ormaechea 2013). More to the east, an extensive area named the ‘Patagonian steppe’ (85% of the Santa Cruz province), is characterized by natural rangelands that are used for extensive sheep production in large paddocks (2000–5000 ha) on a year-round basis, with stocking rates ranging from 0.13 to 0.75 head ha−1 year−1 (Cibils & Coughenour 2001). These two contrasting ecosystems (forest and steppe) span a precipitation gradient that decreases from west to east, but have in common the cold semi-arid climate with high wind speeds, principally from the S-SW and water deficits in summer. It is known that in arid and semi-arid grasslands, belowground components are an important fraction of the total C assimilated (Milchunas & Lauenroth 2001; Peri & Lasagno 2010) and could be characterized by slow decomposition rates (Gill et al. 2002). In the same way, N. antarctica trees allocate higher proportions of biomass to roots in sites with low fertility or water deficit (Gargaglione et al. 2010). These two functional groups (grasses and trees) may differ in their decomposition rates and nutrient release due to contrasting chemical composition of their respective fine roots, differences in root diameter (Fan & Guo 2010; Sun et al. 2013) or due to dissimilar environmental conditions (climate and soil) since steppe is drier than forest. However, in these austral sites, the high latitude, which includes a complex interaction of limiting factors related to climate, soil and biological factors under cold arid conditions may overlap the impacts of other factors that affect fine root decomposition in other biomes. For this reason, we made an assay comparing fine root decomposition and nutrient release of (i) grasses in the steppe; (ii) grasses and N. antarctica fine roots in primary forests and (iii) grasses and tree fine roots decomposing in open forest (silvopastoral system). We hypothesized that (i) root decomposition of grasses would be slower in the steppe ecosystem compared with primary forests; (ii) root decomposition of trees and grasses would be faster in forests with more open canopy conditions (e.g. silvopastoral systems) compared with primary forests; (iii) in forests, root nutrient release would be different between grasses and trees.
Material and Methods
Study sites
The study was carried out in the southern region of Santa Cruz, an Argentinian province located in Patagonia, in two different biomes: the Patagonian steppe and the Nothofagus antarctica forests. The driest site corresponded to the Patagonian steppe, where three sampling points were located as replicates, two in Los Pozos ranch (51° 29′ 53′′ S; 69° 19′34.5′′ W; and 51° 17′49.8′′ S; 69° 14′ 56.5′′ W) and one in INTA Potrok Aike experimental site (51° 56′ 57′′ S; 70° 24′ 42′′ W). In this area, called ‘Dry Magellanic Steppe (DMS)’, the mean long-term annual precipitation is 235 mm year−1 and the mean air temperature (MAT) is 7.1°C. The vegetation is dominated by grasses such as Festuca gracillima and short grasses such Poa spiciformis, Rytidosperma virescens and Carex andina. Soils generally have sandy texture and are generally aridisols and mollisols.
The others sites used in this study were located in the western part of Santa Cruz province, at the base of the Andes mountains, where N. antarctica forest predominate. Here three different sites (replicates) were also selected: Morro Chico ranch (51°57′ 24″ SL; 71°31′ 48″ WL), Cancha Carrera ranch (51° 13′ 21 SL″; 72° 15′ 34″ WL) and Tres Marías ranch (51° 19′ 05 SL′′; 72° 10′ 47 WL′′), where mature (120–150 years) N. antarctica stands were selected. The mean annual precipitation in this area is 390 mm year−1 and the MAT is around 4.9°C. The soils are mollisols and inceptisols with sandy loamy texture and slightly acid pH. Understory vegetation is dominated by grasses such as Agrostis capillaris, Bromus setifolium, Dactylis glomerata, Deschampsia flexuosa, graminoids of the genus Carex and, in lower proportion, Festuca gracillima. In these forest sites, two different use conditions were evaluated: forest under silvopastoral use (SPF) with a crown cover around 50–60% which are grazed by cattle or sheep; and primary forest (PF) with higher crown cover (80–90%) and without grazing by domestic animals.
Environmental measurements
Five composite samples of five soil cores were randomly taken (to 30 cm depth) to characterize soil at each site. Samples were air dried and transported to a laboratory to determine: (i) organic carbon (C) with spectrophotometry according to Kumies after wet oxidation in acid medium (Houba et al. 1988); (ii) total nitrogen content (N) determined by the semi-micro Kjeldahl method (Sparks 1996); (iii) available phosphorus content (P) using the Truog method (Sparks 1996); (iv) exchangeable potassium (K) determined using saturation with sodium acetate, washed with ethylic alcohol, displacement through pH 7 buffered ammonium acetate. The pH was obtained by potentiometric measurement in a water saturated paste. The determination of texture was carried out through the densimeter method of Bouyoucos and sieving the sand fractions.
At each site, air and soil temperatures were measured continuously every 2 h with a data logging system (HOBO H8 Family, Onset Computer Corporation, USA). Sensors of air temperature were placed at 0.6 m height from ground level. Soil temperature was measured at 3 cm depth using soil thermometers (HOBO, Model TMC50-HA, USA). At each decomposition sampling date, the volumetric water content (0–20 cm) was measured in each study site using a soil moisture meter Time Domain Reflectometry proven precision equipment (TDR flag Eijkelkamp, Model FM-3-14.62, Santa Barbara, USA).
Decomposition measurements
In steppe, SPF and PF five quadrants of 20 × 40 cm of grasses were excavated to 30 cm of depth and grass root samples were collected from their natural growing sites. The N. antarctica fine roots (<2 mm diameter) were collected in five quadrants in a 70–80 years old pure forest and also from a mature stand under silvopastoral use. In the laboratory, roots were separated from the soil by gently washing them with deionized water. Then, roots were air dried to constant weight, cut into 10 cm long pieces and enclosed in 15 × 20 cm bags (5 g per bag) of polyethylene gauze of 0.35 mm mesh. Five subsamples of roots were weighed and oven dried at 60°C to calculate initial weight of the incubated material and to analyse lignin, C, N, P, K and Ca concentrations for initial conditions. Lignin was determined using the Klason method with sulphuric acid in Ancom system (Theander et al. 1995). C was determined by dry combustion with an elemental analyzer (Leco, model CR-1, USA) and N was determined by semimicro Kjeldahl. Concentrations of P, K and Ca were determined with a plasma emission spectrometer (Shimadzu ICPS-1000 III, Japan).
In November 2012, bags containing steppe grass roots were placed in a plot of 1 m2 in the steppe (8 bags × 3 replicates = 24 bags). In the forest, bags containing forest grass roots from SP were buried in SP forest and grass roots from PF were buried under PF (8 bags × 2 conditions × 3 replicates = 48 bags). The same procedure was followed for tree fine roots. All bags were buried horizontally (10 cm deep) and covered with the removed soil. Samples were collected after 60, 160, 290, 380, 465, 660, 735 and 820 days of decomposition. At each retrieval time, samples were taken to the laboratory where material was removed from the bags, cleaned to remove soil, weighed fresh and then were dried at 60°C for 48 h and then weighed again. Organic matter proportion was determined by loss on ignition (4 h, 500°C), thus inorganic contaminants (mainly soil particles) were also excluded. Additionally, samples were analysed for macro nutrients (N, P, K and Ca). The absolute amount of organic matter and each nutrient were calculated by multiplying the concentration by the corresponding remaining dry mass. The nutrient mass data obtained in each extraction date were expressed as a percentage of the initial values on an ash-free basis.
Data analysis
The decomposition rate (k) was calculated from the percentage of dry mass remaining using an exponential decay model proposed by Olson (1963): mt/m0 = exp (−kt), where mt is the mass at time t, m0 is the estimated initial mass, the constant k is the decomposition coefficient and t is the elapsed time (year−1). With this decay model, we used all data from all plots to calculate mean decomposition constants (k). One-way ANOVA was used to determine the effect of site (steppe, SPF and PF) on grass root decomposition constants (k). Two-way ANOVA was used to determine the effect of root type (grass or tree), forest use (SPF or PF) and their interactions on k in forest sites. Significant differences were analysed using Tukey tests (P < 0.05).
Dynamic of nutrients over time was analysed with repeated measures-ANOVA with the site as between-subject factor and each sampling date as within-subject factor. This analysis was done because the decomposition values are not independent of time. Bonferroni tests were performed to test differences among ecological areas when F-values were significant (P < 0.05). Multiple comparisons were made to detect inter-subject effects (ecological area) for each sampling date. To verify if environment variables influence on decomposition or nutrient release, Pearson correlation analysis was made (P < 0.05).
Results
Soils and root characteristics
Soils from all sites had a sandy texture with pH values between 5.8 and 6.3 (Table 1). No significant differences were found for K, C and N between sites (Table 1). In contrast, significant differences were found in P, where the primary forest had the highest value (43.14 ppm) while steppe had the lowest values (10.6 ppm) (Table 1). The C:N ratio of soils also presented significant differences between sites: forest soils had values around 13–14 and steppe soils had values of around 4.7 (Table 1).
| Site | K (meq/100 g) | C (%) | N (%) | C:N | P (ppm) | pH |
|---|---|---|---|---|---|---|
| Primary forest | 1.21 (0.29) | 7.74 (2.28) | 0.55 (0.20) | 14.23 (1.45) a | 43.14 (3.0) a | 5.89 (0.36) |
| Silvopastoral forest | 1.09 (0.52) | 6.57 (4.86) | 0.50 (0.38) | 13.03 (0.55) a | 23.24 (25.8)ab | 5.84 (0.14) |
| Steppe | 0.33 (0.14) | 1.53 (0.68) | 0.36 (0.18) | 4.73 (1.92) b | 10.6 (3.2) b | 6.27 (0.31) |
| Significance (P < 0.05) | ns | ns | ns | ** | * | ns |
- *P < 0.05; **P < 0.01; n = 5. ns, no significant differences.
In forests, no significant differences were found in initial characteristics between roots from PF and SPF for both, grass roots and tree roots (Table 2). Likewise, there were no significant differences in initial characteristics of root types (tree or grass) or sites (steppe or forest) for most nutrient, with the only exception of K, which had significant higher concentrations in forest grass roots compared with steppe roots and tree roots (Table 2). Tree roots had higher lignin:N ratio compared with grass roots (40–44 vs. 10–15) although these differences were not significant, probably due to the high deviations that tree roots presented (Table 2).
| Site | Lignin (%) | C (%) | N (%) | Lignin:N | C:N | P (%) | N:P | K (ppm) | Ca (ppm) |
|---|---|---|---|---|---|---|---|---|---|
| PF grass roots | 12.45 (0.07) | 40.46 (0.27) | 1.11 (0.05) | 11.2 (0.45) | 36.4 (1.4) | 0.07 (0.007) | 16.9 (0.9) | 5202 a (158) | 7658 (205) |
| SPF grass roots | 12.05 (0.31) | 40.42 (0.21) | 1.15 (0.07) | 10.9 (0.33) | 35.15 (1.50) | 0.08 (0.01) | 14.37 (0.06) | 5130 a (170) | 7521 (450) |
| PF Tree roots | 17.20 (7.07) | 45.43 (0.76) | 0.47 (0.22) | 44.9 (30) | 96.7 (20.9) | 0.18 (0.13) | 8.0 (6.5) | 3911 b (317) | 6488 (3307) |
| SPF tree roots | 19.0 (5.01) | 45.05 (0.31) | 0.55 (0.11) | 40.1 (12.5) | 81.9 (19.5) | 0.22 (0.11) | 5.0 (2.3) | 3750 b (520) | 6792 (1150) |
| Steppe grass roots | 10.05 (0.49) | 42.13 (1.94) | 0.70 (0.20) | 14.8 (3.56) | 60.2 (19.8) | 0.12 (0.07) | 5.8 (4.0) | 3958 b (178) | 4300 (318) |
| ns | ns | ns | ns | ns | ns | ns | * | ns |
- *P < 0.05; **P < 0.01. ns, no significant differences.
Climatic variables
Significant differences in mean air temperature (MAT) were found across the year (P < 0.001) with the highest values being detected in summer (10.5°C) and minimum values in wintertime (1.8°C) (Figure 1a, Table 3). Significant differences in MAT were also found among sites (P = 0.0007), with a higher MAT (8.12°C) in steppe than in forest (7.04°C) (Figure 1a, Table 3). A similar tendency was found for Maximum Air Temperature (MaxT). Steppe had significantly higher values (14.4°C) than forest (13.1°C) (Figure 1b, Table 3). In contrast, no significant differences were found in mean soil temperature (Table 3) with values of 8.1 and 7.8°C for steppe and forests, respectively (Figure 1c). Lastly, significant differences were found in volumetric soil water content according to site, where the forest had higher values (around 12%) than steppe sites (5%) (Table 3).
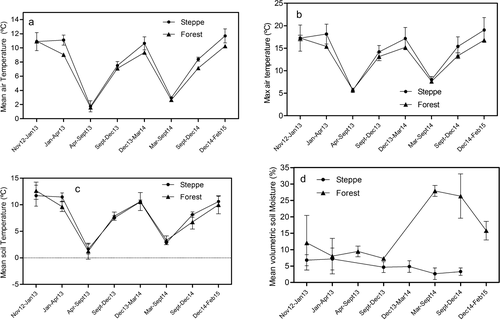
| Mean air temperature | |||||
|---|---|---|---|---|---|
| Variability source | Total Square sum | Freedom degrees | Mean square | F value | P value |
| Model | 357.97 | 15 | 23.86 | 44.85 | <0.0001 |
| Period | 339.49 | 7 | 48.5 | 91.14 | <0.0001 |
| Site | 9.25 | 1 | 9.25 | 17.37 | 0.0007 |
| Tukey test α = 0.05 | |||||
| Site | Mean | N | Significant differences | ||
| Forest | 7.04 | 16 | a | ||
| Steppe | 8.12 | 16 | b | ||
| Period | |||||
| Apr–Sep 13 | 1.77 | 4 | a | ||
| Mar–Sept 14 | 3.13 | 4 | a | ||
| Sep–Dec 13 | 7.13 | 4 | b | ||
| Sept–Dec 14 | 7.27 | 4 | b | ||
| Dec 13–Mar 14 | 9.89 | 4 | c | ||
| Jan–Apr 13 | 10.03 | 4 | c | ||
| Dec 14–Feb 15 | 10.48 | 4 | c | ||
| Nov 12–Jun 13 | 10.97 | 4 | c | ||
| Max air temperature | |||||
|---|---|---|---|---|---|
| Variability source | Total Square sum | Freedom degrees | Mean square | F value | P value |
| Model | 601.89 | 15 | 40.13 | 17.75 | <0.0001 |
| Period | 580.02 | 7 | 82.86 | 36.65 | <0.0001 |
| Site | 13.26 | 1 | 13.26 | 5.87 | 0.0277 |
| Tukey test α = 0.05 | |||||
| Site | Mean | N | Significant differences | ||
| Forest | 13.1 | 16 | a | ||
| Steppe | 14.4 | 16 | b | ||
| Period | Mean | N | |||
| Apr–Sept 13 | 5.7 | 4 | a | ||
| Mar–Sept 14 | 7.83 | 4 | a | ||
| Sept–Dec 13 | 13.68 | 4 | a | ||
| Sept–Dec 14 | 14.38 | 4 | b | ||
| Dec 13–Mar 14 | 16.18 | 4 | bc | ||
| Jun–Apr 13 | 16.78 | 4 | bc | ||
| Nov 12–Jun 13 | 17.25 | 4 | bc | ||
| Dec 14–Feb 15 | 17.93 | 4 | c | ||
| Mean soil temperature | |||||
|---|---|---|---|---|---|
| Variability source | Total Square sum | Freedom degrees | Mean square | F value | P value |
| Modelo | 420.35 | 15 | 28.02 | 20.75 | <0.0001 |
| Period | 413.4 | 7 | 59.06 | 43.73 | <0.0001 |
| Site | 0.72 | 1 | 0.72 | 0.53 | 0.4759 |
| Tukey Test α = 0.05 | |||||
| Period | Mean | N | Significant differences | ||
| Apr–Sept 13 | 1.45 | 4 | a | ||
| Mar–Sept 14 | 3.08 | 4 | a | ||
| Sept–Dec 14 | 7.45 | 4 | b | ||
| Sept–Dec 13 | 7.68 | 4 | bc | ||
| Dec 14–Feb 15 | 10.3 | 4 | cd | ||
| Jun–Apr 13 | 10.55 | 4 | d | ||
| Dec 13–Mar 14 | 10.58 | 4 | d | ||
| Nov 12–Jun 13 | 12.18 | 4 | d | ||
| Mean volumetric soil moisture | |||||
|---|---|---|---|---|---|
| Variability source | Total Square sum | Freedom degrees | Mean square | F value | P value |
| Modelo | 735.87 | 6 | 122.65 | 2.38 | 0.0745 |
| Site | 316.83 | 1 | 316.83 | 6.15 | 0.0239 |
| Period | 419.04 | 5 | 83.81 | 1.63 | 0.2063 |
| Tukey test α = 0.05 | |||||
| Site | Mean | N | Significant differences | ||
| Forest | 12.25 | 16 | a | ||
| Steppe | 4.98 | 16 | b | ||
Root decomposition
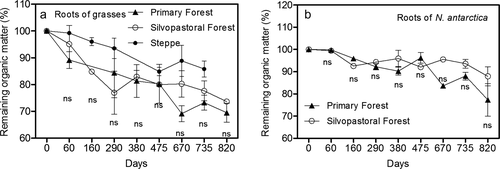
After 2.2 years of decomposition in the field, remaining mass for grass roots was around 69% in primary forest, 74% in the silvopastoral forest and 86% in steppe (Figure 2a). Significant differences between sites (P = 0.0247) were found in decomposition rates (k) (Table 4). According to our hypothesis (i), roots of grasses in the steppe exhibited the slowest decomposition rate (0.09 year−1), whereas roots of grasses in primary forests had the highest (0.15 year−1). The time of 50% decomposition of grasses in the primary forest was 4.9 years, while the time of 50% decomposition of roots in steppe was 8 years (Table 5). Likewise, the time of 95% decomposition ranged from 21 years for roots in primary forests to 34 years for roots in steppe (Table 5).
| ANOVA of decomposition constants (k) of root grasses according to site | |||||
|---|---|---|---|---|---|
| Variability source | Total square sum | Freedom degrees | Mean square | F value | P value |
| Model | 0.01 | 4 | 2.70E-03 | 10.74 | 0.0205 |
| Site | 0.01 | 2 | 2.70E-03 | 10.73 | 0.0247 |
| Error | 1.00E-03 | 4 | |||
| Tukey test α = 0.05 | |||||
| Error | 0.0002 | ||||
| Site | Mean | N | Significant differences | ||
| Steppe | 0.09 | 3 | a | ||
| SPF | 0.11 | 3 | ab | ||
| PF | 0.15 | 3 | b | ||
| ANOVA of decomposition constants (k) according to root types and forest condition in forest sites | |||||
|---|---|---|---|---|---|
| Variability source | Total square sum | Freedom degrees | Mean square | F value | P value |
| Model | 0.02 | 4 | 4.10E-03 | 6.04 | 0.020 |
| Forest condition | 1.70E-03 | 1 | 1.70E-03 | 2.42 | 0.163 |
| Root type | 0.01 | 1 | 0.01 | 13.58 | 0.008 |
| Error | 4.80E-03 | ||||
| Tukey test α = 0.05 | |||||
| Error | 0.0007 | ||||
| Forest condition | Mean | N | Significant differences | ||
| SPF | 0.09 | 6 | a | ||
| PF | 0.11 | 6 | a | ||
| Root type | |||||
| Tree roots | 0.07 | 6 | a | ||
| Grass roots | 0.13 | 6 | b | ||
| Grasses decomposing in different sites | |||
|---|---|---|---|
| k | T 50 | T 95 | |
| Steppe | 0.09 a | 8.0 a | 34.5 a |
| Silvopastoral forest | 0.11 ab | 6.9 ab | 30.0 ab |
| Primary forest | 0.15 b | 4.9 b | 21.1 b |
In forests, our hypothesis (ii) was rejected because no significant differences were found in k between PF and SPF in both grasses and tree roots (Table 4). In contrast, tree roots decomposed slower (k = 0.07 year−1) (P = 0.008) than grass roots (k = 0.13 year−1) (Table 4). The time of 50% decomposition of roots grasses was 5.9 years, while the time of 50% decomposition of tree roots was 11.2 years (Table 6).
| Root type | k | T 50 | T 95 |
|---|---|---|---|
| Ñire roots | 0.07 a | 11.2 a | 85.3 a |
| Grass roots | 0.13 b | 5.9 b | 25.6 b |
From all the environment variables evaluated, only the volumetric soil moisture content had a significant effect on the remaining mass in the forest (P = 0.03) (Table 7).
| Primary forest grasses | Silvopastoral forest grasses | Forest tree roots | |||
|---|---|---|---|---|---|
| Soil Moisture | MAT | MAT | MaxAT | Soil Moisture | |
| OM | −0.55 (0.03) | ns | ns | ns | ns |
| N | −0.7 (0.0027) | ns | ns | ns | ns |
| Ca | ns | 0.54 (0.03) | 0.51 (0.04) | 0.54 (0.03) | 0.54 (0.03) |
Nutrient release during grass roots decomposition
No significant differences in N release during root decomposition were found between forest and steppe or between PF and SPF (Table 8). Initially, N was immobilized by microorganisms, and after 2.2 years, around the 90–100% of the original N was still present in the undecomposed root material (Figure 3a). In contrast, significant differences (P < 0.001, Table 8) were found for P release during fine root decomposition among sites (Figure 3c). In general, grass roots decomposing in steppe had a fast P release at the beginning (47% of the initial P content lost by 30 days) and only 39% of the initial P content remained in undecomposed root biomass after 2.2 years (Figure 3c). Decomposing grass roots in forests showed P retention during the entire evaluation period, with values around 100% (Figure 3c). Significant differences were found according to forest type at 290 and 380 days of incubation, where SPF had lower P amounts than PF in the undecomposed fraction of root biomass (Figure 3c), but these differences disappeared at the end of the decomposition period (820 days).
| Nitrogen | Phosphorus | Potassium | Calcium | |||||
|---|---|---|---|---|---|---|---|---|
| F value | P value | P value | P value | F value | P value | F value | P value | |
| Grass-roots | ||||||||
| Sites (Steppe, SPF and PF) | 0.21 | 0.817 | 63.85 | <0.001 | 0.74 | 0.515 | 0.94 | 0.44 |
| Tree roots | ||||||||
| Sites (SPF and PF) | 1.25 | 0.327 | 2.68 | 0.177 | 0.43 | 0.548 | 5.13 | 0.086 |
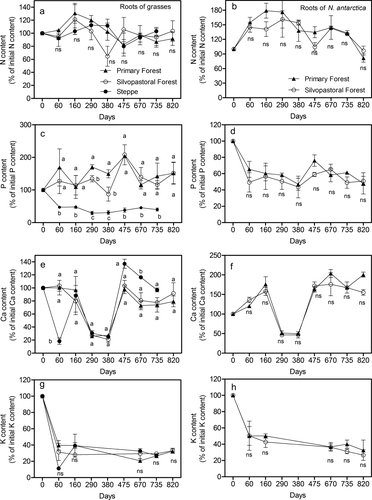
Calcium release showed different fluctuations over the 820 days (Figure 3e). In forest, no significant differences were found between SPF and PF (Table 8), showing a Ca release at 290 and 380 days with a remaining mass of Ca around 30%. Then, at the end of the 820 days, Ca content in the undecomposed root biomass was 80% of the initial Ca content (Figure 3e). Grasses in steppe showed a similar tendency but differed significantly at 60 and 670 days of decomposition, with lower and higher values than forest grasses, respectively (Figure 3e, Table 8). For K release during decomposition, no significant differences were found between grass roots in steppe and forests (Table 8), with a fast release after 60 days and subsequent K content in undecomposed root biomass of 30% of initial values for the remainder of the incubation period (Figure 3g).
Significant correlations were found for the release of some nutrients in forest grass roots and environmental factors. For example, N release was strongly correlated with soil moisture (r = −0.7), whereas Ca release was correlated with mean (r = 0.51) and maximum air temperature (r = 0.54) (Table 7). In contrast, no significant correlations were found between environmental factors and nutrient release for steppe grasses.
Nutrient release during tree fine roots decomposition
Nutrient release during decomposition did not differ for tree fine roots among forest types (Table 8). For N, there was evidence of N retention with the exception of the last sampling date (820 days), at which time some N release was observed. At the end of the 820 day period the undecomposed biomass retained around 80% of the initial N content (Figure 3b). A different pattern was observed for P, which had a high initial release (40–50%) after 60 days, whereas release rate slowed down as time progressed (Figure 3d). Ca release pattern in forest roots showed a similar tendency to forest grasses, with some immobilization at the beginning, Ca release at 290 and 380 days of incubation (50%) and then immobilization again, from 475 days to the end (Figure 3f). For K, the nutrient retention was the lowest. K was released fast at the beginning (60%) and only 20–30% of the initial K content was retained in undecomposed biomass at the end of the evaluation period (Figure 3h).
A weak positive correlation between Ca release and soil moisture in primary forest was observed (Table 7).
Differences in nutrient release between grasses and trees in forest sites
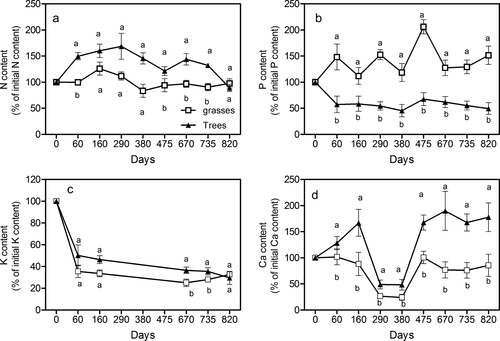
Both tree and grass roots tended to retain N across the decomposing period, but significant differences were found at 60, 475, 670 and 735 days, where tree roots had higher N retention values than grass roots (Figure 4a, Table 9). However, both root types had similar values of N remaining at the end of the decomposition period (around 90%) (Figure 4a). In the case of P, significant differences were found between grasses and trees across all sample dates (Table 9), where grasses showed P retention or even immobilization at all sampling time (remaining values around 140–150%), whereas trees showed evidence of P release especially during the early stages of the incubation period (57% of P remaining at 60 days) (Figure 4b). K release pattern was similar for tree and grass roots, but this release was significantly higher in the grass roots at 670 and 735 days (Figure 4c, Table 9). Significant differences were found in Ca release pattern between grass and tree roots across the complete 820 days (Table 9). In this case, tree roots showed a strong retention or even immobilization during the initial period (160% of the remaining Ca after 160 days), whereas grass roots showed a slow Ca release (88% of the remaining Ca after 160 days) (Figure 4d). Both type of roots released some amount of Ca between 290 and 380 days followed by another period in which little Ca was liberated (Figure 4d). At the end of the experiment (820 days) undecomposed grass and tree roots had 85% and 177% of the initial Ca content, respectively (Figure 4d).
| Days of decomposition | Nitrogen | Phosphorus | Potassium | Calcium |
|---|---|---|---|---|
| 60 | 0.003 | 0.011 | 0.520 | 0.008 |
| 160 | 0.055 | 0.049 | 0.326 | 0.040 |
| 290 | 0.151 | <0.0001 | – | 0.003 |
| 380 | 0.100 | <0.0001 | – | 0.013 |
| 475 | 0.019 | <0.0001 | – | <0.0001 |
| 670 | 0.028 | 0.018 | 0.022 | 0.001 |
| 735 | 0.001 | 0.001 | 0.011 | <0.0001 |
| 820 | 0.044 | 0.002 | 0.955 | <0.0001 |
- (–) Indicate data missing.
Discussion
Nutrient concentrations in grass roots and tree fine roots
Initial concentrations of N, P, K and Ca in steppe grass roots were similar to those reported by Peri and Lasagno (2010) for roots of Stipa chrysophylla growing in steppe near this study site. Likewise, Moretto and Distel (2003) reported similar values of N, P and C:N values for two species (Poa ligularis and Stipa gynerioides) in arid grasslands of La Pampa, in central Argentina. In contrast, Vivanco and Austin (2006) working with P. ligularis in Northern Patagonia reported higher initial values of N (1.04%) and P (0.11%) than those found in this study. Nitrogen concentrations in roots of grasses growing in forests sites in this study are similar to those presented by Gargaglione et al. (2014) for grasses in N. antarctica forest under silvopastoral use in south Patagonia. In the case of N. antarctica roots, values of P, K and Ca were similar of those presented by Peri et al. (2006), whereas N values found in this study were higher. Nothofagus antarctica nutrient concentration in plant organs generally reflect site fertility (Gargaglione et al. 2013).
Root decomposition
Decomposition rates of grass roots found in this work were lower than those reported for Moretto and Distel (2003) for grass species in arid grasslands in central Argentina, with similar precipitation but higher mean air temperatures (15°C) than our sites. Likewise, k values in our study (0.09–0.15 year−1) were lower than those reported for temperate grasslands (0.27 year−1) in Germany (Solly et al. 2014) and lower than a group of species (0.38 year−1) in middle latitude areas (≥30°) in a global analysis (Zhang & Wang 2015). One factor that could explain this discrepancy is the relatively cold and dry conditions in these ecosystems. Parton et al. (2007) observed that leaf and root decomposition were slowest in cold dry regions such as tundra and boreal forests and fastest in tropical regions. It is possible that low temperatures in our extreme southern sites, combined with aridity, limited microorganism's activity (Couteaux et al. 1995; Aerts 1997) and in consequence reduced decomposition rates. This confirms our hypothesis that root decomposition of grasses was slower in the steppe ecosystem compared with native forests. Many authors support that decomposition is influenced by ambient conditions and for the quality of the material (i.e. Aerts 1997; Silver & Miya 2001; Bontti et al. 2009). In our case, because we did not find differences in quality between roots of grasses from steppe and forest, so probably grass roots decomposed faster in forests due to moister conditions, since steppe presents more aridity than forest (Kreps et al., 2012).
There are no published data about Nothofagus root decomposition. However, values found in this work (71–88% mass that remained undecomposed) were similar to those reported for fine roots (<3 mm) of Fagus sylvatica in forests of Germany (Scheu & Schauermann 1994) and for Picea abies forest in eastern Finland (Palviainen et al. 2004) after 3 years of decomposition. Root decomposition was not influenced by the use of the forest, with similar values in PF and SPF for both grasses and trees fine roots. In contrast, decomposition rates of trees roots were slower than grasses probably due to differences in root diameter (Fan & Guo 2010; Sun et al. 2013). In addition, the higher initial lignin content of N. antarctica roots (17%) compared with grass roots (12%) may control fine root decomposition rate (i.e. Zhang & Wang 2015). This could also be due to higher C:N ratios of tree roots (60 vs. 36) that are known to negatively affect decomposition. In concordance with our results, Solly et al. (2014) reported higher decomposition rates in grasslands (0.21 year−1) than in Fagus sylvatica forests (0.09 year−1) in temperate ecosystems in Germany.
Nutrient release in decomposing grass roots
Grass roots decomposing in steppe and forests showed strong N retention for most of the evaluation period. The initial C:N ratios of grass roots found in this study were 36 in forest and 60 in steppe, respectively. These values are in the range where immobilization occurs according to the theory of the ‘critical value’ (Kaye & Hart 1997). This theory posits that when detritus C:N ratio is above of 30, heterotrophic microorganisms are N limited and acquire N from sources exogenous to the litter and convert it to microbial biomass or exoenzymes. In contrast, when C:N ratio from detritus is below of 30, in general microbes can meet their N requirements directly from the litter they are consuming and net N mineralization occurs (i.e. Kaye & Hart 1997). There are no studies of grass root decomposition for these southern latitudes, but Bahamonde et al. (2012) working with decomposing leaves of grasses in N. antarctica woodlands also reported N immobilization. Yahdjian et al. (2006) found N immobilization during the initial phases of decomposition and N release after 7 months in Stipa speciosa leaves decomposing in Río Mayo, Patagonia.
On the other hand, P release had a different pattern depending on site. In steppe, decomposing roots grasses released P from the beginning of the incubation, meanwhile grasses in forest tended to immobilize P. This could be related to the fact that the N:P ratio in grass roots was lower in the steppe (4.2) than in the forest sites (16.9), indicating that P is more limiting in forest. Thus, when N:P ratio is below 15, P mineralization could occur, as we observed in steppe roots. Our results are thus concordant with Güsewell and Freeman (2005) who reported for nine herbaceous species at different N:P ratios that litter with N:P ratios >22 always had P-limited decomposition, but litter with lower N:P may be P limited (with immobilization) or P not limited (with P mineralization).
Calcium dynamics in decomposing grass roots also were slightly different depending on site. In steppe, initial Ca release was observed followed by periods of Ca recovery and release. In forests, decomposing grass roots showed some initial retention followed by periods of release and then retention again. Laskowski et al. (1995) postulated that the mechanism behind Ca retention and loss may be related to litter Ca:C ratio and could be similar to that regulating N:C nutrient dynamics of decomposer organisms. According to this, it is known that Ca:C ratio of different mycelium fungi is in the range of 0.015–0.07 (Staaf & Berg 1982). As our Ca:C ratio was 0.019 and 0.01 for grasses in forest and steppe, respectively, thus our results are consistent with this theory for the forest sites but not for the steppe sites. Information about nutrient release in grass roots in these southern sites is scarce, but Bahamonde et al. (2012) studying grass leaf decomposition in N. antarctica forests also found Ca immobilization-release patterns during an extended period of decomposition.
Potassium was the only nutrient that showed consistent release from the beginning of the experiment for grass roots and sites. This is concordant with several studies that showed the same pattern for different species and sites (Laskowski et al. 1995; Osono & Takeda 2004). Since this nutrient is not a structural material and exists mainly in solution in plant cells, K is mobile and thus leached quickly from decomposing litters (Osono & Takeda 2004).
Nutrient release in decomposing tree roots
Despite there being some studies on leaf litter decomposition in Nothofagus spp. (Decker & Boerner 2006; Bahamonde et al. 2012; Moretto & Pastur 2014), information about Nothofagus roots decomposition is limited. We found different patterns according to each particular nutrient. Nitrogen was immobilized meanwhile P and K were released from the onset of decomposition. For Ca, there was an initial period of immobilization followed by release and then immobilization again. Our results are consistent with Hobbie et al. (2010) who found that the majority of tree species had N immobilization in Poland. Parton et al. (2007) studying global patterns in N release reported that some species such as Drypetes glauca showed net N immobilization in early decomposition for a range of sites meanwhile other species, as for example pines, had N release without immobilization.
With respect to P dynamics, we found an important release from the onset of decomposition, and for this nutrient several authors have reported both patterns along different species: P release or P immobilization in fine root decomposition (i.e. Ostertag & Hobbie 1999; Bachega et al. 2016). Thus, the process that predominates may be related with litter, soil and/or microorganisms characteristics.
Our results for Ca are concordant with several authors that have reported a two-phase pattern in Ca dynamics for different aerial litters in cool temperate forests, with an increase phase early followed by a decrease phase later (Berg et al. 1987; Laskowski et al. 1995; Osono & Takeda 2004). Despite differences between aerial and belowground litter, this pattern also may occur in decomposing roots. In this sense, Osono and Takeda (2004) postulated that leaching or decomposition of more readily available organic components may cause the relative increase in Ca concentration without a net increase in Ca mass, independent of initial Ca content. In contrast, in the later decrease phase, the release of Ca was dependent on the decomposition of structural components and the initial Ca concentration.
Potassium dynamics for tree roots were quite similar to those described for grasses.
Comparing grasses vs. tree roots for nutrients release in forests
The hypothesis that in forests, root nutrient release would be different between grasses and trees was partially supported by our results. In general, decomposing roots of grasses and trees had similar patterns for K and N, although trees had significant higher values of N immobilization, indicating that this nutrient could be more limiting for trees. Previous studies indicated that N is the most limiting nutrient for almost all Nothofagus species in Patagonia (Diehl et al. 2003). These authors also reported that, although there were low P concentrations in soils, this was not limiting for tree growth. This could be related with Nothofagus′s ectomycorrhizae which could facilitate P acquisition (Aerts 2002). Likewise, mycorrhizas could explain differences in Ca patterns, since trees showed a strong immobilization at the initial period, whereas grasses showed a slow Ca release. It has been reported that various forms of Ca in mycorrhizal tissue, such as Ca-polyphosphates on the cell surface near the cytoplasmic membrane, may also contribute significantly to the overall root Ca levels when such associations occur (Kulaev 1975; Peterson & Howarth 1991).
With respect to forest use, we found that the silvopastoral use apparently did not affect grass or tree root decomposition and/or nutrient release, since no significant differences were found for any nutrient. In concordance with these results, Carrera et al. (2008) reported that grazing disturbances affected leaf litter decomposition in grasses but not root decomposition process in Northern Patagonian.
Conclusions
This study provide important information about roots decomposition rates in austral sites in south Patagonia, Argentina. We studied grass root decomposition in steppe and forest resulting in lower values than those reported in northern sites, probably due to the lower temperatures. Despite the low decomposition rates, grass roots decomposed more slowly in steppe than in forests. Likewise, in forests, decomposition rates of tree roots were slower than grass roots.
Nutrient dynamics or release differed between sites and roots types. In all cases, N was immobilized following the order: tree roots > forest grasses > steppe grasses. P was immobilized by grass roots in forests but released by both trees and steppe grasses. For Ca, trees showed a strong immobilization initially whereas grasses showed a slow Ca release. K was the only nutrient that had a strong release from the beginning in grass and tree roots. Lastly, we found that the silvopastoral use of N. antarctica forests did not affect grass or tree roots decomposition and nutrient release. All this information is important to understand nutrient cycling in very austral sites, were ecosystem processes are usually slower than in other ecosystems.
Acknowledgements
This study was supported by PICT 2012-2335 from the FONCYT agency, government of Argentine. We thank the cooperation of Jorge Birgi and Francisco Mattenet for their help in the field and to Esteban Galli, Santiago Fernandez and Halliday family by allowing us to work in their ranches.




