Betel quid: New insights into an ancient addiction
Abstract
The use of areca nuts (areca) in the form of betel quids constitutes the fourth most common addiction in the world, associated with high risk for oral disease and cancer. Areca is a complex natural product, making it difficult to identify specific components associated with the addictive and carcinogenic properties. It is commonly believed that the muscarinic agonist arecoline is at the core of the addiction. However, muscarinic receptor activation is not generally believed to support drug-taking behaviour. Subjective accounts of areca use include descriptions of both sedative and stimulatory effects, consistent with the presence of multiple psychoactive agents. We have previously reported partial agonism of α4-containing nicotinic acetylcholine receptors by arecoline and subsequent inhibition of those receptors by whole areca broth. In the present study, we report the inhibition of nicotinic acetylcholine receptors and other types of neurotransmitter receptors with compounds of high molecular weight in areca and the ability of low molecular weight areca extract to activate GABA and glutamate receptors. We confirm the presence of a high concentration of GABA and glutamate in areca. Additionally, data also indicate the presence of a dopamine and serotonin transporter blocking activity in areca that could account for the reported stimulant and antidepressant activity. Our data suggest that toxic elements of high molecular weight may contribute to the oral health liability of betel quid use, while two distinct low molecular weight components may provide elements of reward, and the nicotinic activity of arecoline contributes to the physical dependence of addiction.
1 INTRODUCTION
The chewing of preparations of areca nut (areca) with other constituents, commonly known as betel quids, is an ancient custom throughout much of South Asia, with physical evidence for areca use on human dental remains predating written history.1 With an anecdotally estimated 600 million users, betel quid is the fourth most commonly used addictive substance in the world, after alcohol, caffeine, and tobacco.2
The essential ingredient of all forms of areca is the fruit of the palm, Areca catechu (Figure 1). Areca nuts are prepared in various ways by different cultures, and these preparations have many different names.3 One of the most common formulations is the “betel quid,” the preparation of which begins with a leaf of the vine Piper betle, believed to be the source of the somewhat misleading association of areca and “betel quid” (or sometimes, “betel nut”). While some cultures use dry, ripe areca nuts, popular in India, others use fresh, often immature nuts (Figure 1), sometimes including the husks4 that are chewed without betle leaf. As is often the case with drug use habits, areca nut chewers often change their preferences over time and with their age, starting with sweetly flavoured quids, such as meetha paan masala, popular in India. While the amount of nicotine in areca is negligible,5 it has been shown that the primary areca alkaloid is a partial agonist for brain nicotine receptors.6 Additionally, the spread of tobacco products through Asia in recent centuries has also affected betel quid use, so that presently, on average, about half of adult betel chewers add tobacco to their quid.7 As accommodations to modern mass marketing, areca is also available in foil packets premixed with other ingredients. These are sold as “pan masala” when formulated without tobacco and as “gutka” when processed smokeless tobacco is included. Asian specialty stores in the United States commonly carry raw areca products and these prepared packets.8

Areca users have a twofold higher chance of oral cancer than the general population and a 50- to 100-fold higher chance of precancerous disease such as oral submucosal fibrosis,9 making it a serious health problem in countries where betel quid use is high.
The areca alkaloid arecoline, first isolated in 1891, has been known to be a muscarinic agonist since the time of the earliest studies of autonomic pharmacology.10 As the muscarinic activation of salivary glands is the most overt indication of betel quid use, it has been hypothesized that the muscarinic activity of arecoline in the brain is sufficient to account for the short-term reinforcing effects of areca. Although the muscarinic antagonist scopolamine is considered a deliriant hallucinogen,11 muscarinic agonists have no history of being drugs of abuse, aside from the unsubstantiated supposition that arecoline must account for areca addiction. As is the case with tobacco, it is difficult, even for users, to identify the psychoactive effects of areca that are likely to account for its use-promoting reward. Anecdotal accounts sometimes liken betel quid effects to a strong cup of coffee. In a survey of 370 addicted betel quid users in Karachi,12 47% of the subjects identified betel quid as a central nervous system (CNS) stimulant. However, 14% identified it as a CNS depressant, and 38% believed it had no CNS effects. Nonetheless, in the same study, 74% of the respondents believed that chewing betel quid could cause cancers of the mouth or throat. Similar results were recently reported in a study of betel quid users in Myanmar.13
In the present work, we further study the toxic effects of areca nut compounds of high molecular weight (HMW) on neurotransmitter-activated ion channels.6 We also report previously unknown activities mediated by distinct low molecular weight (LMW) compounds in areca. We find that areca contains high concentrations of GABA and will therefore stimulate peripheral GABA receptors. LMW fractions of areca also contained blockers of the both the dopamine transporter (DAT) and the serotonin transporter (SERT), suggesting important effects on the dopamine (DA) and serotonin (5HT) brain systems and associated behaviours. These activities are likely to account for the mixed stimulant and antidepressant effects reported by users and may be at the root of the addiction-promoting reward of betel quid use.
2 MATERIALS AND METHODS
2.1 Commercial reagents
Acetylcholine chloride (ACh), arecoline, and other reagents were purchased from Sigma-Aldrich Chemical Company (St. Louis MO, USA). Cyclothiazide (CTZ) was purchased from Research Biochemicals International (Natick MA, USA). Additional Areca alkaloids were purchased from Toronto Research Chemicals, (Toronto, Canada). Isoarecolone was provided by the NIDA drug supply programme. Fresh stock solutions of 10 mM ACh and serotonin were made in Ringer's solution each day of experimentation. Stock solutions of the other reference agonists, kainic acid and γ-amino-n-butyric acid (GABA), were made in Ringer's solution and kept at 4°C and used within 7 days. Working solutions were prepared freshly at the desired concentration from the stored stocks.
2.2 Areca infusion
Whole ripe A. catechu nuts (supari), sourced from India were purchased through eBay from Rider International Health Foods, Nuts & More, Chicago IL, and from World Wide Export, Bloomingdale IL. The dry areca nuts, weighing approximately 10 g each, were broken into small bits (roughly 0.5 cm cubes) with a hammer and pruning shears, then ground with an electric coffee grinder or a heavy-duty spice grinder, and sieved through 1/16th inch screen. The resulting powder was weighed, mixed at 200 mg per ml purified water, and stirred 90 min at 37°C. This was vacuum-filtered through coarse filter paper and then through a sterile 0.2 μm nylon filter into a sterilized glass bottle. The pH was measured at this point to be 5.4. This broth was further processed as described below or brought up to pH 7.2 with appropriate buffer for oocyte electrophysiology experiments.
Additional samples of fresh and younger areca nuts were obtained from various vendors on eBay (Figure 1). Except that their soft fresh condition required less rigorous pre-processing, these were processed as described above. However, due to limited supplies of these nuts, unless otherwise indicated, experiments relied on the dry ripe nuts from India.
2.3 Ultrafiltration and ion-exchange chromatography
The whole areca broth was additionally subjected to ultrafiltration using the Amicon Stirred Cell (MilliporeSigma, Burlington MA, USA) and regenerated cellulose filters sequentially with 100, 10, and 1 kDa cutoffs for low molecular weight (LMW) areca broth, using nitrogen gas for pressure. LMW areca solution was further fractionated by ion exchange chromatography with components eluted with water through either Amberlite IR120 cation exchange or Dowex-1 anion exchange resins. Neutral components and negatively charged compounds flow through the cation exchange while neutral and positively charged components flow through the anion exchange column.
2.4 Determination of areca GABA and glutamate content
To determine GABA and glutamate content, electrochemical analysis of ortho-phthalaldehyde-derivatized free amino acids was performed. Areca LMW fractions were diluted 1:3 in methanol followed by 20 min at −20°C. Samples were filtered through 0.22 μm PVDF centrifugal filters (UFC30GV0S) at 12 000 G for 5 min. After centrifugation, samples were further diluted to 1:1000 in mobile phase. Samples were analysed using the EICOM HTEC-510 HPLC automated system with a FA-3ODS separation column.
2.5 Dopamine and serotonin transporter measurements
2.5.1 Cell culture, transfections, and treatments
Chinese hamster ovary (CHO) cells were purchased from American Type Culture Collection. CHO cells were cultured in Ham's F-12 medium supplemented with 10% foetal bovine serum (FBS, Gibco, Amarillo TX, USA), 1 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma-Aldrich). The human DAT and SERT cDNAs were cloned into the pEYFP-C1 vector and used to transfect CHO cells with Lipofectamine 2000 (Invitrogen, Waltham MA, USA). For stable transfections, DAT- and SERT-expressing single clones (CHO-DAT and CHO-SERT cells, respectively) were selected with G418 (Gibco), verified by DAT and SERT immunoblot, and maintained in Ham's F-12 media containing 0.5 mg/ml G418. DAT and SERT functions were analysed via DA and 5-HT uptake experiments, which were conducted 24–48 h after seeding cells in 24-well plates coated with Poly-D-Lysine (Sigma-Aldrich). For the treatments, cells were incubated with different drug conditions for 10 min at 37°C. Conditions are described as follows: Areca extract ≤1 kDa fraction (supari) tested at 6 mg/ml dry weight, Areca anion-exchange fraction tested at 0.72 mg/ml dry weight, Areca cation-exchange fraction tested at 0.72 mg/ml dry weight, 10 μM GBR12935 (DAT blocker, only for CHO-DAT cells, Sigma-Aldrich), and 0.5 μM Fluoxetine (SERT blocker, only for CHO-SERT cells, Sigma Aldrich).
2.6 Dopamine and serotonin transporter assays
[3H]-DA or [3H]-5HT uptake measurements in CHO cells transfected with human DAT or SERT were performed as previously described.14 Briefly, cells seeded in 24-well plates at 80% confluency were pre-incubated for 5 min at 37°C with 200 μl of uptake buffer (4 mM Tris base, 6.25 mM HEPES, 120 mM NaCl, 5 mM KCl, 1.25 mM CaCl2, 1.25 mM MgSO4, 0.57 mM ascorbic acid, 5.6 mM glucose, 1 mM tropolone, pH 7.4) containing the tested areca fractions. Uptake assays were conducted in the presence of 60 nM of [3H]-DA (3,4-[7-3H] dihydroxyphenylethylamine, Perkin Elmer, Waltham, MA, USA) or 50 nM of [3H]-5HT (5-Hydroxytryptamine Creatinine Sulfate, Perkin Elmer) at 37°C for 5 min. After loading with [3H]-DA or [3H]-5HT, cells were washed with 1 ml ice-cold uptake buffer. Samples were solubilized in 0.4 ml of 1% SDS and incubated at room temperature for 1 h. Samples were then transferred to scintillation vials and filled with 4 ml scintillation counting fluid (RPI Bio-safe IITM). Counts per min (cpm) were obtained using a LS-6500 liquid scintillation counter (Beckman Coulter, Brea, CA, USA). Experiments were conducted three times with four replicates in each experiment (total n = 12). The values of [3H]-DA or [3H]-5HTwere analysed using one-way ANOVA with Bonferroni post-hoc test. Minimal significance was set at p < 0.05.
2.7 Heterologous expression of receptors in Xenopus laevis oocytes
Human nicotinic acetylcholine receptor (nAChR) clones were obtained from Jon Lindstrom (University of Pennsylvania, Philadelphia, PA). Rat α1, β2, and γ2(long) GABAA receptor clones were obtained from David Weiss (University of Texas, San Antonio, TX). Human α1 and γ2(short) GABAA receptor clones were obtained from Philip Ahring (University of Sydney, Australia), and the human β2 GABAA receptor clone was obtained from Trevor Smart (University College London). Clones for the rat AMPA-type glutamate receptor subunits GluA1 and GluA2 were a gift from Steve Heinemann (Salk Institute, LaJolla CA). The clone for the mouse 5HT3A receptor was obtained from Steve Traynelis (Emory University, Atlanta GA). Subsequent to linearization and purification of the plasmid cDNAs, RNAs were prepared using the mMessage mMachine in vitro RNA transcription kit (Ambion, Austin TX, USA).
Oocytes were surgically removed from mature X. laevis frogs (Nasco, Ft. Atkinson WI, USA) and injected with appropriate receptor subunit RNAs as described previously.15 Frogs were maintained in the Animal Care Service facility of the University of Florida, and all procedures were approved by the University of Florida Institutional Animal Care and Use Committee (approval #202002669). Stage V oocytes were isolated and injected with 50 nl of 5–20 ng subunit RNA. Recordings were carried out 2–7 days after injection.
2.8 Two-electrode voltage clamp electrophysiology
Experiments were conducted using OpusXpress 6000A (Molecular Devices, Union City CA. USA).15 Both the voltage and current electrodes were filled with 3 M KCl. Oocytes were voltage-clamped at −60 mV for cells expressing nAChR, 5HT3A, and glutamate receptors. In order to more reliably generate inward currents, cells expressing GABAA receptors were voltage clamped at −80 mV. The oocytes were bath-perfused with Ringer's solution (115 mM NaCl, 2.5 mM KCl, 1.8 mM CaCl2, 10 mM HEPES, and 1 μM atropine, pH 7.2) at 4 ml/min. To evaluate the effects of experimental compounds compared to control agonist-evoked responses of various receptors expressed in oocytes, baseline conditions were defined by two initial applications of control agonist made before test applications. Drug applications were 6 s in duration followed by 241 s washout periods. A typical recording for each oocyte consisted of two initial control applications, an experimental test application, and then follow-up control applications to determine the desensitization or rundown of the receptors. For the GABA concentration-response studies, applications alternated between controls and experimental tests. The control concentrations were 100 μM ACh for α3β4 and 30 μM ACh for α4β2 nAChR, 10 μM GABA for GABAA receptors, 100 μM kainic acid for glutamate receptors, and 10 μM serotonin for 5HT3A receptors. The responses were measured as peak current amplitudes.
Data were collected at 50 Hz, filtered at 5 Hz, analysed by Clampfit 9.2 (Molecular Devices) and Excel 2003 (Microsoft, Redmond WA, USA), and normalized to the averaged response of the two initial controls for each oocyte.15 Data were expressed as means ± SD from at least four oocytes for each experiment and plotted by Kaleidagraph (Synergy Software, Reading PA, USA). Multi-cell averages were calculated for comparisons of complex responses. Averages of the normalized data were calculated for each of the 10 500 points in each of the 210 s traces (acquired at 50 Hz), as well as the standard errors for those averages. In the ANOVA, Bonferroni correction for multiple comparisons or Dunnett post-hoc tests were applied. ANOVA results are provided in Supplemental Data.
3 RESULTS
3.1 Neurotoxic activity of areca
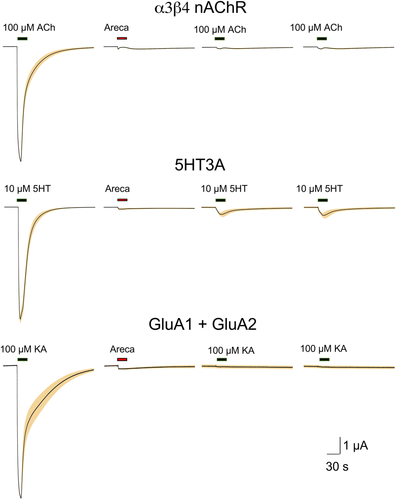
The heterologous expression of neurotransmitter receptors in Xenopus oocytes is a well-proven method for studying the effects of addictive drugs such as nicotine16 and alcohol.17 We have previously reported6 that areca broth has, for want of a better term, a neurotoxic activity that suppresses subsequent ACh-evoked responses of α7, α4β2, and α6-containing nAChR. This activity, which is not due to the presence of the nicotinic partial agonist arecoline, was not decreased when the broth was co-applied with ACh (Figure 2A). Neither the competitive antagonist dihydro-β-erythroidine (DHβE) nor the noncompetitive antagonist mecamylamine protected α4β2 nAChR receptors from the antagonist activity of the areca broth (Figure 2B; see Supplemental Data for ANOVA). The stability of this inhibition was tested by making multiple control applications of ACh at four-minute intervals, and there was no detectible recovery of ACh sensitivity over this time period (Figure 2B). Similar inhibition (p < 0.0001; see Supplemental Data for peak currents ANOVA) was observed with α3β4 nAChR (Figure 3). The toxic activity was also evident for 5HT3A receptors (p < 0.0001; see Supplemental Data for peak currents ANOVA), which have a high structural homology to nAChR,18 as well as for the unrelated AMPA-type glutamate receptors formed by the co-expression of GluA1 and GluA2 subunits19 (Figure 3, p < 0.0001; see Supplemental Data for peak currents ANOVA).
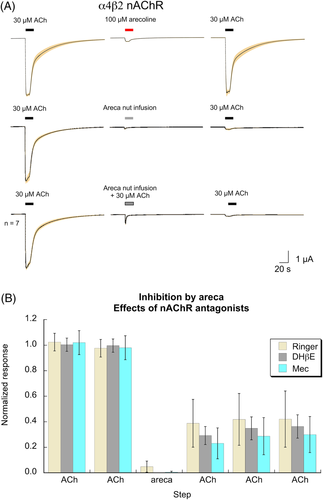
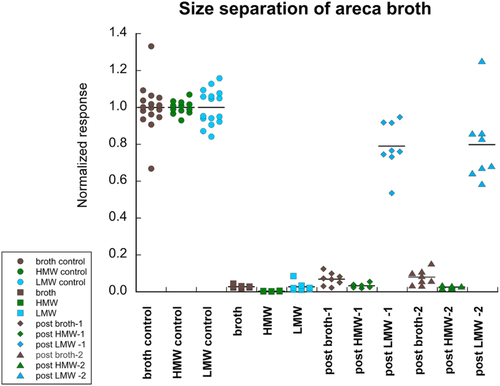
Areca broth was subjected to two steps of ultrafiltration that yielded two fractions, one enriched with components of high (≥10 kDa) molecular weight (HMW), and the other of low molecular weight (LMW) components (≤1 kDa). The stable neurotoxic activity for α4β2 nAChR that was present in the whole broth was retained in the HMW fraction (p < 0.0001) and absent in the LMW fraction (Figure 4; see Supplemental Data for ANOVA). The red colour and tendency to form precipitates was retained in the HMW, and the LMW fraction was clear and pale yellowish.
3.2 GABAA receptor activity of areca
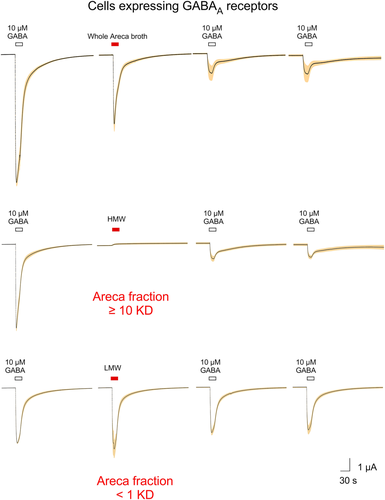
When whole areca broth was applied to cells expressing rat GABAA receptors formed from the co-expression of rat α1, β2, and γ2(long) subunits, we observed a large transient activation of the receptors followed by a reduction in the subsequent GABA-evoked responses (Figure 5, upper traces). Application of the HMW fraction produced no activation but did cause inhibition of control GABA-evoked responses (Figure 5, middle traces), consistent with the HMW activity on other receptor types. In contrast, the application of the LMW fraction activated GABA receptors without detectable inhibition of subsequent controls (Figure 5, lower traces). Statistical analysis of these responses was included in the analyses for Figure 6. The activation of GABAA receptors could not be attributed to arecoline or the other alkaloids commonly identified in areca. The related compound isoarecolone likewise had no GABAA activity (Figure 6; see Supplemental Data for ANOVA).
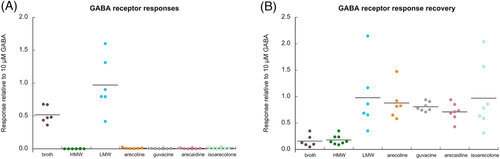
We also evaluated the effects of LMW areca obtained from either fresh nuts from Vietnam (Figure 1) or dried Indian supari on human GABAA (α1β2γ2) receptors and compared that activity to a range of GABA concentrations (Figure 7, Table 1). The LMW supari preparation was additionally concentrated by drying under vacuum. Samples of tested LMW solutions were dried completely to obtain actual dried weights after filtrations. The dry weight of the LMW fresh nuts from Vietnam at the highest concentration tested was 13.75 mg/ml, and the dry weight of the supari LMW highest concentration tested was 142.38 mg/ml. For the purpose of comparisons, the molar concentrations of GABA were converted to mg/ml, based on the molecular weight of GABA. Peak-current responses were initially measured relative to 10 μM GABA controls and then adjusted by the ratio of the GABA controls to the empirically observed GABA maximum response.
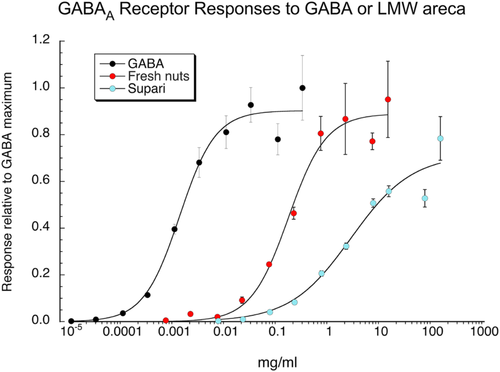
| EC50 (mg/ml) | IMax | χ2 | R | |
|---|---|---|---|---|
| GABA | 0.0013 ± 0.0003a | 0.90 ± 0.04 | 0.026 | 0.991 |
| LMW fresh nuts | 2.37 ± 0.44 | 0.90 ± 0.04 | 0.022 | 0.991 |
| LMW supari | 40.8 ± 23.8 | 0.72 ± 0.09 | 0.029 | 0.979 |
- a This converts to a GABA EC50 = 124 ± 23 μM.
3.3 HPLC analysis of LMW areca
We tested the hypothesis that the GABAA receptor activation by the LMW areca fraction might be attributed to the presence of GABA in the LMW solution by conducting an HPLC analysis of the LMW solution (Figure 8A). A GABA peak was observed and confirmed by comparison to a 1 μM GABA standard. Serial dilutions of GABA were analysed and fitted to a linear calibration curve, with sensitivity from 1 nM to 10 μM, which was used to determine total GABA content of areca extract. Our estimate of the areca GABA content was compared to literature reported values for GABA in tobacco20 and other foods,21 data which were obtained using spectrophotometric enzymatic activity assay and HPLC analysis of AQC-derivatized amino acids, respectively (Figure 8B).
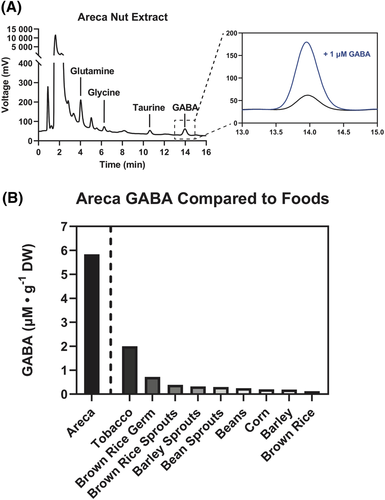
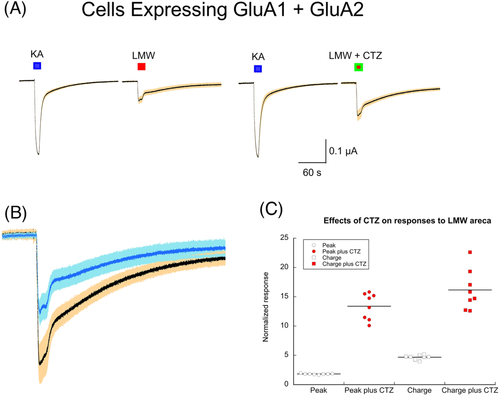
We also noted peaks for taurine, glycine and glutamate. In order to confirm the presence of glutamate, we applied LMW areca to oocytes expressing GluA1 and GluA2 subunits and compared the responses to 100 μM kainic acid controls (Figure 9A). Note that the most abundant post-synaptic glutamate receptors in the brain are heteromeric assemblies of GluA1 and GluA2 subunits and are typically characterized as AMPA-selective. However, these receptors desensitize rapidly to AMPA, making AMPA unsuitable for the slow drug application protocols of the oocyte expression system. In contrast, responses to kainic acid desensitize more slowly, so the use of kainic acid was instrumental in the discovery of the first glutamate receptor clones and remains useful as a standard control. The GluA1 and GluA2-expressing cells responded to the application of LMW areca, and those responses were amplified (p < 0.0001) by the AMPA receptor-selective modulator cyclothiazide (CTZ), consistent with the presence of glutamate in the LMW areca (Figure 9B,C).
3.4 Sensitivity of neurotransmitter transporters to components of LMW area
The LMW areca solution was further fractionated by ion exchange chromatography with components eluted with water through either Amberlite IR120 cation exchange or Dowex-1 anion exchange resins. Neutral components and negatively charged compounds would be expected to flow through the cation exchange while neutral and positively charged components would flow through the anion exchange column.
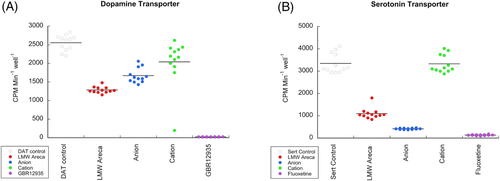
Anecdotal reports of stimulant-like properties in areca12, 13 suggested the presence of amphetamine-like compounds in the LMW areca fraction, encouraging us to look for activity of the LMW in assays that measure the block of neurotransmitter reuptake. CHO cells, transfected with neurotransmitter receptor or transporters have been widely used to study the effects of addictive drugs on the molecular mediators of addiction. We found that the LMW fractions caused significant block (p < 0.0001) of DA reuptake from DAT-transfected CHO cells.22 This activity was essentially retained in the elute from the anion exchange column. The eluate from the cation exchange column (Figure 10A) retained some activity (p < 0.05 with the Bonferroni correction). The LMW fraction was also determined to reduce 5HT reuptake by SERT-transfected CHO cells24 (p < 0.0001). This activity was greater in the anion exchange eluate and absent from the eluate of the cation exchange column (Figure 10B), indicating that these activities come from different molecules in the LMW areca fraction.
4 DISCUSSION
Historically, betel quid users attributed numerous positive effects to betel including reducing bad breath, curing an itching throat, strengthening of the teeth, beautifying the mouth (a very subjective opinion), stimulating appetite, aiding digestion, activating taste buds, curing toothache, providing a sense of well-being, expelling worms, heightening alertness, and kindling passion.25 Given our current knowledge of the risks for oral disease and cancers associated with betel use,7 the most ironic of these attributions are probably those related to oral health. Nonetheless, during Victorian times areca was a popular ingredient for toothpastes used by British citizens both in England and in the Raj.25 A recent study reported the antibacterial properties of toothpaste formulated with areca.26 We attempted to conduct bacterial assays of mutagenesis with the HMW areca fraction and found that all dilutions tested, to 54-fold dilution of the original broth, effectively killed the bacteria used for the assay (unpublished), consistent with a general toxicity associated with the high molecular weight organic factors in areca. It is reasonable that these factors could contribute to the development of conditions like oral submucosal fibrosis common in chronic users of areca products.9
As Western societies have in recent decades been trying to deal with tobacco use, Asian countries are now beginning to enact new public policies to deal with the public health cost of betel use.27, 28 However, on the level of the individual, awareness of a health risk is generally not sufficient motivation for a person to quit an addiction,29, 30 providing a reason for developing targeted cessation therapies.
While the effects of coffee and alcohol are easily appreciated by the consumer of these beverages, like tobacco, areca is relatively subtle in regard to its subjective effects in the CNS.12 Profuse salivation, the most obvious effect of chewing betel quid, is certainly a cue that the betel quid user will associate with their drug,13 but not being a CNS effect, it is unlikely to relate to the actual addiction. Addictions typically have two components, the first being a short-term reinforcing effect that targets the brain's reward mechanism associated with neurotransmitters like dopamine.31 The second component of a serious addiction is the physical dependence produced by the chronic use of the drug that leads to withdrawal symptoms should the user try to quit. The nicotinic activity of arecoline has been proposed to relate to this second component, as well as to contribute to the predilection for adult betel users to add tobacco to their quid.6
Although the primary addictive substances in areca are not definitively identified, our data along with other studies27 provide insights connecting areca use to brain neurotransmitters associated with addiction, consistent with fMRI studies that have indicated that areca use increases activity in brain reward circuits.32 The short-term reinforcing effects of several types of drugs have been associated with increases in brain catecholamines, most especially dopamine.33 Cocaine, for example, inhibits DAT, while amphetamine blocks both DAT and the vesicular dopamine transporter, and nicotine increases the firing of mesolimbic dopamine neurons.34 Another mechanism through which catecholamines may be increased in the brain is through inhibition of their metabolism. In this regard, inhibition of monoamine oxidase (MAO) has been proposed as a potential mechanism for the addictive properties of areca.35 Arecoline has been shown to inhibit the expression of MAO-A mRNA and protein expression,36 and a dichloromethane fraction from A. catechu was found to inhibit MAO activity.37 These effects might synergize with the DAT and SERT inhibition determined in our studies.
The presence of GABA in areca is a novel observation. Although it seems unlikely that very much of the GABA from areca will make it into the brain or account for the reinforcing effects of betel quid use, it has been reported that radiolabeled GABA injected intraperitoneally into rats can be detected in the brain within just a few minutes.38 It has also been reported that the GABA transporter GAT2/BGT-1 is expressed at the blood brain barrier.39 Outside of the CNS, it is possible that the GABA in areca may modulate the activity of GABA receptors in various peripheral tissues including pancreatic islets, smooth muscle, and the bladder.40 High concentrations of GABA are found naturally within the cytosol of the insulin-producing beta cells of the islets, secreted at a period of 5 min.41 Autocrine and paracrine feedback loops have been described, with GABA receptors present in alpha, beta, and delta cells. Activation of these GABA receptors upon external application of GABA results in a decrease in both glucagon and insulin secretion.42, 43 Incidentally, disrupted GABA signalling in the pancreas has been observed in islets from patients with type 2 diabetes, although the cause of disruption is unknown.41 Given the high concentration of GABA in areca, and that GABA is readily absorbed in the intestines and reaches maximal blood concentration only 30 min post-ingestion,44 continued use of areca may interfere with pancreatic islet function. Consistent with this, in studies of Taiwanese men, areca use has been associated with metabolic syndrome and a higher risk for type 2 diabetes and central obesity.45
Our discovery of DAT and SERT inhibition by small molecules in areca offers potentially important new insights into the initiation and maintenance of the betel quid habit. In a study of betel quid users in Myanmar, users reported boredom as the most common factor contributing to their use of the drug,13 and this may be indicative of depression. Other recent studies have pointed towards an important relationship between areca use and depression, although whether this association is correlational or causal remains unclear. A recent study of betel quid use in Assam29 indicated that 68% of betel quid users in their study chewed areca to relieve stress, and stress is a large factor in the aetiology of depression.46 One pilot clinical study reported that patients with depression had a higher prevalence of betel-quid chewing habits, and patients who chewed betel quid showed more severe depressive symptoms.47 After antidepressant therapy, the addictiveness of betel-quid was significantly reduced, an observation that begs the question of whether the quid users were depressed because they chewed areca or whether they were betel quid users because they were depressed and betel quid chewing was a form of self-medication. Ethanolic extract of A. catechu has been reported48 to have antidepressant-like action in behavioural models of depression in rats, and another study49 reported potential antidepressant activity of areca via elevation of serotonin and noradrenaline in the hippocampus of rats. A preclinical study examined the effects of antidepressants on an animal model of areca use50 and found various sorts of antidepressants, including selective serotonin reuptake inhibitors (SSRIs), monoamine oxidase inhibitors (MAOIs), and tricyclic antidepressants to be partially successful at reducing areca self-administration in rats. A small-scale randomized, double-blind, placebo-controlled trial of male betel quid users in Taiwan was conducted to determine the potential efficacy of antidepressants to promote betel quid cessation.51 The MAOI moclobemide and the SSRI escitalopram both had modest and equivalent effects (approximately 33% six-month quit rates, compared to 5.4% for placebo controls). This would be consistent with the medications functioning as a sort of replacement therapy for the natural antidepressant effects of betel quids. One important caveat regarding the Taiwan study is that in this population, areca is used without tobacco, whereas in countries like India and Myanmar, areca is normally combined with substantial amounts of tobacco,13 so that in those populations, nicotine dependence is likely to be coincident with betel quid use. For such populations of betel quid users, perhaps a better approach would be to use the alternative antidepressant bupropion,52 which has the additional indication as a smoking cessation aid.
Clearly, an immediate challenge would be to identify the specific molecules in areca related to the SERT and DAT inhibition. Sorting out the addicting and/or potential beneficial elements of areca, combined with a better understanding of its toxicities and possible carcinogens, may point towards useful therapies. However, for such therapies to be appealing to current users of betel quid, they may also have to provide for the accustomed cue of salivary stimulation with a therapeutically safe agent such as pilocarpine.53
Betel quid was once considered by Europeans as a quaint and somewhat curious custom, and by South Asians as a societal norm. Nowadays it is largely unknown by Americans, outside of the substantial Asian-American communities. Based on data from the U.S. census, there are a total of approximately 9 million first generation immigrants in the United States from countries where areca product use is high, including India, the Philippines, Vietnam, Pakistan, Cambodia, Taiwan, and Bangladesh. Additionally, areca use is prevalent in the U.S. territory of Guam (population of 168 000). As with other cultural traditions, this use may be passed on to second, third, and even further generations, especially in tight social communities, such as those of the Indian-Gujarati immigrants. It is reasonable to estimate the total number of people in the United States who have a cultural tradition of areca use to be close to 20 million, and based on the limited survey data available,54, 55 more than 1 million people in the United States may be current users, with elevated risks for oral disease and cancers.9 The sum impact of areca product use is probably hundreds of preventable cancers in the U. S every year and much more precancerous oral disease. The path to prevention of areca-related disease is the management of the addiction, and with accumulating new insights into the root causes of areca addiction, we can hope to find that path.
ACKNOWLEDGEMENTS
The authors thank Marta Quadri, Lu Wenchi Corrie, Joseph Dragone, and Kofi Ofosu for technical assistance. This work was supported by National Institute of General Medical Sciences (GM57481), Fondecyt Initiation Fund 11191049 (JAP). Research in the Phelps laboratory was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK124267.
CONFLICT OF INTEREST
The authors declare that there are no conflict of interest.
AUTHOR CONTRIBUTIONS
Clare Stokes prepared areca nut broths and fractions, conducted oocyte experiments, analysed data, and assisted in writing the paper. Jose A. Pino designed and conducted the neurotransmitter uptake experiments, analysed those data, and assisted in writing the paper. D. Walker Hagan conducted the HPLC analysis of the low molecular weight areca fraction, researched the published studies of areca composition, and assisted in writing the paper. Gonzalo E. Torres supervised the neurotransmitter uptake experiments and assisted in writing the paper. Edward A. Phelps supervised the HPLC analysis of the low molecular weight areca fraction and assisted in writing the paper. Nicole A. Horenstein, conducted the ion exchange preparation of the low molecular weight areca fraction, advised on the size fractionation, and assisted in writing the paper. Roger L. Papke designed experiments, analysed data, prepared figures, and wrote the manuscript with assistance from the co-authors.
Open Research
DATA AVAILABILITY STATEMENT
Data in this manuscript are available from the corresponding author on request.




