Street fighters: Bite force, injury rates, and density of urban Australian water dragons (Intellagama lesueurii)
Abstract
In an increasingly urbanized world it is imperative that we understand how wildlife responds to this novel anthropogenic landscape, both at the individual- and population-level. Urbanization generally reduces biodiversity, but can also favour particular species and increase their abundance relative to wild populations. When population density increases, so too does the frequency and cost of social interactions. We studied Australian water dragons (Intellagama lesueurii), a species common in urban, semi-natural and natural areas, to firstly test the prediction that urban populations occur at higher densities, and then determine the consequences of urbanization for combat rates (quantified using wounding) and bite force. We established that urban populations are denser than ones from semi-natural and natural habitats. We also recorded significantly more wounds in females from urban populations than females from both natural and semi-natural populations. Urban males also had significantly higher incidence of wounding than males from natural populations. We did not find a difference in male or female bite force among any populations across the urban-natural gradient. Overall, we found evidence that urbanization results in a higher population density of water dragons and more frequent conspecific combat, but this was not associated with an increase in bite force. These finding suggests that there may be a physiological cost to living in urban habitats related to increased contest rates and wounding.
Introduction
Competition for mates and limited resources has a profound impact on the behavioural and social interactions of many vertebrates and invertebrates (Greenberg & Crews 1983; Ostfeld 1990; Maher & Lott 2000; Losin et al. 2016). These fundamental processes can be exacerbated when habitat is disturbed or fragmented, like during urbanization (see review by Banks et al. 2007). Compared to undisturbed habitats, urban landscapes frequently contain a host of novel ecological challenges, including altered food webs, altered thermal regimes, anthropogenic mortality sources, increased vectors for disease and parasites, limited suitable habitat, and novel predators (McIntyre 2000; Shochat et al. 2006; Hamer & McDonnell 2008). All these factors can influence social interactions and spatial organization in wildlife (see review by Maher & Lott 2000). Furthermore, urban habitats are also often isolated or limited in size, functioning much like islands (Davis & Glick 1978; Soulé et al. 1988; Littleford-Colquhoun et al. 2017), and provide an excellent opportunity to examine the behavioural and spatial ramifications of urbanization on wildlife within a natural experiment (Banks et al. 2007).
Some species are able to thrive in urban areas because they utilize human resources, and this often results in higher population densities compared to conspecific populations in natural habitats (termed ‘urban exploiters’; Blair 1996; Francis & Chadwick 2012; Teixeira et al. 2016). A higher population density, however, can present a unique set of challenges, such as increased social interactions, which may require adaptation if urban populations are to persist (Shochat et al. 2006; Alberti et al. 2017). Many urbanized species have responded to the challenges of finite suitable habitat behaviourally, with populations becoming more crowd-tolerant (i.e. increased home-range overlap and decreased conspecific aggression). This phenomenon has been reported in mammalian carnivores (Atwood & Weeks 2003), lizards (Johnston & Bouskila 2007), and snakes (Mitrovich et al. 2009; Corey & Doody 2010). For example, urban coyotes (Canis latrans) increase home range overlap and group size as an adaptive response to clumped urban resources (Atwood & Weeks 2003), as do coachwhip snakes (Masticophis flagellum) in human-disturbed areas (decreased home range size and distance to their nearest neighbour; Mitrovich et al. 2009). Alternatively, animals in denser urban environments may become more aggressive towards conspecifics, as reported in some birds (Abert's towhees, Melozone aberti, and curve-billed thrashers, Toxostoma curvirostre; Fokidis et al. 2011) and lizards (Cuban rock iguana, Cyclura nubile; Lacy & Martins 2003). Although effective in the short-term, these altered behavioural responses may be a stopgap that allows species to persist in urban areas until natural and sexual selection can drive mechanistic urban adaptations, like morphological and physiological responses, that confer fitness benefits (Shochat et al. 2006). Adaptive traits related to conspecific contest competition, such as enhanced bite force and other animal weaponry (structures specifically used in contests; Emlen 2008), could be used to respond to altered population densities and social interactions (Knell 2009). For example, increased population density of wall lizards (Podarcis sicula) in an island habitat resulted in a higher frequency of wounding and also, a higher bite force (Vervust et al. 2009). Bite force has been suggested as a key determinant of contest outcome in lizards (Herrel et al. 2001; Lailvaux et al. 2004; Vanhooydonck et al. 2005; Husak et al. 2006), and is therefore predicted to be the target of selection when contest competition drives access to territories and mates. Higher bite force in male collared lizards (Crotaphytus collaris) correlates with an individual's ability to win contests against rivals and increased inferred fitness (Husak et al. 2009); however alternative mating tactics that avoid costly contests also have fitness benefits (see Baird et al. 2007; Braun 2016). Therefore, if urban populations are adapting to the disrupted social interactions and spatial organization caused by urban habitats, then we expect both behavioural adaptations (crowd-tolerance or aggressive encounters) and physiological responses, to occur.
We examined the effect of urbanization on Australian water dragons (Intellagama lesueurii), a lizard species thriving across a variety of human-modified habitats, ranging from natural bushland to urban cores. We examined nine water dragon populations varying in their level of urbanization (urban, semi-natural, and natural), to determine if urbanization has altered this species’ social interactions and population density. Adult water dragons are sexually dimorphic, with adult males demonstrating a larger body length, mass, and a proportionally larger head (Thompson 1993; Harlow 2001; Gardiner et al. 2014). Male water dragons are also combative, with some contests lasting upwards of two hours (Baird et al. 2012; Baird 2013). They engage in contests to establish and defend territories or adopt a satellite non-territorial reproductive tactic until an opportunity arises to challenge a territorial male or a territory becomes available (Baird et al. 2012, 2014). Conversely, female water dragons do not defend territories and tend to be more aggregative; an individual's home range overlaps with multiple males and females, and females tend to form strong social associations with one another unrelated to kinship (Strickland et al. 2014). As such, we expected urbanization to affect the rate of social interactions of sexes differently. We predict that population density will increase with urbanization. If this is the case, then we expect that male urban water dragons will not become crowd-tolerant, and instead, engage in higher rates of combat, which will result in more wounds in urban habitats than semi-natural and natural habitats. In contrast, we expect that the wounding rate in females will be similar among habitat types. In addition, if urban males do exhibit more wounds, due to more frequent combat, then we predict higher bite force for males within urban habitats compared to semi-natural and natural habitats. In contrast, females are likely not under the same selective pressures, due to different social and spatial organization (Strickland et al. 2014), and so we expect that female bite force will be similar among habitat types.
Methods
Study species and sites
Australian water dragons are large agamid lizards (maximum snout-vent length [SVL]: 304 mm; Thompson 1993) distributed along Australia's east coast, and are common around bodies of freshwater within forested areas (Cogger 2014). However, they are also found in urban areas (Littleford-Colquhoun et al. 2017) and their distribution overlaps a majority of the Australian human population (approx. 80%; Australian Bureau of Statistics 2011). Previous research has documented distinct genetic and morphological difference between water dragon populations in cities (Littleford-Colquhoun et al. 2017), suggesting that the selective pressure within urban areas may be altering phenotypic traits, such as behaviour and biology.
We collected dragons at nine sites (three urban, four semi-natural, two natural) in a 50 km radius within the greater Sydney area in New South Wales, Australia, from October 2015 to March 2017 (see Appendix S1 for detailed locations). Urban sites had a landscape that was widely human-modified (e.g. buildings, concrete, gardens, roads, etc.), and a dense local human population. Semi-natural sites had natural features, such as waterways with treed shorelines, but were close to urban/suburban areas and had a moderate daily human presence (e.g. park visitors). Natural sites, although not completely free from human disturbance, were associated with large green spaces, waterways with treed shorelines, and a relatively low direct human presence or footprint.
Population density index
We created a population density index using line transect counts (Overton 1971; Vervust et al. 2009). Survey dates ranged across the dragon's post-nesting active season (December to March) of 2016–2017 and 2017–2018. Surveys were conducted mid-morning on days when the weather was optimal for lizard basking (clear or partially clear skies with an air temperature between 25 and 35 °C). We performed five surveys along a fixed transect at each of the nine sites, and recorded the number of dragons observed within 10 m of the primary surveyor; which remained the same during each survey for consistency. Transects ranged from 0.30–3.00 km in length (mean 1.08 km ± 0.13 SE), and followed a linear pathway (either walking trail or waterway) depending on the site and terrain. Typically, surveys took between 1 and 2 h, depending on terrain and number of dragons sighted.
Handling, morphology, wounding, and bite force
All dragons used in the wounding and bite force analysis (n = 190) were captured either by hand or using a noose-pole, and sampling was conducted both during the day and night (depending on terrain and location). Once captured, adult dragons were sexed using secondary sexual characteristics (e.g. relative head size and chest colouration). We measured individual snout-vent-length (SVL) with a clear plastic ruler (±1 mm) and recorded the dragon's surface body temperature with a handheld laser thermometer (±0.1 °C; RIT310, Ryobi, Doncaster, Australia) held to the dragon's abdomen prior to measuring bite force (Berg et al. 2015). Bite force was measured using an isometric Kistler force transducer (type 9203; Kistler Inc. Wintherthur, Switzerland), mounted on a retort stand, and connected to a Kistler charge amplifier (type 5995). All animals were coerced to bite on two parallel plates (fixed at a distance of 3 mm), by stroking both sides of the dragon jaw simultaneously with the researcher's index finger and thumb. Each individual dragon was tested three consecutive times, and the maximum bite force recorded and used in analyses (Anderson et al. 2008).
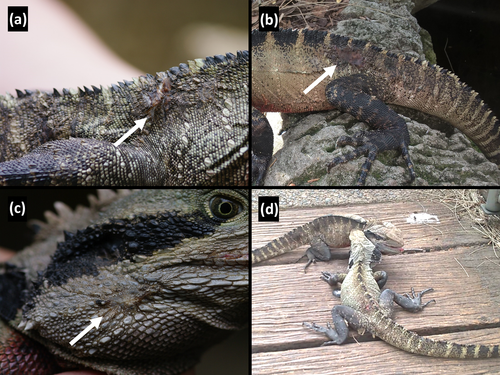
Fresh wounds, scars, obvious broken bones, and missing digits were counted and recorded as a measure of wounding rate (tally of the total number of injuries per individual). The purpose of collecting this information was to glean which individuals were being injured more often, as a proxy for the rate of conspecific combat. As such, and to maintain statistical power, we pooled injuries of different types into one metric ‘wounding’. Of the injuries, the most commonly recorded wounds were V-shaped scars over the lower back, hips, and base of the tail, and scarring around the head and jowls, which are indicative of combat (Fig. 1). We also commonly recorded missing digits. We did not include broken and regenerating tails or tail tips, as injuries such as these have a higher likelihood of being related to predation attempts (Hayes et al. 2012). Once measured, the lizards were immediately released back at their site of capture.
Analyses
Data are presented as means ± standard error (SE). All summary statistics and data visualizations were calculated from raw data, and unless otherwise stated they were predicted from the models. All statistical tests were conducted in R version 3.2.3 (R Core Team 2015). Before beginning our analyses, we explored each dataset following the protocol outlined in Zuur et al. (2010). During our data exploration, we did not find any outliers or strong collinearity between our predictor variables within the same model.
Population density index
We compared population densities between habitat types using a visualizations linear mixed effects model with a Poisson distribution using the function glmer within the R package lme4 (Bates et al. 2015). The total number of dragons observed per survey was the response variable which we offset by the transect distance (km). We included the fixed effect of habitat type (categorical with three levels: urban, semi-natural, and natural), and the random intercept of site to control for dependencies within our data relating to population-specific differences. For this and all models below, we ensured that model assumptions were met and that Poisson models were not overdispersed (as per Zuur et al. 2010). To generate contrasts among habitat types we used the function lsmeans from the lsmeans R package (Lenth 2016). P values generated for comparisons among habitat type were corrected using Tukey's HSD using a multiplicity adjustment (Lenth 2016).
Wounding
We examined differences in wounding separately for females (nNATURAL = 26; nSEMI-NATURAL = 34; nURBAN = 35) and males (nNATURAL = 23; nSEMI-NATURAL = 32; nURBAN = 40), because of the known sexual differences in both ecology, morphology, and social behaviour (Thompson 1993; Baird et al. 2012, 2014; Strickland et al. 2014). The data were zero-inflated with 44% of females and 16% males not having wounds. Therefore, we used a zero-inflated mixed-effect Poisson regression, with the function glmmadmb in the R package glmmADMB (Skaug et al. 2014), to test for differences in wounding rates among habitat types (categorical with three levels: urban, semi-natural, and natural). We controlled for differences in body size by SVL (mm) as a fixed effect, and, as above, we accounted for dependencies in our data related to population-specific effects by including the random intercept of site. We conducted pairwise comparisons between habitat types using the same protocol as above (lsmeans from the lsmeans R package; Lenth 2016).
Bite force
We used linear mixed effect models (with a Gaussian distribution using the function lmer from the R package lme4; Bates et al. 2015) to examine differences in the maximum bite force (N) of water dragons among habitat types. Again, we examined each sex separately (sample size listed above). We controlled for differences in body size (SVL; mm) and body temperature (°C) by including these variable as fixed effects. Both maximum bite force and SVL were log-transformed in the analysis, due to a non-scalar linear relationship between these two variables (Lailvaux et al. 2004). We also included a random intercept for site, and tested contrasts between all habitat types using the same method as above (Lenth 2016).
Results
Population density index
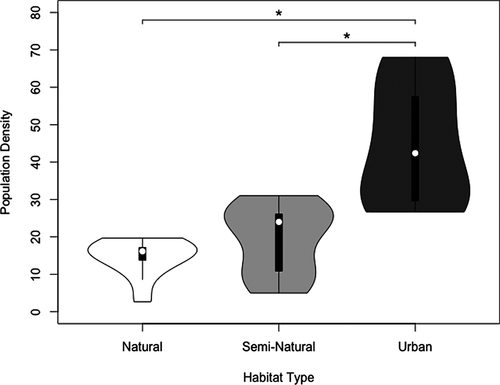
Urban areas had significantly higher population densities than both semi-natural (β = −0.868 ± 0.263, z = −3.302, P = 0.003) and natural areas (β = −1.094 ± 0.290, z = −3.780 P = 0.001; Fig. 2; see Appendix S1 for specific densities). Population densities did not differ significantly between semi-natural and natural areas (β = −0.226 ± 0.309, z = −0.731 P = 0.745; Fig. 2).
Wounding rate
In both male and female water dragons, the wounding rates were significantly, positively related to SVL (females: β = 0.025 ± 0.009, z = 2.93 P = 0.003; males: β = 0.020 ± 0.003, z = 6.52, P < 0.001). This relationship was controlled for in our examination of wounding rate (results below).
On average, female water dragons (n = 95) were observed with 1.2 ± 0.5 wounds (median = 1, range = 0–6). The wounding rate for female water dragons was significantly higher in semi-natural than natural areas (Table 1; Fig. 3), and significantly higher in urban than natural areas (Table 1). The wounding rate for female dragons was not significantly different between urban and semi-natural areas (Table 1).
| Habitat type | Females | Males | ||||||
|---|---|---|---|---|---|---|---|---|
| β | SE | z | P corr | β | SE | z | P corr | |
| Natural vs. Semi-Natural | −0.996 | 0.352 | −2.830 | 0.013 | −0.395 | 0.345 | −1.146 | 0.486 |
| Natural vs. Urban | −0.802 | 0.344 | −2.331 | 0.052 | −1.129 | 0.320 | −3.533 | 0.001 |
| Semi-natural vs. Urban | 0.194 | 0.443 | 0.438 | 0.900 | −0.734 | 0.455 | −1.611 | 0.241 |
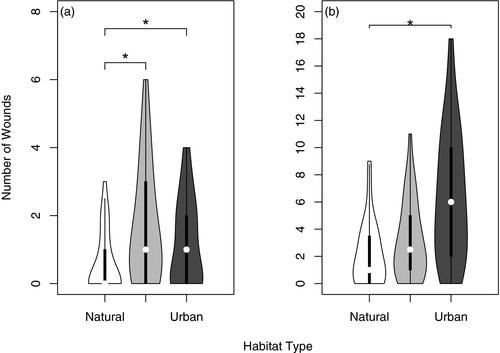
On average, male water dragons (n = 95) were observed with 4.3 ± 0.4 (median = 3, range = 0–18) wounds. The wounding rate for male dragons was significantly higher in urban compared to natural areas (Table 1; Fig. 3). The wounding rate for male dragons between natural and semi-natural areas, and semi-natural and urban areas was not significantly different (Table 1).
Bite force
Female water dragons had an average maximum bite force of 47.9 ± 2.9 N (ranging from 4.9 to 176.5 N), and males had an average maximum bite force of 317.9 ± 18.0 N (ranging from 30 to 642 N). Maximum bite force did not differ significantly among habitat types in either female or male water dragons (Table 2). Body size (log-transformed SVL) was significantly related to female (β = 4.414 ± 0.696, t = 6.643, P < 0.001) and male maximum bite force (β = 7.033 ± 0.544, t = 12.936, P < 0.001), while body temperature was not (female: β = 0.000006 ± 0.009, t = 0.0006, P = 0.100; male: β = −0.002 ± 0.007, t = −0.257, P = 0.798).
| Habitat type | Females | Males | ||||||
|---|---|---|---|---|---|---|---|---|
| β | SE | z | P corr | β | SE | z | P corr | |
| Natural vs. Semi-Natural | 0.157 | 0.364 | 0.431 | 0.903 | 0.097 | 0.083 | 1.177 | 0.467 |
| Natural vs. Urban | −0.372 | 0.349 | −1.068 | 0.534 | 0.070 | 0.080 | 0.871 | 0.659 |
| Semi-natural vs. Urban | −0.529 | 0.310 | −1.707 | 0.202 | −0.028 | 0.077 | −0.360 | 0.931 |
Discussion
Urban water dragons occurred in higher densities than conspecifics living in semi-natural and natural habitats. Furthermore, both male and female water dragons from urban populations have significantly higher wounding rates than natural populations. Females from urban population also bore significantly more wounds than semi-natural populations. These findings support our predictions that urban water dragons live in higher population densities than their counterparts in wild populations and male water dragons within these urban areas are engaging in higher rates of male-male combat, while our prediction regarding female wounding rate (i.e. no difference) was not supported. As expected, we did not observe an increase in female bite force related to urbanization; however, contrary to our expectations, we also did not find evidence for increased bite force in urban males. This suggests that although water dragons are living in higher density, and engaging in conspecific fights more often, this has not resulted in stronger selection for enhanced bite force in urban individuals.
Human-subsidized resources and altered predator-prey relationship are factors that cause the population density of particular species, often termed ‘urban-exploiters’, to increase in urbanized habitats (Blair 1996; Francis & Chadwick 2012; Teixeira et al. 2016). Urban water dragon population densities were significantly higher than densities in natural habitats, which suggests water dragons may fit the classification of an urban exploiter. Traditionally, much of the research into urban exploiting species has focused on birds (Gering & Blair 1999; Shochat 2004; Kark et al. 2007; Evans et al. 2011; Meillère et al. 2015); however, arthropods (McIntyre 2000) and mammals (Prange et al. 2004; Teixeira et al. 2016) have garnered some attention. Generally, native reptile species are under-represented in studies examining urban exploiters; yet there are observations of higher than natural population densities of urbanized lizards and turtles (Henle 1990; Souza & Abe 2000; Germaine & Wakeling 2001; Johnston & Bouskila 2007). For example, tree dtella geckos (Gehyra variegata) occur in a density an order of magnitude higher on buildings compared to their natural habitat (trees) due to a higher prey abundance (Henle 1990), which follows assertions that increased resources can result in increased population density in urban areas (Blair 1996; Francis & Chadwick 2012). Similarly, water dragons may be able to exploit the vast array of potential food sources in urban areas, as they are generalist omnivores (Baxter-Gilbert 2014). However, although an increase in population density in urban areas may appear to be beneficial for a species’ persistence, living in higher densities may come with substantial costs (e.g. body condition, clutch size, and lifespan; Shochat 2004) or require divergent behavioural or morphological phenotypes to promote urban survival (Tomkins & Brown 2004; Banks et al. 2007; Knell 2009).
For many species, suitable urban habitats are finite in size (Davis & Glick 1978; Soulé et al. 1988) and increased population density within these areas may require individuals to become either more crowd-tolerant (Johnston & Bouskila 2007; Mitrovich et al. 2009; Corey & Doody 2010) or more aggressive towards conspecifics (Lacy & Martins 2003; Fokidis et al. 2011) to compensate for increased conspecific interactions. We found no reduction in wounding rate between urban water dragon populations and the other habitat types, so we can infer from our findings they are not becoming more crowd-tolerant. Instead, we found there was a significant increase in wounding of both urban female and male water dragons compared to conspecifics from natural areas, which suggests they are being exposed to increasing rates of aggressive encounters. Our findings reflect what has been found in wall lizards (Podarcis sicula; Vervust et al. 2009) and Eurasian badgers (Meles meles; Macdonald et al. 2004), wherein higher population density increased male wounding rates. But, in contrast, fan-fingered geckos (Ptyodactylus guttatus) that live on buildings and occur at a density 195 times higher than natural populations living on rock faces and caves, which do not exhibit an increase in aggressive encounters (Johnston & Bouskila 2007). These geckos instead used mate guarding and social-sorting to compensate for the dramatically inflated population density (Johnston & Bouskila 2007). It is likely that the behavioural responses a particular species exhibits due to increased urban population density (e.g. increased crowd-tolerance or aggression) is related to the degree of sexual selection from contest competition and mating strategy; however, this postulate requires further research. Interestingly, the increased wounding rate in females suggest that in urban areas where population density is higher, and suitable habitat is finite, females may be altering their normal social behaviour to become more aggressive (choosing to engage in more conspecific combat). Previous research in collar lizards has seen female aggressive encounters increase when key resources are limited (elevated rock perching, Baird & Sloan 2003; Baird 2013). An alternative rationale, however, is that increase female wounding a merely a result of individuals suffering the brunt of male-based aggression or biting through potential coercion from males. Future studies focused on determining the demographics of contest instigators is required to better understand the source of increased wounding rates in urban female water dragons. Overall, our findings suggest that urban living can result in higher rates of wounding of both males and females, reflecting increased conspecific aggression, and suggests that there may be a substantial physiological cost of living in urban areas.
Population density is often a key factor in the evolution of divergent phenotypes, such as sexual size dimorphism (Stamps 1983) and male weaponry (Tomkins & Brown 2004; Knell 2009). Increased population densities of male European earwigs (Forficula auricularia) on islands has resulted in higher levels of male competition, which in turn has selected for exaggerated weaponry (pincer size and head shape; Tomkins & Brown 2004). This positive relationship between contest competition and population density has also been observed in insects (Tomkins & Brown 2004; Buzatto et al. 2012) and reptiles (Vervust et al. 2009); although a negative relationship has been observed in frogs (Buzatto et al. 2015; Lüpold et al. 2017) and ungulates (Jorgenson et al. 1998; Kruuk et al. 2002). For example, male quacking frogs (Crinia georgiana) use their forearms during precopulatory male combat (Buzatto et al. 2015). At low density these frogs increase combat success and mating opportunities by enlarging their forearms, however at high density their arms are reduced in size, with energy being diverted to increased sperm production and quality instead (Buzatto et al. 2015). Interestingly, we did not detect any significant relationship, positive or negative, between water dragon bite force and urbanization, for either females (as predicted) or males (contrary to our predictions). A potential explanation for a lack of positive directional selection on male urban water dragon bite force could be related to morphological and physiological constraints. Male water dragons naturally engage in male combat and territoriality (Baird et al. 2012; Baird 2013), thus selection may have already driven this species to it functional maxima, and any exaggerations in weaponry may be detrimental or not physiologically or morphologically possible (Kokko & Brooks 2003). However, further investigation into the kinematics and physiological constraints of water dragon skull morphology, musculature, and bite force are required to test this hypothesis. Anecdotally, we observed two separate instances of fatal male-combat both related to bite wounds. In one case a male suffered widespread deep tissue damage along the mid-body and limbs leading to a fatal infection, while in the other case a male had a large section of its lower jaw removed during combat (SM Fig 1). This suggests that the bite force in male water dragons is already a significant weapon, and increased bite force may not actually provide any further advantage. Furthermore, investment in animal weaponry is to incur a trade-off and can come at a cost to other morphological structures. For example, in multiple species of Onthophagus dung beetles, horn size is traded-off against the size of antennae, eyes, testes, and wings (Emlen 2001; Simmons & Emlen 2006). Alternatively, selection may be driving increased combat potential is through jaw muscle stamina, rather than maximal bite force. We recommend future research examine if increased urban population density and aggressive encounters has driven selection for increased combat endurance, or if selection is operating on other sexual traits, beyond male-combat weaponry, such as sperm competition (Buzatto et al. 2015; Lüpold et al. 2017) or density-dependant alternative mating tactics (Kokko & Rankin 2006).
Overall, our study found that urban water dragons are living at higher densities, suggesting they are an urban exploiting species (Blair 1996; Francis & Chadwick 2012), and are engaging conspecific contests more often. This increased rate of aggressive encounters has not, however, translated into increased bite force. This raises questions regarding the physiological cost of living in urban areas (e.g. wounding, healing, and stress), and what traits are being selected for to deal with these challenges both behaviourally and biologically. What is clear, is that more research is required to uncover how these urbanized lizards are responding to not only a novel ecosystem, but also a novel social and spatial landscape and how this may effect mating systems and population sex ratios. In general this study contributes to the small, but growing, field of mechanistic urban ecology (Shochat et al. 2006), and furthers our understanding of an iconic Australian reptile as it persists in an ever-changing urban world.
Acknowledgements
We would like to thank P. Bolton, T. Damasio, C. Fryns, G. Hughes, F. Kar, S. Klopper, L. Monk-Whipp, M. Mühlenhaupt, and D. Noble for their assistance in the field, as well as all our colleagues at the Lizard Lab at Macquarie University for their support. Thanks to J. Riley for assistance with statistical analysis, and editing previous versions of this manuscript, as well as B. Philips, T. Baird, and B. Bateman. We would also like to thank Taronga Zoo, notably S. Brown and the team at Backyard to Bush, P. Harlow, and the veterinary staff at the Taronga Wildlife Hospital who performed the post-mortem necropsies. This research was supported by Macquarie University and Natural Sciences and Engineering Research Council of Canada. Experimental protocols were approved by the Macquarie University Animal Ethics Committee (ARA # 2015/023), Taronga Zoo Animal Ethics Committee (ARA # 3b/08/15), and New South Wales National Parks and Wildlife Services (scientific license # SL100570).




