Sodium acamprosate and calcium exert additive effects on nucleus accumbens dopamine in the rat
Funding information: Swedish Medical Research Council, Grant/Award Numbers: 2018-02814, 2020-00559, 2020-01346, and 2020-02105; Stiftelsen Professor Bror Gadelius minnesfond; Systembolagets alkoholforskningsråd, Grant/Award Number: FO2020-0089.
Abstract
Acamprosate (Campral® – calcium-bis[N-acetylhomotaurinate]) is one of few available pharmacotherapies for individuals suffering from alcohol use disorder. Previously, we suggested that acamprosate reduces ethanol intake by increasing dopamine in the nucleus accumbens (nAc), thereby partly substituting for alcohol's dopamine releasing effect. An experimental study suggested the calcium moiety of acamprosate to be the active component of the drug and to mediate the relapse preventing effect. The aim of the present study was to, by means of reversed in vivo microdialysis, elucidate if the dopamine elevating properties of acamprosate are mediated by N-acetylhomotaurine or by the calcium moiety. Male rats were equipped with a microdialysis probe in the nAc and received acute local treatment with regular acamprosate (CaAcamp 0.5 mM), calcium chloride (CaCl2 0.5 mM), sodium acamprosate (NaAcamp 0.5–1 mM), the glycine receptor (GlyR) antagonist strychnine (Stry 20 μM), or vehicle. In all experiments, extracellular levels of dopamine and taurine were examined. We found that local perfusion with both CaAcamp and CaCl2 increased dopamine levels in a GlyR-dependent manner. NaAcamp did not influence dopamine levels, but concomitant administration with CaCl2 resulted in an additive dopamine output compared to the drugs administrated alone. We also found CaAcamp and the combination of CaCl2 and NaAcamp to increase accumbal taurine levels, suggesting that CaAcamp may act indirectly on GlyRs via taurine release. The present results indicate that both N-acetylhomotaurine and the calcium moiety of acamprosate have dopamine elevating properties within the nAc and that, in this respect, these substances are beneficial in combination.
1 INTRODUCTION
Alcohol addiction and the manifested use of alcohol in an abusive way account for a pronounced quantity of the total burden of disease and injury worldwide.1 Alcohol use disorder (AUD) is a chronic relapsing brain disease, involving several neuronal pathways and circuits including the mesolimbic dopamine system, a major part of the brain reward pathway.2, 3 Ethanol-induced activation of the mesolimbic dopamine system increases dopamine levels in the nucleus accumbens (nAc), a phenomenon common to most drugs of abuse and associated with reward and positive reinforcement.4, 5
One of the few pharmacotherapies available to treat AUD is acamprosate (calcium-bis[N-acetylhomotaurinate]; Campral®),6, 7 a drug initially found to have an ethanol intake-reducing effect in rat.8 Early clinical studies, with different biological and clinical endpoints, found acamprosate to generate a positive outcome with reduced alcohol intake and relapse frequency in patients with AUD.9, 10 Furthermore, a meta-analysis with data originating from both alcohol-dependent men and women demonstrated acamprosate to be beneficial relative to placebo when comparing four efficacy endpoints with no differences in drug response between the sexes.7 In several rodent models, acamprosate treatment was shown to attenuate alcohol seeking behaviour and decrease preference for ethanol,11, 12 as well as to reduce the frequency of relapse to high ethanol intake in a dose-dependent manner.13-15 Acamprosate was put on the market in France in 1989,16 that is, more than 30 years ago, and the mechanism of action is still not fully understood. In the development of new molecules targeting cellular or molecular mechanisms related to AUD, it is of importance to gain knowledge of the mechanism of action for existing treatment methods.
Acamprosate is a homotaurine derivative sharing structural analogies with both the γ-aminobutyric acid (GABA) and the endogenous amino acid taurine, a glycine (GlyR) and GABAA receptor agonist with a number of physiological functions including possessing osmoregulatory properties.6, 17 Several hypotheses have been put forward on the mechanism underlying the ethanol-intake reducing effect of acamprosate suggesting a modulatory role on both GABA and glutamate neurotransmission by direct or indirect interaction with GABAA-, NMDA- and mGluR receptors, respectively.8, 18-22 We previously demonstrated that GlyRs in the nAc and nicotinic acetylcholine receptors in the ventral tegmental area also participate in mediating the effects of acamprosate.23, 24 In line with others, we found acamprosate to have dopamine elevating properties, following both systemic and local administration in the rat, to a similar degree as previously demonstrated for ethanol25, 26 and taurine.27, 28 Thus, acamprosate may partially act as a neurochemical substitution for ethanol in its mesolimbic dopamine activating effect. Interestingly, an experimental study suggested that the calcium moiety of the calcium-bis(N-acetylhomotaurinate) compound is solely responsible for the drug effect on ethanol consumption.29 This conclusion was made because the sodium salt of N-acetylhomotaurinate failed to reduce excessive alcohol drinking, alcohol-seeking and relapse-drinking behaviour, whereas calcium administration alone was successful. It was further suggested that N-acetylhomotaurine lacks a binding site within the central nervous system and is biologically inert.29
As the mechanism of action underlying the effects of acamprosate is still elusive, we aimed to perform an in depth in vivo microdialysis study exploring the acute effects of different salt forms of N-acetylhomotaurinate and calcium on dopamine output in the nAc. Although it has been suggested that N-acetylhomotaurine is biologically inert, we hypothesised that homotaurine may act together with calcium to raise accumbal dopamine levels, and thus, that regular acamprosate could possess two active ingredients. We further postulated that regular acamprosate and calcium would elevate accumbal dopamine levels in a GlyR-dependent manner, thereby resembling the mechanism by which ethanol activates the brain reward system. Because acamprosate shares the same structural backbone as the amino acid taurine, we also aimed to characterise the tentative effects of these drugs on extracellular levels of taurine.
2 MATERIAL AND METHODS
2.1 Animals
Naïve male Wistar rats (Envigo, the Netherlands) weighing 280 to 340 g, corresponding to an age of 9–10 weeks, were used in the experiments. From arrival until the microdialysis probe-placement surgery, the animals were housed in groups of three to four animals in each cage, at constant room temperature (20–22°C) and humidity (55–65%) and were kept under a regular 12-h light/dark cycle (lights on at 7:00 AM and lights off at 7:00 PM). The animals had access to standard rodent chow and water ad libitum during the entire experiment and were allowed to adapt to the novel environment during 1 week before any experiments were initiated. The study was approved by the Ethics Committee for Animal Experiments, Gothenburg, Sweden.
2.2 Drugs and chemicals
Calcium-bis(N-acetylhomotaurinate) (CaAcamp) (kindly provided by Merck, Lyon, France), sodium-N-acetylhomotaurinate (NaAcamp) (TCI, Zwijndrecht, Belgium), CaCl2 (Fisher Scientific, Gothenburg, Sweden) and strychnine hydrochloride (Sigma-Aldrich, Stockholm, Sweden) were dissolved and diluted in Ringer's solution (consisting of [in mmol/l]: 140 NaCl, 1.2 CaCl2, 3.0 KCl and 1.0 MgCl2). CaAcamp, NaAcamp and CaCl2 were all diluted to a concentration of 0.5 mM. NaAcamp was also diluted to an additional concentration of 1 mM, because this concentration equals 0.5 mM CaAcamp with respect to the stoichiometry of CaAcamp (two N-acetylhomotaurinates to one calcium ion; NaAcamp = one N-acetylhomotaurinate to one sodium ion). The GlyR antagonist strychnine was diluted to a concentration of 20 μM. All drugs used were administered via reversed microdialysis. Using this type of drug administration, the actual concentration of substance passing over the probe membrane in vivo is difficult to estimate. However, perfusion with 0.5 mM calcium-bis(N-acetylhomotaurinate) was previously demonstrated to yield approximately the same dopamine output within the nAc as the physiological relevant dose of 200 mg/kg i.p.
2.3 In vivo microdialysis
The microdialysis experiments were performed in awake and freely moving rats (n = 88). Two days prior to the experiment, the rats were anaesthetised by isoflurane (Baxter, Kista, Sweden), mounted into a stereotaxic instrument (David Kopf Instruments, Lidingö, Sweden) and placed on a heating pad to prevent hypothermia during the surgery. Two holes were drilled for anchoring screws and one for the placement of a custom-made dialysis probe, with an active space of 2 mm, allowing passive diffusion of solutes. The probe was lowered into the nAc core/shell borderline region (A/P: +1.85, M/L: −1.4 mm relative to bregma, D/V: −7.8 relative to dura30) and was, together with the anchoring screws, fixed to the scull with Harvard/dental cement (DAB Dental AB, Gothenburg, Sweden). To prevent dehydration, the animals received saline (2 ml 0.9% NaCl s.c.) and were allowed to recover in individual cages before the experiment.
On the day of the microdialysis experiment, the sealed inlet and outlet of the probe were cut open and, via a swivel, connected to a microinjection pump (U-864 Syringe Pump, AgnTho's, Lidingö Sweden) allowing the animals to move around freely in its home cage. The probes were perfused with Ringer's solution at a rate of 2 μl/min, and dialysate samples (40 μl) were collected every 20 min during the entire experiment. Two hours prior to baseline sampling began, the rats were perfused with Ringer's solution to allow for equilibrium. Drugs were administered locally in the nAc after a stable dopamine baseline was obtained, with accepted normal fluctuation of ±10%. Instantly after the experiment, the animals were sacrificed, brains were removed, fixed (Accustain, Sigma-Aldrich, Sweden) and stored (−4°C) until verification of probe placement 5–7 days later. Only rats with a correct probe placement and no visual signs of brain tissue damage were included in the statistical analysis (Figure 1). A total of 10 rats were excluded in this study.
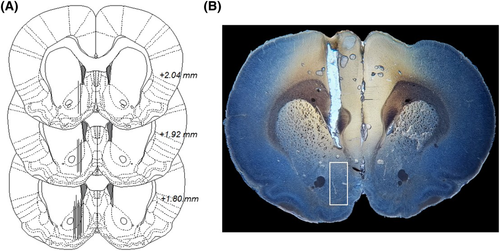
2.4 Biochemical assays
The dialysate sample was split and analysed for dopamine and taurine separately. Dopamine was separated and detected using two different simultaneously running high-performance liquid chromatography (HPLC) systems with electrochemical detection, as previously described.31 To identify and quantify the dopamine peak, an external standard containing 3.25 nM of dopamine was used. The dopamine samples were analysed online. In order to avoid degradation of taurine, sodium azide (50% v/v) was added to each individual sample before storage in −20°C. Taurine was detected using an HPLC system with fluorescence detection, as previously described.32 Identification and quantification of taurine was accomplished by using two external standards containing 0.5–1.0 μM of taurine.
2.5 Statistical analysis
The content of dopamine and taurine in each sample was expressed as the percentage of the average pre-treatment baseline. From the dialysates collected, the overall drug effect was calculated as the average alteration of dopamine and taurine content after the drug administration (0–180 min or 20–180 min) and was statistically evaluated using two-way analysis of variance (ANOVA) with repeated measures (treatment group × time). One-way ANOVA followed by Tukey's post hoc analysis was used for statistical evaluation of the area under the curve (AUC). All data are presented as mean ± SEM, and a probability value (p) less than 0.05 was considered statistically significant.
3 RESULTS
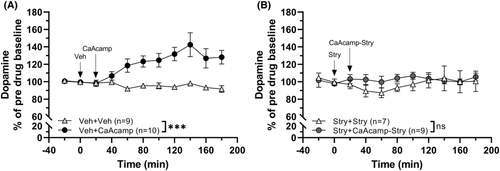
3.1 Calcium acamprosate elevates dopamine in the nAc but not when perfused in combination with strychnine
In the first set of experiments, we aimed to verify the dopamine elevating effect of acamprosate and the role of GlyRs in mediating this effect. Animals were pre-treated with either vehicle (Ringer's solution) or the GlyR antagonist strychnine (20 μM in the perfusate) 20 min before concomitant application of CaAcamp (0.5 mM) via the microdialysate probe. Administration of CaAcamp within the nAc increased the extracellular levels of dopamine as compared to vehicle (Veh) treatment (two-way ANOVAt = 0–180 min: group effect F1, 17 = 15.05, p = 0.001, η2 = 0.46; time effect F9, 153 = 4.00, p < 0.001, η2 = 0.19; interaction F9, 153 = 6.20, p < 0.001, η2 = 0.26; Figure 2A). However, pre-treatment with strychnine completely blocked the dopamine elevating effect of CaAcamp (two-way ANOVAt = 0–180 min: group effect F1, 14 = 0.681, p = 0.423; time effect F9, 126 = 0.821, p = 0.598; interaction F9, 126 = 0.759, p = 0.654; Figure 2B).
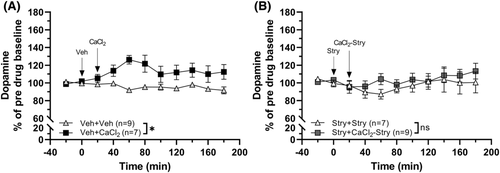
3.2 Calcium chloride elevates dopamine in the nAc, an effect prevented by strychnine
In the next set of experiments, we examined if calcium itself had the capability to increase nAc dopamine. Administration of CaCl2 (0.5 mM) alone resulted in a modest increase of dopamine compared to vehicle perfused animals (two-way ANOVAt = 0–180 min: group effect F1, 14 = 20.31, p < 0.001, η2 = 0.59; time effect F9, 126 = 0.935, p = 0.497; interaction F9, 126 = 2.00, p = 0.045, η2 = 0.12; Figure 3A). In similarity with CaAcamp, the previously observed calcium-induced dopamine elevation was absent in animals pre-treated with strychnine (20 μM; two-way ANOVAt = 0–180 min: group effect F1, 14 = 0.592, p = 0.455; time effect F9, 126 = 1.70, p = 0.096; interaction F9, 126 = 0.549, p = 0.836; Figure 3B).
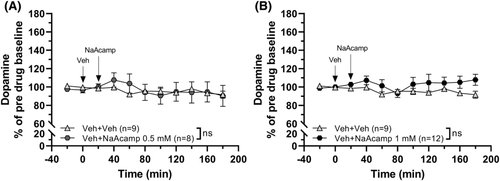
3.3 Sodium acamprosate administration does not increase nAc dopamine alone but in combination with calcium chloride
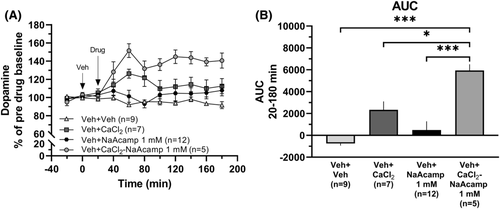
In order to evaluate whether the N-acetylhomotaurine part of CaAcamp influences nAc dopamine, we explored the effects of the sodium salt of the drug (NaAcamp). Administration of NaAcamp (0.5 mM or 1 mM) did not significantly increase dopamine over time (two-way ANOVAt = 0–180 min; 0.5mM: group effect F1, 15 = 0.018, p = 0.894, time effect F9, 135 = 1.55, p = 0.137, interaction F9, 135 = 0.806, p = 0.611; 1 mM: group effect F1, 19 = 2.15, p = 0.159, time effect F9, 171 = 0.790, p = 0.626, interaction F9, 171 = 0.965, p = 0.471; Figure 4A, B). To further investigate the possible effects of NaAcamp, we explored concomitant administration of CaCl2 (0.5 mM) and NaAcamp (1 mM). Perfusion with the combination of the drugs produced a significant elevation of dopamine in comparison to both vehicle-treated animals and to each of the drugs locally perfused alone (one-way ANOVA of AUCt = 20–180 min: F (3, 29) = 13.22, p < 0.001, η2 = 0.58; post hoc analysis: Veh versus CaCl2-NaAcamp p < 0.001, CaCl2 versus CaCl2-NaAcamp p = 0.023, NaAcamp versus CaCl2-NaAcamp p < 0.001; Figure 5A, B).
3.4 Acamprosate increases taurine levels in the nAc
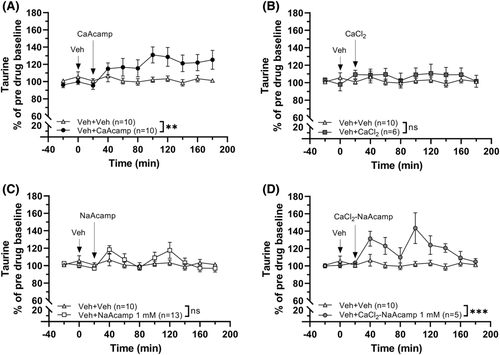
As acamprosate is a homotaurine analogue, where the carbon backbone has an acetyl group added at the amino end to facilitate absorption, we wanted to investigate taurine levels in the nAc following the administration of the different salt forms of acamprosate. We also examined the levels of taurine following CaCl2 treatment alone and when combined with NaAcamp. The amino acid analysis showed that CaAcamp (0.5 mM) has taurine elevating properties compared to vehicle-administrated animals (two-way ANOVAt = 0–180 min: group effect F1, 18 = 4.16, p = 0.056, time effect F9, 162 = 2.83, p = 0.004, η2 = 0.14, interaction F9, 162 = 3.19, p = 0.001, η2 = 0.15; Figure 6A). Treatment with CaCl2 (0.5 mM) or NaAcamp (1 mM) alone did not significantly elevate taurine levels (two-way ANOVAt = 0–180 min; CaCl2: group effect F1, 14 = 0.794, p = 0.388, time effect F9, 126 = 0.568, p = 0.821, interaction F9, 126 = 0.721, p = 0.689; NaAcamp: group effect F1, 21 = 0.251, p = 0.622, time effect F9, 189 = 3.07, p = 0.002, η2 = 0.13, interaction F9, 189 = 1.79, p = 0.073; Figure 6B, C), whereas the two substances combined increased the accumbal taurine output compared to vehicle treatment (two-way ANOVAt = 0–180 min: group effect F1, 13 = 8.03, p = 0.014, η2 = 0.38; time effect F9, 117 = 4.13, p < 0.001, η2 = 0.24; interaction F9, 117 = 3.85, p < 0.001, η2 = 0.23; Figure 6D).
4 DISCUSSION
Contradicting the idea that the acamprosate molecule is biologically inactive, the findings presented here show that there is an additive effect produced by calcium and N-acetylhomotaurine on dopamine as well as a potentiated effect on taurine output in the nAc following local drug administration. In addition, this dopamine increase appears to depend on GlyR activation as the acamprosate-induced dopamine elevation was prevented by strychnine pre-treatment. Even though acamprosate previously was demonstrated not to directly interact with α1 subunit containing GlyRs when using a Xenopus oocyte model,33 the drug could interact with other α subunit containing GlyRs or act indirectly through the release of taurine. Regardless, GlyRs appear to be a major participant in the effects of acamprosate as GlyR antagonism also has been shown to reverse the ethanol intake reducing effect of the drug.24
In the search for a mechanism of action for acamprosate, it was suggested that the calcium moiety of acamprosate is solely responsible for the effects of the drug because different forms of calcium mimicked the effects of acamprosate whereas the sodium salt of N-acetylhomotaurinate did not.29 By means of in vivo microdialysis, we show in this study that local nAc administration of calcium chloride increases accumbal dopamine. Interestingly, this effect also appears to involve GlyRs, as strychnine prevents the calcium chloride-induced dopamine elevation. When comparing the dopamine increases produced by regular acamprosate and calcium chloride, we found a larger increase by acamprosate indicating a possibility of multiple mechanisms of the compound on dopamine output. Administration of the corresponding sodium salt formulation of acamprosate did not produce a significant alteration of dopamine on a group level, supporting previous studies concluding that the calcium moiety of acamprosate is responsible for the drug's effect and that N-acetylhomotaurine is biologically inactive.29 Nevertheless, our study exploring nAc dopamine levels demonstrated an enhanced elevation of dopamine following local concomitant administration of calcium chloride and sodium acamprosate. This finding strengthens the idea that calcium and N-acetylhomotaurine exert multiple effects, which may influence dopamine neurotransmission in an additive or even synergistic manner. Hence, N-acetylhomotaurine does not appear to be biologically inert, as suggested by Spanagel and co-workers. It should be noted, however, although we employed the Wistar rat, the Sprague–Dawley strain was used in the former study, a difference which could account for this discrepancy.
Dopamine has been suggested to have a major impact on ethanol consumption, not just as a pharmacological consequence of passive (injections) or active (voluntary intake) ethanol exposure, but the endogenous dopamine tone in the mesolimbic dopamine system appears to differentially drive the individual towards consumption. A spontaneously low dopaminergic tone, or a decreased dopaminergic tone as a consequence of long-term alcohol consumption, appears to be associated with a high voluntary ethanol consumption both in rats and humans.34-36 The increase in dopamine following acamprosate administration has not received much attention when examining the mechanism underlying the anti-alcohol effect of the drug. However, in a previous study, we found a link between the ethanol-intake reducing effect of acamprosate and its ability to increase dopamine.37 Apart from increasing dopamine by itself, acamprosate prevented ethanol from an additional influence on dopamine in the nAc. Conversely, following repeated administration of acamprosate, there appeared to be a tolerance towards the ethanol-intake reducing effect of the drug, and once this phenomenon occurred, acamprosate had also lost its ability to raise nAc dopamine. Whether the decline of effect of acamprosate following long-term treatment is species specific and only present in the rat14, 37-39 is not known, the dopamine elevating properties of acamprosate may indeed be central to its effect on alcohol consumption.
The link between acamprosate and GlyRs may not be direct, but rather indirect as indicated by a previous study.33 A systemic injection of a very high dose of acamprosate (1 g/kg) was shown to increase nAc taurine,40 indicating the possibility of an indirect influence of acamprosate on GlyRs. Although previous interpretations included an acamprosate and taurine-induced normalisation of a hyperglutamatergic state, when both acamprosate and taurine were found to block the glutamate increase following ethanol withdrawal,41, 42 activation of GlyRs may be another or additional effect of the drug. In the present study, we used a lower dose of acamprosate (0.5 mM, corresponding to 200 mg/kg i.p. with regards to influence on nAc dopamine23) but were still able to detect a significant increase of taurine. We have previously speculated that acamprosate acts as a neurochemical substitute for ethanol in the mesolimbic dopamine system,37 because the drug, just as previously shown with ethanol, increases dopamine.23, 43 Here we thus demonstrate another ability of acamprosate that mimics that of ethanol, the ability to increase taurine.44 Considering that taurine increases dopamine in the nAc,27 this further supports the idea that acamprosate may partially act as a substitution for ethanol with regards to dopamine elevation. Indeed, as low dopamine levels may motivate for further ethanol consumption,45 the acamprosate/taurine-induced activation of GlyRs and the ensuing dopamine elevation may be the underlying reason to decreased ethanol intake, a suggestion further supported by the finding that strychnine locally applied in the nAc reversed the ethanol intake reducing effect of acamprosate.24 However, this indication is based on the postulate that acamprosate acts as a neurochemical substitute for ethanol in the nAc and needs to be further confirmed in future studies to include or exclude other known or unknown mechanisms.
Neither calcium chloride nor sodium acamprosate significantly altered nAc taurine following local administration via the dialysis probe. Because calcium chloride increased dopamine levels without impacting taurine, calcium may have dopamine modulating properties by mechanisms separate from that of N-acetylhomotaurine. Indeed, calcium is known to impact neurotransmission including primary effects on both neurons and astrocytes,46, 47 and further studies are needed to unravel the specific mechanisms underlying the calcium-induced dopamine elevation relevant to acamprosate treatment. Regardless, because the calcium-induced dopamine elevation was absent following strychnine pre-treatment, the mechanism of action appears to involve GlyRs. Furthermore, sodium acamprosate treatment did not induce a significant elevation of taurine. Further support of separate mechanisms acting in an additive manner of calcium and N-acetylhomotaurine was found following co-administration of the drugs. Although each drug on its own was unable to increase taurine, the combination of calcium chloride and sodium acamprosate elevated extracellular taurine levels to approximately the same extent as regular acamprosate. Whether additive or synergistic impact of calcium and N-acetylhomotaurine only holds true relative to dopamine neurotransmission or if this also extends to voluntary ethanol intake remains to be established.
In the present study, we used sodium acamprosate synthesised according to what was previously described.29 For administration of acamprosate locally in the nAc, we used the same concentration (0.5 mM) as in a previous publication,23 a concentration equivalent to a physiologically relevant systemic dose of 200 mg/kg i.p. Using calcium chloride, we aimed to obtain approximately the same concentration (0.5 mM) for equivalent amounts of Ca2+ ions. However, as the drugs were administered by reversed microdialysis, the excovery of the compounds could be somewhat different, but we do not estimate this to have a significant impact on the findings presented. Using sodium acamprosate, we perfused two different concentrations (0.5 and 1 mM). The higher concentration equals to the perfused dose of regular acamprosate, as the original acamprosate salt dissociates completely into its constituents following dissolution in a hydrophilic media.22 The lower concentration contains equivalent amounts of ions (Na+ versus Ca2+) with regards to the perfused dose of regular acamprosate and calcium chloride. Furthermore, as taurine is an osmoregulator and is released into the extracellular space upon alterations in the osmotic environment,17, 48, 49 we considered the difference in osmolarity between the various drug solutions perfused. As no differences with respect to dopamine or taurine output were observed following sodium acamprosate (0.5 mM: dopamine Figure 4A, taurine data not shown; or 1 mM: dopamine Figure 4B, taurine Figure 6C), we chose to only use the higher concentration in combination with calcium chloride to correctly compare 1 mM of sodium acamprosate with 0.5 mM regular acamprosate.
In conclusion, in the present microdialysis study, we show that regular acamprosate increases both dopamine and taurine in the nAc and that the dopamine elevation is GlyR dependent. In contrast to others, we do not find that the calcium moiety of acamprosate is solely responsible for the biological effects of the compound, but instead that the calcium ion and N-acetylhomotaurine act in an additive manner to elevate dopamine in the nAc. The exact mechanism(s) underlying this dual effect of the compound remains to be established, but both increased levels of taurine and activation of GlyRs appear to be important for the dopamine elevating properties of the drug. Whether these additive effects are functionally relevant following systemic administration and with respect to voluntary ethanol intake remains to be established.
ACKNOWLEDGEMENTS
We want to acknowledge Rosita Stomberg for technical support and expertise as well as Esi Domi for statistical aid.
CONFLICT OF INTEREST
The authors report no conflict of interest.
AUTHOR'S CONTRIBUTION
M.E. was responsible for the study concept and M.E. and K.A. for the study design. K.A. performed the experiments and performed the statistical analyses. M.E. and K.A. drafted the manuscript. M.E., L.A. and B.S. provided critical revision of the manuscript for intellectual contents. All authors critically reviewed the content and approved the final version for publication.
Open Research
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.




