Towards meaningful monitoring: A case study of a threatened rodent
Abstract
Detecting trends in species’ distribution and abundance are essential for conserving threatened species, and depend upon effective monitoring programmes. Despite this, monitoring programmes are often designed without explicit consideration of their ability to deliver the information required by managers, such as their power to detect population changes. Here, we demonstrate the use of existing data to support the design of monitoring programmes aimed at detecting declines in species occupancy. We used single-season occupancy models and baseline data to gain information on variables affecting the occupancy and detectability of the threatened brush-tailed rabbit-rat Conilurus penicillatus (Gould 1842) on the Tiwi Islands, Australia. This information was then used to estimate the survey effort required to achieve sufficient power to detect changes in occupancy of different magnitudes. We found that occupancy varied spatially, driven primarily by habitat (canopy height and cover, distance to water) and fire history across the landscape. Detectability varied strongly among seasons, and was three times higher in the late dry season (July–September), compared to the early dry season (April–June). Evaluation of three monitoring scenarios showed that conducting surveys at times when detectability is highest can lead to a substantial improvement in our ability to detect declines, thus reducing the survey effort and costs. Our study highlights the need for careful consideration of survey design related to the ecology of a species, as it can lead to substantial cost savings and improved insight into species population change via monitoring.
Introduction
The loss and fragmentation of natural habitats, introduction of non-native species and global climate change are driving declines in species distribution and abundance worldwide (Chapin et al. 2000; Butchart et al. 2010; Barnosky et al. 2011). Effective conservation depends on the ability to detect population trends through reliable, effective and efficient monitoring programmes (Reynolds et al. 2011). Ecological monitoring refers to the process of gathering information about an ecological variable (e.g. species distribution) at different points in time and space to assess change (Yoccoz et al. 2001). But despite their importance, monitoring programmes are often designed without regard for their ability to deliver the types of information required by land managers (Legg & Nagy 2006; Guillera-Arroita et al. 2010; Peel et al. 2015). Disregarding imperfect detection (when a given method does not detect a species where it occurs) can reduce the reliability of estimates of population trends, particularly when detection varies in space or time (MacKenzie et al. 2002; Wintle et al. 2004; Field et al. 2005). The purpose of monitoring programmes differs from that of baseline surveys, which are largely designed to collect information on species distribution and richness (i.e. the number of distinct species that occur within a region). Baseline surveys may not be suitable for collecting the types of data required to infer population trends in some or all of the species they report on, but they do provide valuable data that can be used to inform monitoring programmes.
A useful variable in ecological monitoring is occupancy (the proportion of an area occupied by a species) (Holt et al. 2002). Monitoring occupancy is typically cheaper and less technically demanding than measuring population abundance or density, which can be expensive to implement on large scales (Nimmo et al. 2015); consequently monitoring abundance may suffer from limited statistical power to detect change (Field et al. 2005), despite the available statistical methods to account for imperfect detectability (Buckland et al. 1993; Royle 2004; Borchers et al. 2012). Change in occupancy is considered an important measure of extinction risk, for example in the International Union for the Conservation of Nature (IUCN) Red List of Threatened Species (IUCN 2012). Furthermore, occupancy methods that account for imperfect detection (MacKenzie et al. 2002) are commonly used for large-scale monitoring programmes, and have been applied across diverse taxa including mammals (Wibisono et al. 2011), birds (Royle & Kéry 2007), reptiles (McGrath et al. 2015), amphibians (Petitot et al. 2014) and invertebrates (MacKenzie 2003).
Inadequate survey design can lead to low statistical power to detect trends of interest (Guillera-Arroita & Lahoz-Monfort 2012). Key decisions in the design of occupancy surveys include the total survey effort required to detect effect sizes of ecological relevance with confidence, when and where to monitor and how to allocate a survey budget, given the recognised trade off between the effort applied at each given site (and thus the quality of site-level data) (Mackenzie & Royle 2005; Bailey et al. 2007). One way to guide monitoring design decisions is to use existing data to inform the likely values of relevant system parameters. Based on these, the expected performance of alternative monitoring strategies in meeting the objectives of the monitoring programme can be explored.
In this study, we use existing data to examine the effectiveness of alternative monitoring strategies for the threatened brush-tailed rabbit-rat Conilurus penicillatus (Gould 1842), in one of its last remaining safe havens, the Tiwi Islands in northern Australia. Australia has suffered a remarkably high rate of mammal extinctions over the past two centuries (Woinarski et al. 2015), amounting to loss of at least 30 terrestrial mammals (Fisher et al. 2014). The Tiwi Islands are now one of the few areas in Australia to retain a complete pre-European assemblage of mammals, but recent evidence suggests that small mammal populations, including C. penicillatus, are in decline (Firth et al. 2006a; Davies et al. 2016). The distinct Tiwi Islands subspecies (C. penicillatus melibius, Thomas 1921; Kemper & Schmitt 1992) has also been highlighted as one of the 20 mammals most likely to go extinct in the next two decades (Geyle et al. 2018), suggesting that emergency action must be taken to ensure its’ ongoing persistence.
We estimated the occupancy and detectability of C. penicillatus using baseline data collected across the Tiwi Islands in 2000–2002. We then used this information to examine the statistical power of different monitoring strategies for detecting declines of relevance to the IUCN Red Listing. We note here that the aim of this study is not to make recommendations to the IUCN for listing or assessment, but to advise on how much monitoring effort is required to confidently detect a decline when one occurs. With this, we address in part the priority need to establish an appropriate monitoring programme for this species (see Woinarski et al. 2017).
Methods
Study area
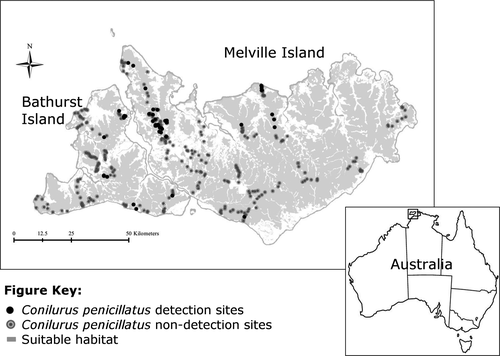
The Tiwi Islands comprise Melville (5788 km2) and Bathurst (1693 km2) Islands, and are ~20 km north of mainland northern Australia. Both islands have similar environments and experience a highly seasonal (wet–dry tropical monsoonal) climate (average rainfall of 1860 mm and 146 mm in the wet and dry seasons respectively) (Bureau of Meteorology 2015). Vegetation includes savanna woodland and open forest dominated by eucalypts Eucalyptus and Corymbia spp., with smaller areas of Melaleuca woodland, sedgeland, grassland, rainforest, mangrove and coastal dunes. Approximately 5% of the islands are covered in short-rotation Acacia mangium forestry plantations, mineral sand mining and urban areas (Richards et al. 2012).
Study species
Conilurus penicillatus is a semi-colonial, medium-sized (150 g) native rodent with a now patchy distribution in northern Australia and southern New Guinea (Firth et al. 2010). It is listed as Vulnerable under the IUCN Red List (Burbidge & Woinarski 2016), and under Australian (Environment Protection and Biodiversity Conservation Act, Australian Government 1999) legislation. It is listed as Endangered under Northern Territory legislation (Northern Territory Parks & Wildlife Conservation Act, Northern Territory Government 2012). The species has suffered a dramatic range contraction, most likely in response to increases in the frequency, intensity and size of landscape fires, and a consequent simplification of vegetation structure (Firth et al. 2010), which may make them more susceptible to predation by feral cats and other predators (Woinarski et al. 2011; Davies et al. 2016). Conilurus penicillatus mostly occurs in tall open eucalypt forests and woodlands that burn infrequently, with a sparse to moderate mid-storey and an under-storey of perennial grasses (of which the seeds and stems are primary diet items, Firth et al. 2005, 2010). Breeding in C. penicillatus is seasonal, occurring over at least 4 months with juveniles predominantly entering populations in the mid to late dry season (June–September) (Taylor & Horner 1971; Firth 2007).
Survey data
We used data collected as part of a larger baseline wildlife survey conducted in the early 2000s (Firth et al. 2006a). A total of 338 sites were sampled in native vegetation across the Tiwi Islands (223 sites on Melville Island and 115 on Bathurst Island) (Fig. 1). Each site was visited only once between 2000 and 2002. Approximately 53% of sites were sampled during the early dry (Apr–Jun) season, while 33% and 14% of sites were sampled during the late dry (Jul–Sep) and late wet (Jan–Mar) seasons respectively. No sampling took place during the early wet (Oct–Dec) season. Sampling followed a protocol widely used across northern Australia (Woinarski & Ash 2002); each site consisted of a 50 × 50 m quadrat, and included twenty Elliott traps (33 × 10 × 9 cm) distributed evenly around the perimeter, and one large cage trap (56 × 20 × 20 cm) located at each corner (four in total), set for three consecutive nights and checked early each day. All individuals caught were released unmarked at the site of capture. The total number of individuals captured was recorded for each trapping night. Note that this sampling encompassed the entire known range of the subspecies C. p. melibius.
Predictor variables
We selected covariates for inclusion in our occupancy models based on environmental and other variables considered important for C. penicillatus, taken from published peer-reviewed literature (in particular Firth et al. 2006a) (see Table 1 for a detailed description justifying the inclusion of each covariate). These included field-measured site characteristics and remotely-sensed variables (i.e. geospatial layers). We had reason to suspect that C. penicillatus detectability may vary seasonally (based on expert knowledge), and thus explored this by including ‘season’ as a survey-specific covariate. We tested for collinearity between each of the predictor variables, finding no correlation coefficients larger than 0.7.
| Predictor | Justification for inclusion | Measurement | Reference(s) |
|---|---|---|---|
| Canopy height | An indicator of habitat suitability | Height of tallest woody plants | Firth et al. (2006a) |
| Canopy cover | An indicator of habitat suitability | Percentage foliage cover of canopy | Firth et al. (2006a) |
| Mean annual rainfall | An indicator of productivity | Mean average rainfall in 10 years preceding survey, derived for BOM gridded data | J. Woinarski, pers obs |
| Total grass cover | An indicator of potential food availability | Percentage ground cover of grass (annual and perennial) | Firth et al. (2005, 2006a, 2010) |
| Fire impact | Fire impact may have a strong influence on critical resources required for species survival (i.e. food availability, hollow logs) | Five-point scale measuring the apparent severity of fire impact, from 1 (no sign of fire) to 5 (evidence of severe crown fire) | Firth et al. (2010, 2006b); Woinarski et al. (2011); Yates et al. (2008) |
| Fire frequency | See above | The number of times a site has burnt in the 4 years preceding survey. Derived from Landsat satellite imagery | See above |
| Distance to nearest watercourse | A highly significant finding in previous analysis across all quadrats, indicative of variation in habitat | Measure in metres. Derived from a digital elevation model | Firth et al. (2006a) |
| Foliage projection cover | An indicator of habitat suitability | The percentage of the site occupied by the vertical projection of foliage or measure of green vegetation on the ground. Derived from Landsat TM satellite imagery | Walker and Hopkins (1990) |
| Basal area of large trees | An indicator of a critical resource (i.e. hollows – more likely to be present in larger trees) | Total basal area (m2 ha−1) of trees with diameter at breast height >50 cm Derived from two sweeps of bitterlich gauge | Firth et al. (2006a,b); Bennett et al. (1993); Whitford (2002); Woolley et al. (2018) |
| Island | A surrogate for the overall population size (due to area of habitat) and related meta-population dynamics, differences between disturbance histories, predator densities and composition of vegetation | 155 sites on Bathurst Island and 223 on Melville Island. Modelled as a binary predictor |
Occupancy-detectability analysis
Single-species, single-season occupancy models (MacKenzie et al. 2002) were used to estimate occupancy and detection probabilities of C. penicillatus across the Tiwi Islands. We summarised survey data as binary detection/non-detection histories at each sampling site, considering each trapping night (here, meaning the entire set of traps deployed at each site on each night) as one detection attempt. For reference, we first calculated the species’ ‘naïve occupancy’ – the estimate of site occupancy disregarding imperfect detection (i.e. the proportion of sites with at least one detection across three trapping nights). We then fitted models (MacKenzie et al. 2002), which are formulated in terms of parameters ψi and pij, where ψi (occupancy) is the probability that sampling site i is occupied by the species and pij (detectability) is the probability of detecting the species at sampling site i during survey j, conditional upon its presence. In its basic formulation, the model structure assumes independence among sites and detections, no changes in the occupancy status of sites (i.e. a site is either occupied or empty across the whole survey period) and no false positive records. We first fitted a model assuming constant detection and occupancy probabilities (null model) to the data set. From the estimated detection probability and assuming independence, we calculated the probability of detecting the species at a presence site in at least one of k visits, as p* = 1 − (1 − p)k. This quantity therefore reflects species detectability given the cumulative effort applied to the site (Kéry 2002). We then extended our models to incorporate covariates (MacKenzie et al. 2002) for occupancy and detectability (Table 1), to explore how these probabilities vary in response to different site characteristics. Covariates were related to these probabilities via a logit-link function; thus the resulting models are effectively an extension of the traditional logistic regression model to account for imperfect detection.
We ran preliminary models to determine which covariates were likely to be good predictors of occupancy and detectability using a step-wise approach, where individual variables were dropped if considered unimportant (i.e. where confidence intervals overlapped zero), finding only two important predictors of detectability (season and fire frequency). We then fitted all possible models resulting from combinations of our chosen covariates: (two for detectability and 10 for occupancy, leading to 4096 models in total). We tested for nonlinear relationships for two covariates, foliage projection cover (FPC) (in occupancy) and fire frequency (in occupancy and detectability). Preliminary results showed very little evidence of nonlinear relationship in these two covariates, so all models were fit with linear relationships.
We used the Akaike Information Criterion (AIC) to rank and identify the best performing models for the observed data set (Burnham and Anderson 2002). The fit of the most saturated model was assessed with a goodness-of-fit test based on parametric bootstrapping and three test statistics: Pearson's chi-square, the sum of squared residuals (SSE) and the Freeman-Tukey chi-square. This method simulates data sets based upon a fitted model, refits the model and evaluates whether the observed frequency of histories has a reasonable chance of happening if the model assessed is assumed to be correct. We calculated Akaike weights (wi,) for each model and summed the contributions of each covariate (i.e. the sum of the Akaike weights ∑ wi) to provide an indication of which covariates had substantial support for explaining the observed data (but see Cade 2015). We conducted all analyses in R (R Development Core Team, 2014), fitting models within the maximum-likelihood framework of inference using the R-package ‘Unmarked’ (Fiske et al. 2010).
Power analysis
Using the methods outlined in Guillera-Arroita and Lahoz-Monfort (2012), we identified the survey effort requirements to detect C. penicillatus occupancy declines of different magnitudes with a given statistical power. These methods provide approximations (equation 1) to calculate how the power of a given occupancy-detection study changes depending on the allocation of survey effort (i.e. number of sites and replicate visits), assuming a standard sampling design with k replicate surveys (here trap nights) carried out at S sampling sites, and constant probabilities of occupancy and detectability. The calculations assume that two data sets are collected (one at time 1 and one at time 2), analysed, and their estimated occupancy probabilities with associated uncertainties compared to assess whether there is evidence of a decline between these two times. The probability of observing a significant difference in occupancy (i.e. power), given a significance level α, is
 (1)
(1)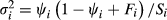 is the variance of the occupancy estimator, and F =
is the variance of the occupancy estimator, and F =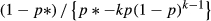 . For convenience, hereafter we defined R to be the proportional difference in occupancy, so that
. For convenience, hereafter we defined R to be the proportional difference in occupancy, so that 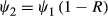 , with R > 0 representing a decline. For a given R, the power to detect the decline increases both as the number of sampling sites (S) and the number of repeat visits (k) increases.
, with R > 0 representing a decline. For a given R, the power to detect the decline increases both as the number of sampling sites (S) and the number of repeat visits (k) increases.We applied equation 1 using the fitted estimates obtained for occupancy and detectability to explore the number of sampling sites required to achieve a given power for detecting changes in C. penicillatus occupancy. We set ψ1 to the occupancy estimated as part of our analysis described above, and set ψ2 to reflect three different magnitudes of decline (i.e. effect size) corresponding to the IUCN Red List decline thresholds for threatened species based on rule A2c (a decline in the area of occupancy where the cause may not have ceased): 80%, 50% and 30% declines over relevant time periods (in the case of C. penicillatus, 10 years, which is greater than three generations) (Burbidge & Woinarski 2016). We did not consider criterion A1 (declines in area of occupancy where the cause of decline has ceased) because there is evidence to suggest some threatening processes are ongoing and could cause rapid declines in this species (Davies et al. 2016; Woinarski et al. 2017).
The calculations above assume the species is monitored twice: once at the beginning and once at the end of the period over which change is considered. More frequent monitoring will yield greater statistical power to detect the same decline, and simulations can be run to compute power for different survey designs (e.g. see Table 1 in Guillera-Arroita & Lahoz-Monfort 2012). The calculations also assume independence in the occupancy status of sites across time steps. Accounting for dependence may lead to increased power to detect declines. Thus, by assuming independence, we are being conservative in our evaluation (i.e. power will be as indicated or greater; see appendix 2 in Guillera-Arroita & Lahoz-Monfort 2012). Where survey data across multiple seasons are available from the same sites, multi-season models can be fitted to parameterise probabilities of extinction and colonisation that reflect the dependence in occupancy status of sites across time, and the information accounted for in sample size assessments (Popescu et al. 2012). This implies that future monitoring should continue sampling the same sites, which is unlikely to be the case here.
For all of our analyses, we set alpha (α) to 0.2 and beta (β) to 0.8. Our rationale behind this choice is that it better reflects the ratio of Type I and Type II costs in threatened species conservation, where committing a Type II error (i.e. not detecting a decline when one has occurred) could have implications that ultimately lead to extinction. In contrast, the general 0.05:0.8 convention assumes that the cost of making a Type I error is four times more important than the cost of making a type II error (for detailed reviews on setting alpha and beta values see Di Stefano 2003). Sample code for the power analysis conducted in this study is available online as a supplementary material in Guillera-Arroita and Lahoz-Monfort (2012).
Monitoring scenarios
We considered three different monitoring scenarios:
In ‘Scenario A’ the assumption is that monitoring will target C. penicillatus likely habitat, excluding the more marginal sites. To calculate average occupancy to inform survey design, we took the 200 sites with the highest probability of occupancy as determined by our best model (Table 1). This eliminated low probability sites (less than 0.07 probability) that largely reflected habitats unlikely to be suitable for C. penicillatus; for example, treeless plains and mangrove forests (Firth et al. 2006a). This monitoring regime also assumes that surveys are conducted during the late dry season (July–September) when C. penicillatus detectability is highest, and therefore assumes a sampling effort of two repeat visits to each site (as this is sufficient for detecting C. penicillatus with greater than 95% confidence, as discussed in the results section below). This is the monitoring regime that takes the greatest account of the model results. Scenario B targets the same type of sites, but assumes surveys are conducted year round (i.e. the design assumes a level of detectability as averaged throughout the year, and thus assumes a sampling effort of four repeat visits to each site. Scenario C takes what may be considered a naïve approach in targeting a random selection of sites and conducting surveys all year round (i.e. detectability averaged throughout the year), effectively ignoring knowledge gained through the modelling process. Like Scenario B, Scenario C too assumes a sampling effort of four repeat visits to each site. Four nights were chosen as this reflects the current standards for sampling of small mammals across the Northern Territory (Gillespie et al. 2015), and thus would realistically be applied if one had not modelled pilot data to better inform monitoring (i.e. the conditions under Scenario A).
For these monitoring scenarios, we considered the extent of sampling required to detect changes relevant to IUCN conservation status categories (i.e. 30%, 50%, 80%) across two monitoring episodes, here assumed to be 10 years apart (i.e. matching the time period relevant to the IUCN criterion A).
Monitoring costs
We calculated the costs associated with conducting monitoring to detect declines in occupancy of differing magnitudes (corresponding to the IUCN Red List Criteria) under each of the three monitoring scenarios described above. This includes the costs associated with equipment, bait for traps, travel and field assistant salaries (Garden et al. 2007; De Bondi et al. 2010) (details of estimated expenditure can be found in Appendix S1). While we provide an estimate of equipment expenses, we focus on the costs required to implement ongoing monitoring under each scenario, including in our calculations only 10% of the initial equipment costs. This was considered appropriate to account for minor repairs and replacement associated with the ongoing use of equipment.
Results
Naïve occupancy (the proportion of sites with C. penicillatus detections) was 0.15. The null model (containing no covariates) estimated an occupancy of 0.18 (SE ± 0.02) and a detectability of 0.47 (SE ± 0.05) (per trapping night). No single model was clearly superior in explaining the patterns of occupancy and detectability (Table 2). Island, canopy height, canopy cover, fire impact, distance to the nearest watercourse, mean rainfall and foliage projection cover were all important predictors (Table 2), featuring in all the top candidate models (those within four AIC units of the best fitting model); the only exception was fire impact which was absent from the last top ranked model. All of the variables considered to be important predictors of occupancy had a summed Akaike weight ≥93% (appendix 2). Basal area of large trees, fire frequency and total grass cover featured in some of the competing models as explanatory variables for the variation in observed occupancy, but had little support (summed Akaike weights ≤36%, Appendix S2). Both season and fire frequency had high support for explaining variation in detectability, featuring in all top ranked models (Table 2) and with summed Akaike weights >99% (Appendix S2). The overall direction and effect size of the estimated relationships (regression coefficients) remained similar for each of the covariates across all top ranked models (Appendix S3). Therefore, we focus on the top ranked model as an explanation for the observed data. The model suggests that the probability of C. penicillatus occupying a site increases with canopy height, distance from the nearest watercourse, foliage projection cover and mean annual rainfall, and decreases with canopy cover, increasing fire impact and Island (with occupancy lower on Bathurst Island) (Fig. 2a).
| ID | Candidate models | AIC | ∆AIC | Wi | Occupancy | Detectability | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| ψ (±SE) | CI | P (±SE) | CI | |||||||
| 1 | P (S + FF) Ψ (IS + CH + CC + FI + DW + RF + FC) | 426.38 | 0.00 | 0.22 | 0.24 (0.07) | 0.13 | 0.34 | 0.45 (0.07) | 0.31 | 0.59 |
| 2 | P (S + FF) Ψ (IS + CH + CC + FI + DW + RF + GC + FC) | 426.48 | 0.10 | 0.21 | 0.25 (0.07) | 0.010.13 | 0.41 | 0.44 (0.07) | 0.30 | 0.58 |
| 3 | P (S + FF) Ψ (BA + IS + CH + CC + FI + DW + RF + GC + FC) | 427.77 | 1.40 | 0.11 | 0.25 (0.07) | 0.12 | 0.42 | 0.44 (0.07) | 0.30 | 0.58 |
| 4 | P (S + FF) Ψ (BA + IS + CH + CC + FI + DW + RF + FC) | 427.82 | 1.44 | 0.11 | 0.24 (0.07) | 0.13 | 0.40 | 0.45 (0.07) | 0.31 | 0.59 |
| 5 | P (S + FF) Ψ (IS + CH + CC + FI + FF + DW + RF + FC) | 428.28 | 1.90 | 0.08 | 0.24 (0.07) | 0.12 | 0.40 | 0.45 (0.08) | 0.31 | 0.59 |
| 6 | P (S + FF) Ψ (IS + CH + CC + FI + FF + DW + RF + TC + FC) | 428.40 | 2.09 | 0.08 | 0.25 (0.08) | 0.12 | 0.42 | 0.44 (0.08) | 0.30 | 0.58 |
| 7 | P (S + FF) Ψ (IS + CH + CC + FI + DW + RF + TC) | 429.25 | 2.87 | 0.05 | 0.25 (0.07) | 0.13 | 0.40 | 0.44 (0.08) | 0.29 | 0.58 |
| 8 | P (S + FF) Ψ (BA + IS + CH + CC + FI + FF + DW + RF + TC + FC) | 429.77 | 3.39 | 0.04 | 0.25 (0.08) | 0.11 | 0.43 | 0.44 (0.08) | 0.30 | 0.58 |
| 9 | P (S + FF) Ψ (BA + IS + CH + CC + FI + FF + DW + RF + FC) | 429.81 | 3.42 | 0.04 | 0.24 (0.07) | 0.11 | 0.41 | 0.45 (0.07) | 0.30 | 0.59 |
| 10 | P (S + FF) Ψ (IS + BA + CH+CC + FI + FF + DW + RF + GC) | 429.84 | 3.45 | 0.04 | 0.25 (0.07) | 0.13 | 0.41 | 0.44 (0.08) | 0.29 | 0.58 |
| 11 | P (S + FF) Ψ (IS + CH + CC + DW + RF + TC + FC) | 430.12 | 3.74 | 0.03 | 0.26 (0.08) | 0.13 | 0.43 | 0.42 (0.07) | 0.29 | 0.56 |
| Null | P (.) Ψ (.) | 496.20 | NA | NA | 0.18 (0.02) | 0.14 | 0.23 | 0.47 (0.05) | 0.37 | 0.57 |
- AIC, Akaike Information Criterion, ∆AIC, Akaike unit difference, Wi, Akaike weight, ψ, mean probability of occupancy, P, mean probability of detection, SE, standard error, CI, confidence intervals, S, season, FF, fire frequency, BA, basal area of large trees, IS, island, CH, canopy height, CC, canopy cover, FI, fire impact, DW, distance to watercourse, RF, mean rainfall, GC, total grass cover and FC, foliar projection cover. [Correction added on 14 November 2018, after first online publication: The Akaike unit difference on row 2 has been corrected from ‘0.01’ to ‘0.10’.]
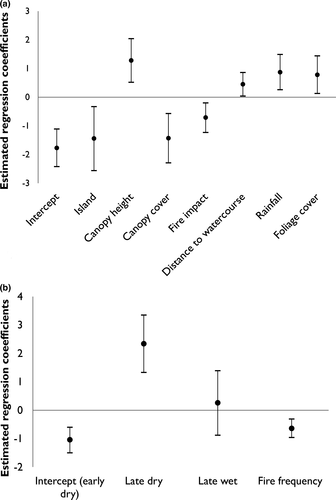
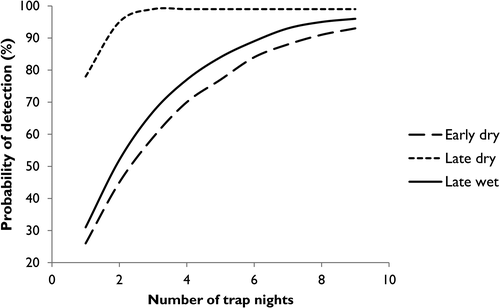
We found that detectability varied seasonally (Fig. 2b), with nightly detection probabilities much higher in the late dry season (July–September), 0.78 (SE ± 0.02), compared to the early dry (Apr–June), 0.26 (SE ± 0.08) or late wet (January–March), 0.31 (SE ± 0.08). This suggests that surveys conducted in the late dry season would require far less effort (i.e. fewer repeat visits) to ensure high certainty that C. penicillatus is detected when present (Fig. 3).
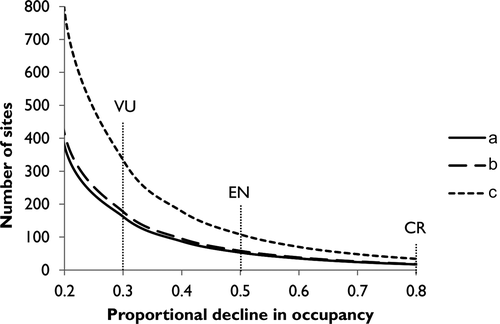
We calculated the number of survey sites required for detecting declines of 30, 50 and 80% in C. penicillatus occupancy under each monitoring scenario (Fig. 4). Our results show that fewer sites and visits were required under Scenario A compared with Scenario B, and less than half the number of sites were required under Scenario A compared with Scenario C to detect declines corresponding to each IUCN threatened category (Vulnerable, Endangered and Critically Endangered) (see Fig. 4 and Appendix S4).
The relative costs associated with the ability to detect declines corresponding to each IUCN threatened category (Vulnerable, Endangered and Critically Endangered) for all monitoring scenarios are outlined in Table 3. Scenario A is the most cost-effective method for detecting declines of a magnitude great enough to nominally qualify C. penicillatus for a threatened (or more threatened) status, saving approximately $11 700, $40 500 and $123 200 compared with Scenario B and approximately $46 800, $150 200 and $467 800 compared with Scenario C (for allocation of Critically Endangered, Endangered and Vulnerable threat categories respectively) (Table 3).
| Proportional decline in occupancy | Costs (AU$) | ||
|---|---|---|---|
| A | B | C | |
| 30% | 265 356 | 388 515 | 733 130 |
| 50% | 86 814 | 127 310 | 237 060 |
| 80% | 27 846 | 39 510 | 74 630 |
Discussion
Monitoring is a critical component of threatened species conservation, but requires sufficient power to detect and reliably estimate population trends (Guillera-Arroita & Lahoz-Monfort 2012). We show how a quantitative assessment of statistical power based on existing data can inform the design of monitoring to ultimately improve our ability to detect policy-relevant species’ declines.
We found that detectability for C. penicillatus is reasonably high (0.45 on average per trapping night), but varies greatly throughout the year: detection rates in the late dry season were three times higher than in the early dry (0.78 compared to 0.26). Although seasonal variability in detection of wildlife is well known for other taxa and generally considered in the timing of surveys, for example in butterflies (Pellet 2008), burrowing owls (Latif et al. 2012), bats in maternity caves (Baudunette et al. 1994) and amphibians (Sewell et al. 2010), there is little evidence in the literature to suggest that such variability has been considered when monitoring mammals in a tropical climate. In highly seasonal environments (i.e. those closer to the poles), seasonal changes (and subsequent changes in detectability) are more obvious, particularly for species that hibernate (i.e. mountain pygmy possums, Geiser & Broome 1991) or go into torpor (i.e. bats, Geiser & Brigham 2000). Here, we show that explicit consideration of monitoring design, based on seasonal variability, can be critical, even in contexts where seasonality and changes in temperature are less apparent (i.e. in areas closer to the equator). Our findings have strong implications for the cost-effectiveness of monitoring and management of C. penicillatus, and potentially other threatened taxa with similar ecologies and life history characteristics in seasonal environments. They also demonstrate the need to account for imperfect detection when analysing survey data, as otherwise, declines may be masked or exaggerated by seasonal inconsistency in sampling and seasonal variation in detectability.
Several factors could explain the higher probability of detection during the late dry season compared with the early dry season. Food resources are more abundant during the early dry season (related to plant productivity following wet conditions), potentially reducing the chance of an individual entering a trap in search of bait. Seasonal variation may also relate to C. penicillatus breeding patterns; high numbers of juveniles at the end of the dry season may result in higher trap success due to increased relative abundance and/or inexperienced, less cautious individuals. Conilurus penicillatus is just one example of a species displaying strong seasonal breeding cycles – it can be expected of other rodents and some dasyurids (i.e. the northern quoll Dasyurus hallucatus) – highlighting the importance of accounting for this when examining population trends.
The most important variables driving occupancy of C. penicillatus (canopy height, canopy cover, fire impact and distance to nearest watercourse) were consistent with a previous analysis of this data (Firth et al. 2006a). This species shows a preference for eucalypt forests with taller trees, less intense fire and drier upland areas. While fire is thought to influence the occupancy of C. penicillatus, the results of this study were somewhat equivocal. Fire frequency was not a strong predictor for occupancy, while fire impact – a field-based measure of the apparent severity of fire – had greater support, and was negatively correlated with occupancy. Firth et al. (2010) found that late dry season fires contribute to a reduction in both juvenile and adult survival probabilities in C. penicillatus due to a greater impact on vegetation cover and the loss of important resources such as den sites (i.e. logs). Similarly, McDonald et al. (2016) concluded that fire is an important driver of grass cover, which influences the occupancy patterns of another rare rodent (the critically endangered central rock-rat Zyzomys pedunculatus). This outcome suggests recent severe fire events are shaping C. penicillatus occupancy rather than the number of fires that has occurred over time. More intense fire may lead to lower perennial grass species diversity, and thus a reduction in the availability and variety of seed (Russell-Smith et al. 2000). Fire impacts may also be synergistic with other potential causes of declines, such as predation by feral cats, with cat abundance and hunting efficiency shown to increase in areas that have been subject to recent intensive fires in other comparable environments in northern Australia (McGregor et al. 2014; Leahy et al. 2015; Davies et al. 2016).
The power to detect population trends can be improved by increasing the sampling effort, but there are always financial and logistical constraints limiting the effort that can be applied to a particular monitoring programme. Alternative methods have been proposed for improving power; one example is excluding sites with a low probability of occupancy (Rhodes et al. 2006). This approach is explored in this study, where we considered a strategy that targeted sites with a probability of C. penicillatus occupancy greater than 7%, therefore focusing our inference on declines in its core distribution. Our results show that to detect smaller proportional changes in occupancy (<50%), a substantial improvement in power can be achieved by targeting suitable habitats, reducing the survey effort (and thus costs) required to detect declines. Conducting sampling when detectability is highest improves power and reduces costs further. Species’ presence can be inferred with high confidence in two repeat visits when monitoring is conducted during the late dry season, while four times as many visits are necessary to infer the same level of confidence in the early dry season.
One note of caution is that, in assessing the power to detect a decline, we are identifying the ability to detect that there is a decline, but this does not necessarily imply that the true magnitude of that decline is estimated. For example, a power of 0.8 for detecting a decline of 30% between two sampling times indicates that, given there is a true decline of 30%, there is an 80% chance that the statistical analysis of the data identifies a decline greater than 0. The estimated decline may be smaller than the true decline, and may be insufficient to allocate to a threatened category. One can formulate a more stringent null hypothesis to guide the survey design. For example, we could design the sampling to ensure there is high power to estimate a decline greater than X, when the true decline is Y, but this naturally leads to greater sample size requirements (Guillera-Arroita & Lahoz-Monfort 2012). Note, however that one cannot set X = Y, as this would require an infinite sample size (to obtain an estimate of the truth with no uncertainty).
Another important consideration is that as a species declines, its detectability may also decline, thus leading to greater difficulty in detecting a change between sampling occasions. Conilurus penicillatus has declined on the Tiwi Islands in the last 15 years, attributed primarily to predation by feral cats (most likely a consequent result of changing fire regimes and associated impacts on vegetation cover). On Melville Island, it is now restricted to areas with low probabilities of cat occupancy and high shrub density, where predation effects are thought to be effectively diminished (Davies et al. 2016). Trap success in 2015 was less than a third of that reported in monitoring conducted in 2000–2002 (Davies et al. 2016), suggesting that the species is likely to have lower probabilities of both occupancy and detectability across the Island. In the light of new data, we must recognise that there is a possibility that two trap nights, sampled during the late dry season when detectability of this species is highest, may now be insufficient for obtaining high (>95%) confidence in detection.
If one has reason to suspect that a decline has occurred between two samples, then it may be worthwhile calculating power against different levels of detectability during the early stages of survey design. This would allow for a more conservative approach to be developed that can meet the project objectives in the event of a decrease in detectability between sampling occasions. In the context of C. penicillatus, implementing alternative trap methods could overcome limitations associated with decreasing detectability through time. Motion-sensor cameras are a non-invasive survey tool that have been successfully used for several mammals of varying sizes (Rendall et al. 2014; McDonald et al. 2015; Welbourne et al. 2015), including C. penicillatus (Davies et al. 2016). Once deployed, cameras may be left in the field for long durations of time, thus collecting data across a greater temporal scale with fewer resources (De Bondi et al. 2010). The data obtained from cameras can be analysed in a similar way to provide insights into survey design and power to detect change (Davies et al. 2016).
Designing an effective monitoring programme will depend upon the objectives of the study, however, if practitioners are interested in detecting a decline of magnitude great enough for allocation to a ‘threatened’ category (Vulnerable, Endangered, Critically Endangered), then implementing a monitoring regime capable of detecting smaller declines (i.e. ≤30%) within an allocated budget would be ideal. As we have shown here, detecting a larger decline requires less resources than detecting a small decline, so designing a monitoring regime that is capable of detecting smaller declines will lead to increased confidence in our ability to detect more catastrophic declines (i.e. >50%).
However, land practitioners must also consider the scale and frequency at which monitoring takes place. The IUCN Red List Criteria applies to declines across a species’ entire distribution, and is generally applied at the species level. Though the population of C. penicillatus on the Tiwi Islands is considered a distinct subspecies (C. p. melibius), a reported decline in this population alone would not be sufficient to upgrade its’ conservation status at the species level. A recent study (Geyle et al. 2018) identifying the Australian mammals most likely to go extinct in the next two decades placed both Australian subspecies (C. penicillatus melibius and the mainland C. penicillatus penicillatus) in the top 20 most at risk suggesting that each may require emergency intervention to ensure their ongoing persistence. Gaining an understanding of population trends for both subspecies is thus crucial if we are to improve their conservation outlook.
The frequency in which monitoring occurs is also important. In this case, we have used a 10-year interval, as it is related to the generation time for which a decline must occur for a species to be eligible for conservation status assessment. However, in practise, if monitoring occurs more frequently, there is more likelihood of detecting a decline in a time-sensitive manner, and subsequently managers will be able to respond more effectively and rapidly to the threats driving such declines.
Despite some limitations of our approach, power analyses provide important insight into whether a study is worth conducting by identifying if the change considered meaningful can be detected with reasonable probability using an affordable sample size. Power analysis is an important tool in the development of effective monitoring regimes capable of achieving the desired study outcomes (Guillera-Arroita & Lahoz-Monfort 2012). We show how existing data can be used to estimate parameters required to determine optimal sample sizes, and thus provide powerful insights into the effectiveness of existing monitoring methods at achieving different research and management goals. Our findings demonstrate that a targeted, fit-for-purpose monitoring protocol has greater power to detect declines for C. penicillatus than a design targeted at multiple species. However, we recognise that in many cases, particularly at large spatial scales, single-species monitoring is often impractical. For example, the Tiwi Islands are home to many threatened species that have suffered widespread declines across northern Australia. Future research should explore ways to optimise power for detecting simultaneous declines in multiple threatened species to ensure better use of resources, especially given the sudden collapse of a wide range of small mammals in Kakadu National Park on the adjacent mainland in recent decades (Woinarski et al. 2011). We suggest targeting several species with similar ecological needs, habitat preferences and life history characteristics, as this study highlights the importance of targeting particular sites based upon the local habitat characteristics present and seasonal fluctuations in detectability.
Monitoring programmes that detect a change in abundance or occupancy, while of great importance, simply identify the problem, which is only one of the steps contributing to threatened species conservation. Ideally, monitoring programmes should provide some insight into the potential causes of such change, and designs should thus synchronously include site level consideration of the putative threatening factors (Lindenmayer et al. 2012). As demonstrated here, good sampling design can provide information not only on trends but also on factors influencing those trends. In this case, our analysis indicates that fire regimes are associated with variation among site occupancy, and hence may be contributing to the observed pattern of decline. Such careful consideration of survey design will ultimately lead to a far greater level of confidence in our ability to detect declines, and understand the reasons for them, which in turn may lead to more informed and better conservation outcomes.
Acknowledgements
We thank the Tiwi Land Council and Traditional Owners for their ongoing support of scientific research on their land, and the Northern Territory Government employees responsible for collecting the baseline Tiwi data set used in this study. This work was supported by the Australian Research Council (DE130100434 and DE160100904), the Centre of Excellence for Environmental Decisions, VESKI and the Victorian Government (through a 2015 Inspiring Woman Fellowship) and the National Environmental Science Program's Threatened Species Recovery Hub. We also thank the four anonymous reviewers whose comments improved this manuscript.




