One-way gates successfully facilitate the movement of burrowing bettongs (Bettongia lesueur) through exclusion fences around reserve
Abstract
When native herbivores are enclosed in fenced reserves without predators or dispersal options then overgrazing can occur, leading to damage to vegetation and co-occurring fauna species. One-way gates that allow medium-sized herbivores to exit fenced reserves may be an effective management tool to address overabundance or facilitate population expansion. We tested the use of one-way gates to facilitate the movement of the reintroduced burrowing bettong (Bettongia lesueur) from inside to outside a fenced reserve in arid South Australia. One-way gates were installed in the exterior fence of the reserve and assessed using remote motion-sensor cameras. The influence of gate position (dune, swale or corner) and provision of food were assessed in relation to gate visits and exits. Animals were trapped inside and outside the gates to determine any population bias in gate exits. Baited gates recorded significantly more exits than unbaited gates and dune gates had higher exit rates than interdunal swale gates. When gates were unbaited, those installed in corners of the reserve showed significantly higher visitation by bettongs and a non-significant trend towards more exits compared to gates placed in straight sections of fence along dunes or swales. There was no sex or age bias of burrowing bettongs using the gates and bettongs travelled between 75 m and 1535 m from their warrens to use the gates. No non-target species gained access to the reserve through the one-way gates and only two non-target animals used the gates to exit the reserve confirming gate specificity for bettongs. During the same period, 96 burrowing bettongs exited the reserve through the one-way gates. One-way gates may be a management strategy for facilitating passive movement of medium-sized herbivores outside of fenced reserves for the purposes of reducing overpopulation or facilitating population expansion outside reserves.
Introduction
Reintroductions have become increasingly popular as a tool in animal conservation in Australia and internationally (Slotow et al. 2005; Clayton et al. 2014). Predation by introduced predators has considerable influence on the success of a reintroduction and is the primary cause of reintroduction failure for mammals (Fischer & Lindenmayer 2000; Short 2009; Moseby et al. 2011; Clayton et al. 2014). Predator-proof fencing is an effective way of limiting the effects of predation on reintroduced populations of mammals and is an increasingly common tool used in wildlife restoration projects (Moseby & Read 2006; Schick & Schick 2012; Hayward et al. 2015). There are several secondary benefits that exclusion fences may offer, including regeneration of native plants and invertebrates, recolonisation by birds (Schick & Schick 2012), increase in abundance of non-target animals (Read & Cunningham 2010) and an increase in species richness (Moseby et al. 2009). However, despite these positive effects, fencing may also exacerbate prey naivety and lead to habitat fragmentation (Boone & Hobbs 2004), restriction of dispersal (Hayward & Kerley 2009) and overpopulation with resultant vegetation damage (Hayward et al. 2007; Linley et al. 2016; Moseby et al. 2018).
Overabundance of herbivores is a potential problem where exotic-introduced predators have been eradicated (Kunovac 2001; De Tores & Marlow 2012; Dexter et al. 2013). In the absence of predators and with restricted dispersal pathways, some herbivores can reach high densities, damaging vegetation (Moseby et al. 2018), impacting other fauna species (Bergstrom et al. 2009; Foster et al. 2014) and reducing the carrying capacity of fenced reserves (Dexter et al. 2013). Population control is sometimes implemented to mitigate overabundance problems in small reserves (Slotow et al. 2005) including fertility control (Adderton 2004; Raiho et al. 2015), culling (De Tores & Marlow 2012) or capture and removal (De Tores & Marlow 2012; Bannister et al. 2016). Ideally, methods to control overpopulation would allow for natural dispersal, be low cost, ethically sound and non-lethal. Currently, new techniques are being investigated such as introducing a suitable native predator (Licht et al. 2010) or developing one-way gates that can be installed in the fence to allow movement beyond the fence (e.g. Crisp & Moseby 2010).
In addition to issues with overpopulation, fencing restricts movement of many reintroduced species and prevents dispersal out into surrounding areas (Krebs et al. 1969). If such dispersal and movement were facilitated, then the benefits of fenced reserves could be expanded, creating additional habitat for threatened species. This could not only benefit the dispersing animals by increasing their range and access to preferred habitat but may potentially have flow-on effects to other animals or plants if the dispersing species is a keystone species (Miskelly et al. 2005; Brudvig et al. 2009). Depending on the method used to facilitate movement of animals, there is the potential for reserve managers to specifically direct animals towards resource-rich habitats adjacent to the fence (Cushman et al. 2016).
One-way gates are a potential option for facilitating the movement of animals outside fenced reserves. One-way gates specifically designed for animal use are used in the agricultural industry for managing livestock such as pigs (Kleinsasser 2011) and cattle (Stefanowska et al. 1997; Barasona et al. 2013). In wildlife situations, one-way gates have been used in England to remove and exclude badgers from setts that require demolishing (Adderton 2011). The use of two-way swing gates in fenced reserves have been reported both in Australia and in Africa allowing non-threatened species to disperse through the landscape (Schumann et al. 2006; Coates 2013; Rust et al. 2015). One-way gates are currently not used in fenced reserves for threatened species management. However, recent issues with overabundance at the Arid Recovery Reserve in South Australia have led to initial trials to design one-way gates that could be used for threatened species including the burrowing bettong (B. lesueur) and greater bilby (Macrotis lagotis) (Crisp & Moseby 2010). Although initial trials suggest that one-way gates may be effective, extensive field trials have not been implemented.
Our aim was to investigate whether one-way gates could be an effective tool for facilitating movement of bettongs outside the Arid Recovery Reserve. In particular, we tested the influence of gate placement and lures on the visitation and exit rate of bettongs and non-target fauna as well as investigating the demography of animals using the gates. We hypothesised that baited gates placed on dunes where bettong warrens are more common would result in the highest gate exits. Additionally, we expected exits of non-target species to be low relative to bettongs based on previous studies that designed and tested gates for specific species, including bettongs (Crisp & Moseby 2010). Results of this trial could be used by managers of fenced reserves where overpopulation of one species is negatively affecting other species or the ecosystem. If effective, the gates have the potential to reduce the population density of a specific species and in turn reduce negative impacts to an ecosystem. Managers could also use the gates to expand populations of threatened species outside fenced reserves.
Methods
Study site
The project was conducted at the Arid Recovery Reserve located in the arid zone of South Australia, 20 km north of Roxby Downs. The fenced area consists of 123 km2 of land dedicated to the restoration and conservation of arid ecosystems. The reserve is dominated by longitudinal sand dunes spaced 0.1–1 km apart separated by interdunal clay swales. The dominant vegetation on the dunes includes sandhill wattle (Acacia ligulata), hopbush (Dodonaea viscosa) and sandhill canegrass (Zygochloa paradoxa), while swales are predominantly chenopod shrubland (Maireana astrotricha and Atriplex vesicaria). Run as an independent, not-for-profit conservation initiative, the reserve works to restore arid ecosystems through eradication of invasive species such as cats (Felis catus), rabbits (Oryctolagus cuniculus) and foxes (Vulpes vulpes), and by reintroduction of locally extinct native animals historically found in the area. Burrowing bettongs were re-introduced into Arid Recovery in 1999 and 2000 when 10 and 20 animals were re-located from Western Australia (see Moseby et al. 2011 for details of the reintroduction). The burrowing bettong reintroduction was a success (Moseby et al. 2011) and the population is now estimated at greater than 8000 individuals and approximately 1 per hectare within the reserve (Moseby et al. 2018).
Study species
Burrowing bettongs (B. lesueur) share their warren during the day with their social group, but forage independently at night (Sander et al. 1997; Short & Turner 1999). The home range of burrowing bettongs at Arid Recovery is between 29 and 35 ha (Finlayson & Moseby 2004) with movements of up to 2 km from their warrens (Short & Turner 2000). Warrens are found throughout the Arid Recovery Reserve in both dune and swale habitat; although, bettongs prefer to build warrens in dunes (Finlayson & Moseby 2004).
One-way gates
One-way gates were based on a combination of two gate designs tested during pen trials by Crisp and Moseby (2010) with some minor modifications. Modifications involved using a metal rather than wooden casing and a lighter metal hinge which reduced the weight of the flap. The gates consisted of a rectangular metal casing 170 mm in width, 160 mm high and 600 mm long. A metal plate extended perpendicularly around three sides of one end of the casing so the gate sat flush with the inside of the fence (Fig. 1). Inside the casing, a clear Perspex™ flap was attached with a hinge to the roof of the gate approximately 300 mm inside the gate entrance. The flap acted like a cat-door flap with the hinge only allowing the door to swing outward when pushed, away from the inside of the reserve. A sock was attached to the end of the metal casing made from aluminum fly mesh, 600 mm long. The sock prevented the incursion of feral animals through the gates from the outside and prevented bettongs trying to re-enter the reserve through the gates. When gates were baited during the baiting trial, small canisters were wired to the sock for the placement of food attractants. These canisters had a screw top lid and small holes scattered throughout allowing the smell to permeate.

Gate exits
Animals leaving through one-way gates entered the buffer zone around Arid Recovery, where trapping and shooting were used to reduce introduced predator abundance. The gates were monitored every second day to check for damage to the gate or fence.
Two camera traps were installed per gate, one on the inside of the reserve facing the entrance of the gate and one on the outside facing the exit. The cameras used were a combination of Reconyx HyperFire, Bushnell NatureView HD and Little Acorn based on the resources available. Camera models were randomly placed at gate sites. The cameras were set to take three photos, one-second apart, after each trigger. The cameras were set at a 45 degree angle facing the gate to capture movement from an animal entering or exiting the gate.
The camera data were analysed to record both visits and exits. A visit to the gate was recorded if a bettong approached a gate inside the reserve within at least 1 m of the entrance including animals that entered gates and then retreated without exiting. A new visit was considered to have occurred if there were 10 min between the last photo in the series and the next trigger of the camera. In order to count the exits, the time stamp of outside and inside camera photos were compared. If there was evidence of a bettong outside the gate within 10 min of one entering the gate, then an exit was assumed to have occurred. Cameras were also used to detect feral animals and non-target species using or visiting gates both inside and outside the reserve.
Gate placement
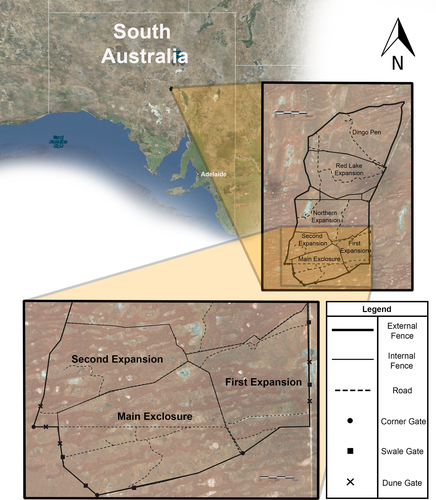
Thirteen gates were constructed and spaced around the reserve within three of the exclosures (Fig. 2) – the Main, First and Second. Five gates were installed at dune bases, at the juncture between the incline of the dune and the flat swale, in straight sections of the fence where it traversed sand dunes. Five gates were installed in straight sections of the fence that traversed the flat, open swale habitat, at least 400 m from a dune. Three gates were placed in corners of the fence line in swale habitat. The gates were placed at least 400 m apart due to previous studies reporting that bettongs travelled approximately 400 m from warrens each night to forage (Finlayson & Moseby 2004). Gates were set for a period of 18 nights in July 2016 and all gates were opened simultaneously.
Effect of supplementary baiting
A cross-over trial was designed to test whether baiting the gates increased the exits. Half of the dune and swale gates were randomly assigned and baited for nine consecutive nights with a mixture of peanut butter and oats placed into the canister wired to the one-way gate sock. After nine nights, the treatments were switched and the other half were baited. Old bait was removed and fresh bait was provided every four nights. The corner gates were never baited and were left open during the cross-over trial.
One-way gate bias
Three cage traps, baited with peanut butter and oat balls, were placed at the exit of each gate outside the reserve for three consecutive nights during the period when the gates were baited. The traps were placed around the exit of the gate with the door pointing towards the opening of the sock approximately 200 mm away from the exit and from each other. The traps were checked early in the morning before sunrise and captured animals were sexed, weighed, measured for pes length and size of testes and the presence of any pouch young recorded. Each animal was tagged with an ear-tag with a unique identifying number in order to record same session recaptures. Any large pouch young ejected from the pouch during handling were returned to the pouch as described in Delroy et al. (1986). Age of bettongs was estimated by body mass with bettongs <950 g classified as sub-adults. The body mass range for adult burrowing bettongs is between 1000 and 1300 g with no difference between males and females (Tyndale-Biscoe 1968; Seebeck et al. 1989).
The body mass, sex ratio and pouch young status were compared between bettongs caught outside one-way gates and those captured during routine monitoring that occurred within the reserve over the same time period. One hundred and sixty cage traps were set inside the Main exclosure along the track network for four nights and baited with peanut butter and oat balls. The cages were checked every morning before sunrise and the animals were released immediately following processing.
Gate reach
Gate reach was defined as how far bettongs would travel from inside the reserve to the gates at the external fence line. Nine bettongs trapped outside the reserve at one-way gates were fitted with VHF radio collars (20 g Sirtrack Pty. Ltd), released at night back inside the reserve at the gate entrance and tracked to their diurnal warren the morning after release. The distance between the warren and the gate where the bettong was trapped was recorded in order to determine the reach of the gates. Cage traps were then placed around the warren to trap the animal and remove the collar.
Statistical analysis
To determine the influence of baiting on gate visits and exits, models were run on data from the cross-over trial which excluded data collected from corner gates which were not baited. Separate generalised linear mixed models were run on gate visit and exit data. These models used a negative-binomial distribution as this best explained the overdispersal in the data. Landform and baiting treatment were the independent variables and gate visits or exits was the dependent variable. These models also included an interaction term between baiting and landform. Generalised linear models were then run using data only from unbaited gates to determine the effect of landform (dune, swale or corner) on the number of visits and exits of bettongs at gates. These models used the poisson distribution and separate models were run for gate visits and exits.
To test for any demographic bias in animals using the gates, a chi-square analysis was conducted comparing the proportion of males and females and the proportion of adult and sub-adult bettongs trapped outside the gate and inside the reserve. To test gate specificity, we used a chi-squared analysis to compare the percentage of visits that ended in exits for each of the re-introduced species in the reserve.
An independent samples t-test was performed comparing the distances that male and female bettongs travelled from their warrens to the gate to see if there was a sex effect.
Results
Over the 18 nights of the trial, a total of 96 burrowing bettongs exited the reserve using the 13 one-way gates. The average number of bettongs leaving through each gate was 0.41 individuals per gate per night.
Baited versus non-baited
Baiting increased the attractiveness of the gates to burrowing bettongs as well as increasing the number of bettongs exiting gates (visits: P < 0.0001, df = 1, F value = 85.08; exits: P < 0.0001, df = 1, F value = 19.46). For baited gates, there was an average of 8.80 visits (±0.88) and 0.63 exits (±0.12) per gate per night compared to an average of 4.34 visits (±0.46) and 0.07 exits (±0.04) when gates were unbaited (Table 1). The percent of visits that ended in exits was higher when gates were baited (8.1%) compared to unbaited gates (2.8%) (Table 1).
| Treatment | Total visits | Average visits (per gate/night) | Total exits | Average exits (per gate/night) | Visits to gates ending in exits (%) |
|---|---|---|---|---|---|
| Baited | 844 | 8.80 (±0.88) | 68 | 0.63 (±0.12) | 8.1 |
| Unbaited | 424 | 4.34 (±0.46) | 12 | 0.07 (±0.07) | 2.8 |
Landforms
During the cross-over trial, significantly more bettongs left the reserve through dune gates compared to swale gates (visits: P = 0.013, df = 1 F value = 6.28; exits: P < 0.0009, df = 1, F value = 13.47). The highest exit rate was at baited dune gates where an average of 1.07 bettongs exited per gate per night. Within this cross-over trial, there was no significant interaction between bait and landform on the number of bettongs approaching or exiting gates (visits: P = 0.834, df = 1; exits: P = 0.313, df = 1). This suggests that the attractiveness of the bait was unlikely to be influenced by gate placement (dune or swale).
When models were run on data from unbaited gates only, there was a significant difference in gate visits between landforms (P = 0.049, df = 2, F value = 3.00) but no significant difference in the exits from corner, dune and swale gates (P = 0.167, df = 2, F value = 1.59). Post-hoc Tukey tests showed gate visits were higher at corner gates (Table 2) compared to swale gates (P = 0.050) with a trend towards more visits to corner gates compared with dune gates (P = 0.745). There was a trend (not significant) towards more exits from corner gates compared to swale (P = 0.224) and dune gates (P = 0.992) (Table 2).
| Treatment | Total visits | Total exits | Average visits (per gate/night) | Average exits (per gate/night) | Visits to gates ending in exits (%) |
|---|---|---|---|---|---|
| Dune | 239 | 10 | 5.01 (±0.79) | 0.11 (±0.75) | 4.2 |
| Corner | 324 | 16 | 5.76 (±1.00) | 0.10 (±0.08) | 4.9 |
| Swale | 185 | 2 | 3.67 (±0.59) | 0.02 (±0.02) | 1.1 |
Gate reach
For the nine bettongs captured outside the gates and re-released back into the reserve, the shortest distance a bettong moved from the diurnal warren to the gate at which it was caught was 75 m and the longest distance was 1535 m (SE = 90, n = 9). On average, females travelled further than males (average male distance: 292.5 m, average female distance: 512.7 m) but this was not significantly different (t-test, P = 0.614, df = 8).
Bias – sex and age
There was no significant demographic bias in gate use between sex or age. Cage trapping inside the reserve in the vicinity of the gates in August 2016 recorded a sex ratio of 238 males to 176 females (1.35:1). In total, 19 male and 20 female bettongs were caught outside the reserve (0.95:1). A chi-squared test supported the hypothesis that there was no bias in the sex of the bettongs using the gates (χ2 = 1.12, P = 0.29, df = 1). Baiting the gates did not lead to any sex ratio bias. Unbaited gates had a ratio of 1:1 males (5) to females (5) and baited gates had a ratio of 0.93:1 males (14) to females (15) (χ2 = 0.009, P = 0.93, df = 1).
Trapping of bettongs inside the reserve in August 2016 recorded 10 sub-adults and 404 adults (0.003:1). This is compared with 2 sub-adults and 37 adult bettongs (0.005:1) trapped outside the gates in this study. There was no age bias in animals using the gates to exit the reserve (χ2 = 1.02, P = 0.31, df = 1). Baiting the gates did not lead to any age bias with 27 adults and 2 sub-adults leaving through baited gates and 10 adults and 0 sub-adults leaving through unbaited gates (χ2 = 0.727, P = 0.394, df = 1). Most (90%) of the 20 female bettongs that were trapped on exiting the gates were carrying pouch young.
Use of gates by other fauna
Outside cameras detected 69 rabbits (O. cuniculus), 37 kangaroos, 32 feral cats (F. catus), no foxes (V. vulpes) and 22 wild dogs (Canis lupus) around the fence line of the reserve from 234 trap nights. No attempts were made by any species to enter the reserve via the gates and no damage to the gates was detected.
One greater stick-nest rat (Leporillus conditor) and one western quoll (Dasyurus geoffroii) used the gates to exit the reserve compared with 96 bettongs (Table 3). When comparing the proportion of visits that ended in exits with a chi-squared test, there was a significant difference between the species (χ2 = 20.53, P = 0.0004, df = 4). Bettongs and quolls had a higher proportion of visits ending in gate exits whilst bandicoots, bilbies and stick-nest rats had a lower proportion (Table 3).
| Species | Total visits | Total exits | Visits to gates that ended in exits (%) |
|---|---|---|---|
| Burrowing Bettong | 1592 | 96 | 6.0 |
| Western Quoll | 5 | 1 | 20.0 |
| Stick-nest Rat | 35 | 1 | 2.9 |
| Greater Bilby | 18 | 0 | 0.0 |
| Western Barred Bandicoot | 14 | 0 | 0.0 |
Discussion
One-way gates were successful in allowing burrowing bettongs to passively exit the reserve. The highest exit rate was observed through baited gates placed at dune bases. These gates successfully enabled an average of 1.07 bettongs per gate per night to exit the reserve. Burrowing bettongs have a known preference for dune habitat for foraging and warren construction (Finlayson & Moseby 2004). The remnant burrowing bettong populations on Dorre and Bernier Islands also favour dune habitat (Short & Turner 1999) suggesting that the higher exits recorded on dunes are likely related to higher density of bettongs living and foraging in dune habitat.
Interestingly, when gates were not baited, there was no significant difference in exits between landforms but the highest bettong exits were from gates installed in the corners of the reserve. Corners may act as funnels for animals within fenced reserves (Crisp & Moseby 2010) which could explain the increased activity at corner gates when gates are not baited.
On average, burrowing bettongs forage close to their warrens (average 320 m, Finlayson & Moseby 2004) closely corresponding with the mean distance bettongs were recorded travelling from their diurnal warren to exit gates (418 m). On Dorre and Bernier Islands, bettongs also typically move short distances with less than 200 m between captures (Short & Turner 1999). Our results suggest that animals do not generally move large distances to exit through a gate regardless of the presence of bait and that gates may need to be relatively closely spaced if managers wish to maximise the number of animals that can access the gates. However, the furthest distance travelled in this study was 1.5 km which is consistent with dispersing bettongs (Short & Turner 2000) and suggests that over longer time periods more bettongs may access gates.
Dispersal is typically male biased in bettongs with males dispersing significantly further than females (Parsons et al. 2002). Other species of bettong including Aepyprymnus rufescens, Bettongia tropica and Bettongia gaimardi also show evidence of male biased dispersal (Taylor 1990; Pope et al. 2005, 2012). The absence of any age or sex bias in bettongs using the one-way gates suggests that the gates are not targeting dispersing individuals or any other demographic group. Interestingly, baiting the gates had no influence on gate bias suggesting that the provision of food is not the reason why gates are attracting a range of demographic groups. The inquisitive nature of bettongs coupled with high population density may be rendering gates attractive to bettongs regardless of sex or age. Burrowing bettongs disperse between 170 and 250 days old and are usually less than 1 kg in weight at this time (Seebeck et al. 1989; Parsons et al. 2002). Our study found that the majority of animals using the gates were above 1 kg in weight and therefore may be older than dispersal age. However, to test whether dispersing bettongs use the gates more than adult animals, this study should be repeated at a time when there is a large proportion of sub-adult bettongs in the population.
The most successful gates used in wildlife management do not require animals to significantly change their behaviour in order to use them. For example, wombat-specific swing gates are placed in areas where wombats are known to dig underneath the fence (Coates 2013). Similarly, one-way deer-specific gates work as they allow the deer to walk along their natural paths (Reed et al. 1974; Ludwig & Bremicker 1983). The gates used in this trial were tested and designed to mimic the size and shape of Sheffield cage traps which readily trap bettongs. Bettongs are known to be ‘trap happy,’ with cage traps consistently used as a trapping method (e.g. Short & Turner 1999; Bannister et al. 2016). Bettongs also have no aversion to novel fixtures in their environment and have been noted interfering with traps set for other species (Moseby et al. 2009). Gates were designed to be flush with the ground, which require no step up, and roughly the same size as a warren opening so the bettong does not need to change its behaviour to enter.
Target specificity of one-way gates is important as it prevents the incursion of unwanted predators and stops the movement of vulnerable non-target species into unprotected areas (e.g. Schumann et al. 2006; Coates 2013). The gates in this trial were designed in accordance with previous trials that tested gate specificity and determined the gate dimensions specific for burrowing bettongs (Crisp & Moseby 2010). Results of the current trial suggested that the visitation rate and the percent of visits that ended in exits were significantly higher for burrowing bettongs than three other threatened species (stick-nest rat, bandicoot, bilby) that were not targeted. Stick-nest rats can exit the reserve if they wish by climbing the fences and fitting through the larger gauge wire (pers. obs Katherine Moseby, 2016). However, greater stick-nest rats are timid making it unlikely that large numbers would visit and use the gates (Moseby & Bice 2004). The only non-target species that had a higher percent of visits that ended in exits than bettongs were the western quoll with five visits and one exit. This species is partly arboreal (Glen & Dickman 2006) and also exits the reserve by climbing over the wire netting fences (Arid Recovery 2017) suggesting that one-way gates are unlikely to result in increased exit rates for this species.
Mammalian pests constantly test exclusion fences, pushing against them regularly, and are able to exploit a weakness within 24 h (Connolly et al. 2009). While this study detected feral animals outside of the reserve, there was no evidence of feral animals trying to breach the fence through the one-way gates suggesting one-way gates are unlikely to pose an incursion risk if they are maintained and checked regularly. Despite poison baiting and trapping programmmes implemented immediately outside the fenced reserve, predators were regularly recorded outside one-way gates on cameras and thus continue to pose a threat to bettongs that exit the reserve via the gates. It is imperative that predator control methods such as trapping and baiting are intensified and maintained alongside any gate programmme in order to increase the survival probability for exiting animals.
Conservation applications
The elevated population densities of burrowing bettongs within the Arid Recovery Reserve has resulted in vegetation damage (Linley et al. 2016; Moseby et al. 2018). One-way gates could be used to address the negative effects arising from overpopulation of herbivores in fenced reserves by allowing animals to disperse from the reserve when the population reaches a certain threshold. Under this management scenario, overgrazing of native plant species can be reduced as animals are allowed access to a new food source (Boone & Hobbs 2004). As the number of bettongs exiting the reserve is dependent on the number of gates installed, the presence of bait and the proportion of gates open at any one time, one-way gates have the potential to be manipulated to achieve sustainable population targets.
One-way gates could also be used as a tool to increase the effective area of reserves. Bettongs can have a significant positive influence on the environment and can increase the biodiversity of an area and improve ecosystem functioning when at sustainable densities (Martin 2003; Estes et al. 2011; Fleming et al. 2014). Allowing animals to disperse outside the reserve may facilitate positive ecosystem benefits to unfenced areas. Additionally, the targeted one-way gate system allows reserve managers to facilitate movement into specific areas such as new habitats during a favourable season (Cushman et al. 2016) or into areas with high levels of predator control adjacent to the fence.
Limitations and future studies
Although bettongs successfully used the one-way gates, the percent of visits that ended in exits was still low, even at baited dune gates where only 8.1 percent of visiting bettongs exited through gates. Increasing the exit rate would considerably increase the efficacy of one-way gates and while baiting has been shown to be a successful tool for increasing gate exits it does require increased maintenance. Scent lures may be a way to overcome the challenges associated with baiting; however, food baits are often better at attracting animals than scent lures alone (Espartosa et al. 2011). In some species, visual, olfactory and auditory lures can increase the attractiveness of a trap (Edwards et al. 1997; Moseby et al. 2004; Warburton & Yockney 2009; Hanke & Dickman 2013) and these could be trialled to increase both exit rates and specificity of one-way gates.
Our study suggests that one-way gates may be an effective tool for facilitating movement of medium herbivores outside of fenced reserves. Regardless of whether one-way gates are installed for management of overpopulation or expansion of population range, intensive predator control should be implemented outside fenced reserves in order to help animals survive after exit. Previous trials have shown that some predator control methods implemented in unfenced areas in Australia are not adequate and populations of native herbivores often fail to establish outside fenced reserves (Bellchambers 2001; Moseby et al. 2011; Bannister et al. 2016; Hardman et al. 2016). Therefore, there are ethical concerns associated with allowing animals to exit reserves without consistent and regular predator controls in place. If these predator control methods are implemented, threatened prey species may survive long enough to establish resident populations outside reserves thus increasing the effective population size of threatened fauna.
Acknowledgements
This study was supported by Arid Recovery, a joint conservation initiative between BHP Billiton, The University of Adelaide, SA Department for Environment, Water and Natural Resources and the Roxby Downs community. Funding and resources for this project were provided by the Arid Recovery, Nature Foundation SA Inc., NRM Research and Innovation Network Honours Scholarship Program 2016, and the School of Ecology and Environmental Science, University of Adelaide. The authors thank Monadelphous and the Frankston Rotary Club for constructing and developing the gates. Special thanks to Kath Tuft and the rest of the Arid Recovery staff, the Green Army and Steve Butler for their invaluable help with this project. Ethics approval for this project was obtained from the University of Adelaide Animal Ethics Committee.




