Gonadal hormones and sex chromosome complement differentially contribute to ethanol intake, preference, and relapse-like behaviour in four core genotypes mice
Funding information: The National Institute on Alcohol Abuse and Alcoholism (R15 AA027915; AKR), the National Institute of Neurological Disorders and Stroke (F99 NS118727; EAS), and the National Institute of Child Health and Human Developoment (R01 HD076125; APA) and by the College of Arts and Sciences, the Office of Research for Undergraduates, and the Department of Psychology's Broadening Undergraduate Research Participation in Behavioural Neuroscience programme at Miami University.
Abstract
Alcohol use and high-risk alcohol drinking behaviours among women are rapidly rising. In rodent models, females typically consume more ethanol (EtOH) than males. Here, we used the four core genotypes (FCG) mouse model to investigate the influence of gonadal hormones and sex chromosome complement on EtOH drinking behaviours. FCG mice were given access to escalating concentrations of EtOH in a two-bottle, 24-h continuous access drinking paradigm to assess consumption and preference. Relapse-like behaviour was measured by assessing escalated intake following repeated cycles of deprivation and re-exposure. Twenty-four-hour EtOH consumption was greater in mice with ovaries (Sry−), relative to those with testes, and in mice with the XX chromosome complement, relative to those with XY sex chromosomes. EtOH preference was higher in XX versus XY mice. For both consumption and preference, the influences of the Sry gene and sex chromosomes were concentration dependent. Escalated intake following repeated cycles of deprivation and re-exposure emerged only in XX mice (vs. XY). Mice with ovaries (Sry− FCG mice and C57BL/6J females) were also found to consume more water than mice with testes. These results demonstrate that aspects of EtOH drinking behaviour may be independently regulated by sex hormones and chromosomes and inform our understanding of the neurobiological mechanisms which contribute to EtOH dependence in male and female mice. Future investigation of the contribution of sex chromosomes to EtOH drinking behaviours is warranted. We used the FCG mouse model to investigate the influence of gonadal hormones and sex chromosome complement on EtOH drinking behaviours, including the alcohol deprivation effect. Escalated intake following repeated cycles of deprivation and re-exposure emerged only in XX mice (vs. XY). These results demonstrate that aspects of EtOH drinking behaviour may be independently regulated by sex hormones and chromosomes.
1 INTRODUCTION
Risky drinking behaviours and the development of alcohol use disorder (AUD) is a prevalent health issue in the United States and worldwide.1 Recent research demonstrates that alcohol use and high-risk alcohol drinking behaviours among women are rapidly rising.2 Further, women may progress from initial alcohol experience to alcohol dependence more quickly than men3 and work harder for alcohol following a period of abstinence.4 Paralleling the effects observed in humans, female rodents are known to be more vulnerable to a range of addictive behaviours.5 For example, female rodents consume more ethanol (EtOH) than males6-9 and are more likely to consume EtOH despite the risk of punishment.10-12 The neurobiological mechanisms contributing to these behavioural differences are still relatively unknown.
One potential mediator of sex differences in alcohol drinking behaviours is gonadal hormones. Elevated levels of estrogens have been associated with higher levels of alcohol consumption in adolescent13 and adult women.14 Recent work also suggests that rising progesterone levels may protect against alcohol intake in some women and that gonadal hormone levels may be associated with drinking alcohol to cope with negative emotional states.15 In rodents, elevated levels of EtOH consumption and EtOH reward in females16-18 are at least partially dependent on ovarian hormones including estradiol, which promotes drinking19-24 and EtOH reward.25
Gonad type is influenced by the presence or absence of the Sry gene, which is located on the Y chromosome and is responsible for the development of testes in male mice and the secretion of testosterone.26, 27 As such, gonad differentiation is the result of only one of a number of chromosomal differences between males and females. Indeed, the human Y chromosome encodes 27 proteins and the X chromosome encodes approximately 1500 proteins that could potentially contribute to sex differences in alcohol drinking behaviours and a large number of X-linked genes are important for brain development and function.28-30 Differentiating the influences of gonadal hormones and sex chromosomes on behaviour is, however, difficult, and there are few data on sex chromosome contributions to alcohol drinking.
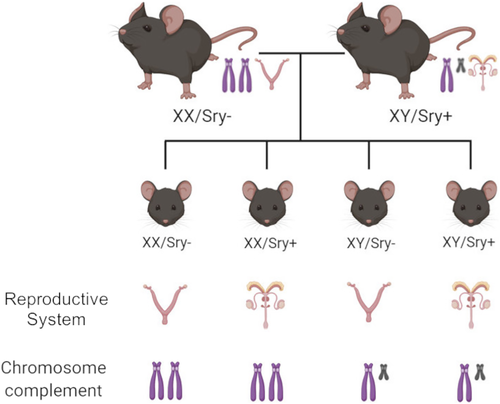
Fortunately, the four core genotypes (FCG) mouse model, in which the Sry gene is absent from the Y chromosome and inserted onto an autosome in the same mice, allows the influences of sex chromosomes and gonadal hormones on behaviour to be assessed independently.26, 27 Without the Sry transgene, XY or XX mice have female gonads (ovaries) regardless of sex chromosome complement, whereas XX or XY Sry+ mice have male gonads (testes) (Figure 1). This results in four groups: XX/Sry−, XY/Sry−, XX/Sry+ and XY/Sry+. Using this mouse model, sex chromosomes have been found to influence aggression, parenting, nociception, social interaction and habit formation.26, 31 In regard to EtOH drinking, female gonads (Sry−) have been associated with increased consumption (paralleling the effects of gonadal hormones described above), while sex chromosomes influenced the development of habitual versus goal-directed responding for EtOH.32
To explore the contributions of gonadal hormones and sex chromosomes to alcohol consumption, preference, and relapse-like behaviour, we examined 24-h EtOH intake and the alcohol deprivation effect in FCG mice. Our results suggest an important role for both sex hormones and chromosomes in mediating female vulnerability to EtOH drinking behaviours.
2 METHODS
2.1 Subjects
Thirty-nine FCG mice (PND 60+) were generated from breeding pairs consisting of Sry+ XY male and C57BL/6J (wild type) XX female mice at the Laboratory of Animal Resources at Miami University.27 FCG breeding pairs used to generate experimental mice were obtained from UCLA. This breeding scheme results in four groups XX/Sry− (n = 10), XY/Sry− (n = 10), XX/Sry+ (n = 9) and XY/Sry+ (n = 10) (Figure 1). Mice were tested in adulthood (XX/Sry−: 27.05 ± 1.85; XY/Sry−: 25.15 ± 2.46; XX/Sry+: 18.57 ± 1.63; XY/Sry+: 20.12 ± 1.84 weeks at the start of testing [all data mean ± standard error of the mean, SEM]). Prior to experimentation, FCG mice were group housed by gonad type (Sry− or Sry+). Fifty-two C57BL/6J mice (male = 20, female = 32) were generated from breeding pairs purchased from the Jackson Laboratory (Bar Harbour, ME) and were grouped housed prior to experimentation or surgery.
One week before the first experimental session, mice were individually housed in standard shoe box udel polysulfone rectangular mouse cages (18.4 cm × 29.2 cm × 12.7 cm) outfitted with two-bottle cage tops. Mice were given standard care and had access to LabDiet 5001 standard chow and reverse-osmosis (RO) filtered water ad libitum. Mice were kept on a 12:12 h light: dark cycle (lights on at 7 AM). All mice were cared for in accordance with the guidelines set by the National Institute of Health and all procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Miami University.
2.2 24-h home cage EtOH drinking and deprivation
Throughout testing, FCG mice had access to two bottles that contained RO drinking water and EtOH in RO water (v/v). Drinking bottles were made from 50-ml conical tubes fitted with ball-bearing sippers weighing approximately 80 g when filled and mice consumed about 2–4 g per session. All bottles were weighed every 24 h using a portable balance (Fisher Science Education, Model: SLF103, readability: 0.001 g). Every 48 h, mice were weighed and solutions were changed out. EtOH concentrations increased over drinking sessions from 5%, 10%, 15%, to 20% (five drinking sessions/concentration) (Figure 2A). After the last 20% EtOH session, mice underwent a 6-day EtOH deprivation period. After the deprivation period, mice were reintroduced to 20% EtOH for 24 h. This cycle of deprivation and re-exposure was then repeated for a total of five deprivation sessions (Figure 3A). Bottles were alternated daily to equate side biases. Two ‘dummy’ cages were outfitted with bottles to account for spillage and evaporation.
2.3 24-h home cage water and sucrose drinking in FCG mice
At least 2 weeks following the last re-exposure session, FCG mice were presented with RO water for one 24-h session. The following day mice were presented with a bottle of 2.5% sucrose or RO water for a total of five 24-h drinking sessions (Figure 4A).
2.4 24-h home cage water drinking in C57BL/6J mice
To follow up on effects observed in the FCG mice, water intake in C57BL/6J mice was assessed in two separate experiments. First, water intake in intact male and female mice was recorded over a 24-h session. Mice (n = 40, male = 20, female = 20) were individually housed at least 3 days prior to experimentation. One water bottle was presented to the mice ab libitum for one 24-h session. Bottles and mice were weighed at the onset and end of the session. Two ‘dummy’ bottles were outfitted on cages to account for any evaporation or spillage.
The influence of circulating ovarian hormones on water intake was assessed in a separate experiment. Female C57BL/6J mice received either ovariectomy (OVX = 16) or sham (SHAM = 16) surgeries as described below. Water intake was assessed over a 24-h session as described above.
2.5 Ovariectomy surgeries
Mice were placed under light anaesthesia using isoflurane and were surgically prepped by removing the fur from the back. The incision sites were cleaned with three alternating swabs of betadine-soaked gauze and EtOH wipes. Next, a 1-cm incision was made through the skin along the abdominal cavity. The ovarian fat pat was identified under the dorsal muscle mass, and a 0.5-cm incision was made through the dorsal muscle mass. The ovarian fat pad was pulled through the incision to expose the ovary. Hemostats were then used to clamp the uterine horn off underneath the ovary to prevent bleeding. A scalpel was used to remove the ovary. Following removal, the uterine horn and fat pad were replaced back into the body cavity. Sutures were made through the muscle and the skin to close the wound. The above steps were repeated for the next side. For sham surgeries, the above steps were the same but the ovary was not removed. After surgery, mice were placed on a heating pad for at least 30 min and were weighed before returning to the colony room. Standard postoperative care was given for 3–5 days, and 50–80 mg/kg/day of ibuprofen was available in drinking water. Experimentation occurred 3 weeks following surgery (after Satta et al. (2018)24).
2.6 Data analysis
Consumption was calculated as (Initial Bottle Weight – Post Bottle Weight) – Average of Dummy Bottles. EtOH consumption was expressed as grams of EtOH consumed per kilogram of body weight. Preference was calculated as ((Volume of EtOH)/ (Volume of EtOH + Water Consumption))*100. Consumption and preference for each concentration were computed by averaging across the five drinking sessions. Total water or EtOH consumption was calculated by summing consumption across all drinking sessions for each individual mouse then averaging for each group. Water and sucrose data were expressed as millilitres of water consumed per kilogram of body weight.
For EtOH consumption and preference data, a three-way analysis of varience (ANOVA) was used with Sry (gonad type) and sex chromosome complement as between-subjects factors and EtOH concentration as the within-subjects factor. Because Sry and chromosome complement were found to interact with EtOH concentration, but not each other, follow-up analyses using a two-way ANOVA were performed on each separate concentration using sex chromosome complement and Sry (gonad type) as between-subjects factors. Dunnett's test corrected for multiple comparisons was used to assess the alcohol deprivation effect, defined as an increase in consumption on re-exposure sessions versus at baseline (= the five sessions immediately preceding the beginning of the first deprivation period). For water and sucrose consumption experiments in FCG mice, a two-way ANOVA was used with Sry and chromosome complement as between-subject factors. In C57BL/6J mice, an unpaired t-test was used between groups.
Sample size was determined a priori based on previous work assessing the alcohol deprivation effect in C57BL/6J mice.33, 34 Partial omega squared (ω2) with 95% confidence intervals were calculated to report effect sizes.35 All data are shown as mean ± SEM, and the alpha level for all comparisons was set at p < 0.05. Analyses were conducted in R v. 4.1.1 and GraphPad Prism v. 8.3. Images were created using GraphPad Prism, BioRender, and GNU Image Manipulation Programme (GIMP) 2.10.
3 RESULTS
3.1 Sex hormones and chromosomes influence EtOH consumption
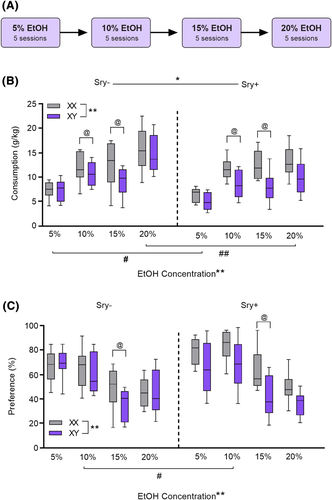
Analysis of EtOH consumption across concentrations revealed influences of both Sry (gonad type) and sex chromosomes (Figure 2B). Sry− mice consumed more EtOH than Sry+ mice and XX mice consumed more than XY mice. A three-way ANOVA identified main effects of the Sry gene (F(1, 35) = 4.704, p = 0.037, ω2 = 0.09 [0.00, 0.30]), chromosome complement (F(1, 35) = 9.839, p = 0.003, ω2 = 0.19 [0.02, 0.41]), and EtOH concentration (F(3, 105) = 55.046, p < 0.0001, ω2 = 0.60 [0.48, 0.68]). There were interactions with EtOH concentration for the Sry gene (F(3, 105) = 3.159, p = 0.028, ω2 = 0.06 [0.00, 0.14]) and chromosome complement (F(3, 105) = 2.838, p = 0.042, ω2 = 0.05 [0.00, 0.13]). The interaction between Sry gene X chromosome complement (F(1,35) = 1.487, p = 0.231, ω2 = 0.01 [0.00, 0.17]) and the three-way interaction of Sry gene X sex chromosomes X EtOH concentration did not reach significance (F(3, 105) = 0.238, p = 0.870, ω2 = −0.02 [0.00, 0.00]).
Follow-up two-way ANOVAs for each concentration of EtOH found a main effect of chromosome complement at 10% (F(1,35) = 5.695, p = 0.023, ω2 = 0.11 [0.00, 0.32]) and 15% (F(1,35) = 12.647, p = 0.001, ω2 = 0.23 [0.03, 0.45]) concentrations but not 5% (F(1,35) = 1.673, p = 0.204, ω2 = 0.02 [0.00, 0.18]) or 20% (F(1,35) = 2.928, p = 0.096, ω2 = 0.05 [0.00, 0.24]). A main effect of Sry gene was uncovered at 5% (F(1,35) = 6.339, p = 0.017, ω2 = 0.12 [0.00, 0.34]) and 20% (F(1,35) = 8.222, p = 0.007, ω2 = 0.16 [0.01, 0.37]) concentrations of EtOH but not 10% (F(1,35) = 0.819, p = 0.372, ω2 = 0.00 [0.00, 0.00]) or 15% (F(1,35) = 0.124, p = 0.727, ω2 = −0.02 [0.00, 0.00]). There were no significant interactions between Sry and chromosomes (p > 0.20 for all; ω2 < 0.01 for all).
Additional analyses on body weights across EtOH concentrations revealed that Sry+ mice weighed more than Sry− mice at 10%, 15%, and 20% concentrations (Table 1). A three-way ANOVA identified a main effect of the Sry gene (F(1,35) = 10.706, p = 0.002, ω2 = 0.21 [0.02, 0.43]) and an interaction between EtOH concentration X Sry gene (F(3,105) = 3.512, p = 0.018, ω2 = 0.06 [0.00, 0.16]). No effect of chromosome complement (F(1,35) = 1.684, p = 0.203, ω2 = 0.02 [0.00, 0.18]) or EtOH concentration (F(3,105) = 1.034, p = 0.381, ω2 = 0.00 [0.00, 0.00]) was observed. The interactions between Sry gene X chromosome complement (F(1,35) = 1.143, p = 0.292, ω2 = 0.00 [0.00, 0.13]), chromosome complement X EtOH concentration (F(3,105) = 0.330, p = 0.804, ω2 = −0.02 [0.00, 0.00]), and the three-way interaction of Sry gene X sex chromosomes X EtOH concentration did not reach significance (F(3,105) = 0.249, p = 0.862, ω2 = −0.02 [0.00, 0.00]).
| Group | 5% EtOH (ml/kg) | 10% EtOH (ml/kg)a | 15% EtOH (ml/kg)a | 20% EtOH (ml/kg)a |
|---|---|---|---|---|
| XX/Sry− | 24.25 ± 0.52 | 22.68 ± 0.35 | 23.95 ± 0.39 | 23.94 ± 0.49 |
| XY/Sry− | 23.40 ± 0.54 | 22.62 ± 0.52 | 23.07 ± 0.58 | 23.11 ± 0.49 |
| XX/Sry+ | 23.85 ± 0.32 | 24.96 ± 0.43 | 25.08 ± 0.43 | 25.22 ± 0.43 |
| XY/Sry+ | 24.32 ± 0.66 | 24.68 ± 0.48 | 24.76 ± 0.52 | 25.02 ± 0.47 |
- Note: Mice with testes weighed more than mice with ovaries. Sry+ weighed more than Sry− mice at 10%, 15%, and 20% concentrations.
- a Main effect of Sry gene, p < 0.01 (two-way ANOVA). Data are averages ± standard error of the mean (SEM).
There were no significant main effects of chromosomes on body weights when follow-up two-way ANOVAs at each concentration were performed: 5% (F(1,35) = 0.138, p = 0.631, ω2 = −0.02 [0.00, 0.00]), 10% (F(1,35) = 2.068, p = 0.159, ω2 = 0.03 [0.00, 0.20]), 15% (F(1,35) = 1.522, p = 0.226, ω2 = 0.01 [0.00, 0.17]), and 20% (F(1,35) = 1.183, p = 0.284, ω2 = −0.01 [0.00, 0.00]). A main effect of Sry gene was found at 10% (F(1,35) = 14.002, p < 0.001, ω2 = 0.25 [0.04, 0.46]), 15% (F(1,35) = 8.281, p = 0.007, ω2 = 0.16 [0.01, 0.38]) and 20% (F(1,35) = 11.440, p = 0.002, ω2 = 0.21 [0.03, 0.43]) concentrations but not at 5% (F(1,35) = 0.234, p = 0.631, ω2 = −0.02 [0.00, 0.00]). There were no significant interactions between Sry and chromosomes (p > 0.20 for all; ω2 < 0.01 for all).
3.2 Sex hormones and chromosomes influence preference for EtOH versus water
Analysis of EtOH preference demonstrated an influence of sex chromosomes and hormones (Figure 2C). Preference was higher in XX (vs. XY mice) and in Sry+ mice (vs. Sry−). A three-way ANOVA revealed a main effect of chromosome complement (F(1, 35) = 8.130, p = 0.007, ω2 = 0.16 [0.01, 0.38]) and EtOH concentration (F(3, 105) = 47.188, p < 0.0001, ω2 = 0.56 [0.43, 0.65]) but no interaction between these factors (F(3,105) = 1.912, p = 0.132, ω2 = 0.02 [0.00, 0.09]). The main effect of Sry gene was not significant (F(1, 35) = 3.136, p = 0.085, ω2 = 0.05 [0.00, 0.25]), but there was a significant Sry gene X EtOH concentration interaction (F(3, 105) = 3.169, p = 0.027, ω2 = 0.06 [0.00, 0.14]). The interactions between Sry gene and sex chromosomes (F(1,35) = 3.555, p = 0.068, ω2 = 0.06 [0.00, 0.14]) and Sry gene x sex chromosomes x EtOH concentration (F(3,105) = 0.117, p = 0.950, ω2 = −0.02 [0.00, 0.00]) did not reach significance.
Follow-up two-way ANOVAs of preference for each concentration of EtOH found a main effect of chromosome complement at 15% (F(1,35) = 12.573, p = 0.001, ω2 = 0.23 [0.30, 0.45]) concentration but not 5% (F(1,35) = 1.297, p = 0.385, ω2 = 0.00 [0.00, 0.15]), 10% (F(1,35) = 3.016, p = 0.091, ω2 = 0.05 [0.00, 0.24]), or 20% (F(1,35) = 2.239, p = 0.144, ω2 = 0.03 [0.00, 0.21]). A main effect of Sry gene was uncovered at 10% (F(1,35) = 6.390, p = 0.016, ω2 = 0.12 [0.00, 0.34]) concentration but not 5% (F(1,35) = 0.774, p = 0.385, ω2 = 0.00 [0.00, 0.00]), 15% (F(1,35) = 3.744, p = 0.061, ω2 = 0.07 [0.00, 0.26]) or 20% (F(1,35) = 0.562, p = 0.459, ω2 = −0.01 [0.00, 0.00]). There were no significant interactions between Sry and chromosomes (p > 0.10 for all; ω2 < 0.04 for all).
Higher EtOH consumption in Sry− mice without corresponding increases in EtOH preference suggested that gonad type also influenced water consumption (Table 2). A three-way ANOVA of water consumption during the 24-h EtOH drinking paradigm in Sry− and Sry+ mice demonstrated that Sry− mice consumed more water than Sry+ mice, as evidenced by a main effect of Sry gene (F(1, 35) = 13.338, p < 0.001, ω2 = 0.25 [0.04, 0.46]). There was also a main effect of EtOH concentration (F(3,105) = 30.486, p < 0.0001, ω2 = 0.45 [0.30, 0.56]) but not chromosome complement (F(1,35) = 3.533, p = 0.069, ω2 = 0.06 [0.00, 0.26]). The interactions between Sry gene X chromosome complement (F(1,35) = 0.858, p = 0.361, ω2 = 0.00 [0.00, 0.00]), Sry gene X EtOH concentration (F(3,105) = 1.555, p = 0.205, ω2 = 0.02 [0.00, 0.06]) and chromosome complement X EtOH concentration (F(3,105) = 1.898, p = 0.134, ω2 = 0.02 [0.00, 0.09]) did not reach significance. The three-way interaction of Sry gene X sex chromosomes X EtOH concentration did not reach significance (F(3, 105) = 0.258, p = 0.855, ω2 = −0.02 [0.00, 0.00]).
| Group | 5% EtOH (ml/kg)a | 10% EtOH (ml/kg)a | 15% EtOH (ml/kg)a,b | 20% EtOH (ml/kg) |
|---|---|---|---|---|
| XX/Sry− | 88.33 ± 13.93 | 88.65 ± 15.66 | 120.02 ± 11.21 | 129.78 ± 14.40 |
| XY/Sry− | 77.95 ± 8.45 | 95.82 ± 17.06 | 153.91 ± 19.45 | 139.10 ± 25.69 |
| XX/Sry+ | 41.02 ± 6.07 | 32.79 ± 10.70 | 61.65 ± 9.93 | 90.84 ± 8.06 |
| XY/Sry+ | 67.09 ± 14.46 | 54.95 ± 12.07 | 107.94 ± 9.92 | 118.12 ± 4.49 |
- Note: Gonad type predicted water consumption. Sry−mice consumed more water when 5% or 10% EtOH was present. Sry− (vs. Sry+) and XY (vs. XX) mice consumed more water when 15% EtOH was present.
- a Main effect of Sry gene, p < 0.05 (two-way ANOVA).
- b Main effect of chromosomes, p = 0.05 (two-way ANOVA). Data are averages ± standard error of the mean (SEM).
Follow-up two-way ANOVAs of water consumption for each concentration of EtOH found a main effect of chromosome complement (XY > XX mice) at 15% (F(1,35) = 8.901, p = 0.005, ω2 = 0.17 [0.01, 0.39]) concentration but not 5% (F(1,35) = 0.563, p = 0.458, ω2 = −0.01 [0.00, 0.00]), 10% (F(1,35) = 1.049, p = 0.313, ω2 = 0.00 [0.00, 0.10]) or 20% (F(1,35) = 1.346, p = 0.254, ω2 = 0.00 [0.00, 0.15]). A main effect of Sry gene was uncovered at 5% (F(1,35) = 5.983, p = 0.020, ω2 = 0.11 [0.00, 0.33]), 10% (F(1,35) = 11.421, p = 0.002, ω2 = 0.21 [0.03, 0.43]) and 15% (F(1,35) = 15.129, p < 0.001, ω2 = 0.27 [0.05, 0.48]) concentrations but not 20% (F(1,35) = 3.578, p = 0.067, ω2 = 0.06 [0.00, 0.26]). There were no significant interactions between Sry and chromosomes (p > 0.10 for all; ω2 < 0.03 for all).
3.3 Sex chromosomes influence the alcohol deprivation effect
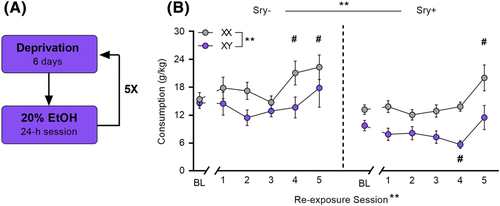
Mice were re-exposed to 20% EtOH following 6-day cycles of deprivation for five total re-exposures (Figure 3A). Previous research suggests that this regimen promotes increases in intake known as the alcohol deprivation effect.34 Analysis of EtOH consumption at baseline (= the five sessions immediately preceding the beginning of the first deprivation period) and on the five deprivation sessions demonstrated that sex chromosomes influenced the magnitude of the alcohol deprivation effect. A three-way ANOVA revealed main effects of the Sry gene (F(1, 35) = 12.075, p = 0.001, ω2 = 0.23 [0.03, 0.45]), sex chromosome complement (F(1, 35) = 13.202, p < 0.001, ω2 = 0.25 [0.04, 0.46]), and re-exposure session (F(5, 180) = 7.9747, p < 0.0001, ω2 = 0.16 [0.06, 0.24]). No interactions were observed between re-exposure session X Sry gene (F(5,175) = 0.970, p = 0.438, ω2 = 0.00 [0.00, 0.00]), re-exposure session X chromosome complement (F(5,175) = 1.719, p = 0.133, ω2 = 0.02 [0.00, 0.05]), Sry gene X chromosome complement (F(1,35) = 0.544, p = 0.466, ω2 = −0.01 [0.00, 0.00]), or re-exposure session X Sry gene X chromosome complement (F(5,175) = 0.521, p = 0.760, ω2 = −0.01 0.00, 0.00]).
Follow-up Dunnett's tests (control condition = baseline) showed that mice in the XX/Sry− group consumed more EtOH on re-exposure sessions 4 (p = 0.039) and 5 (p = 0.047) and mice in the XX/Sry+ group consumed more EtOH on re-exposure session 5 (p = 0.039) compared with baseline (Figure 3B). Mice in the XY/Sry+ group consumed less EtOH compared with baseline on re-exposure session 4 (p = 0.032).
3.4 Sex hormones influence water but not sucrose consumption
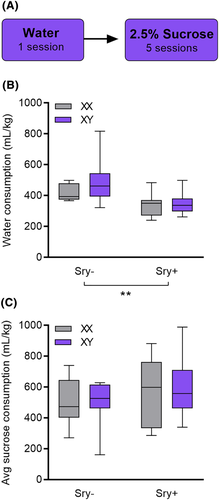
To determine whether the effects of the Sry gene on water consumption could be replicated without concurrent access to EtOH, water consumption in the home cage during a 24-h period was measured in all FCG mice. A two-way ANOVA revealed that Sry− mice drank more water versus Sry+ mice, as evidenced by a main effect of Sry (F(1, 35) = 13.339, p < 0.001, ω2 = 0.24 [0.04, 0.46]) (Figure 4B). The effect of chromosome complement did not reach the threshold of significance (F(1,35) = 1.684, p = 0.203, ω2 = 0.02 [0.00, 0.18]). No interaction was observed between chromosome complement X Sry (F(1,35) = 1.046, p = 0.314, ω2 = 0.00 [0.00, 0.10]).
A final control experiment was conducted to assess whether the observed differences in EtOH consumption, and preference were specific to EtOH reward. FCG mice drank a 2.5% sucrose solution in a two-bottle choice paradigm for five 24-h sessions. A two-way ANOVA identified no main effects of the Sry gene (F(1,35) = 2.585, p = 0.117, ω2 = 0.04 [0.00, 0.22]), chromosome complement (F(1,35) = 0.017, p = 0.898, ω2 = −0.03 [0.00, 0.00]), and no interaction between Sry gene X chromosome complement (F(1,35) = 0.040, p = 0.843, ω2 = −0.03 [0.00, 0.00]) in average consumption of sucrose (Figure 4C).
3.5 Female mice consume more water than male mice
In one 24-h session, female C57BL/6J mice consumed more water than their male counterparts. An unpaired t-test identified a difference between male and female consumption (t(38) = 2.933, p = 0.006, ω2 = 0.16 [0.01, 0.37]) (Figure 5A).
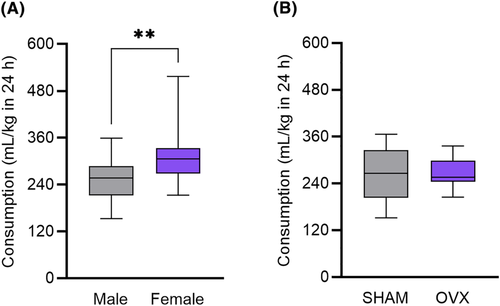
3.6 Water consumption is not dependent on circulating ovarian hormones
In one 24-h session, water consumption in ovariectomized C57BL/6J mice was unchanged compared with controls. An unpaired t-test found no difference between OVX and SHAM water consumption (t(30) = 0.213, p = 0.833, ω2 = −0.03 [0.00, 0.00]) (Figure 5B).
4 DISCUSSION
Using the FCG mouse model in a 24-h continuous access drinking paradigm, we explored how sex hormones and sex chromosomes contribute to female vulnerability to EtOH consumption, preference and relapse-like behaviour and uncovered novel influences of sex chromosomes. Twenty-four-hour EtOH consumption was greater in mice with female gonads (Sry−) and in mice with the XX chromosome complement. EtOH preference was higher in XX versus XY mice. Escalated intake following repeated cycles of deprivation and re-exposure emerged only in XX mice (vs. XY). Follow-up control studies demonstrated that Sry− mice consumed more water than their Sry+ counterparts did. These results concur with prior findings concerning the role of ovarian hormones in female vulnerability to EtOH drinking while revealing an underappreciated role for sex chromosomes in these behaviours.
Because the presence or absence of Sry determines the development of ovaries (Sry−) versus testes (Sry+), behavioural differences between the Sry genotypes are primarily driven by levels of gonadal hormones. It is well-established that ovarian hormones such as estradiol promote consumption and seeking of alcohol in rats and mice.16, 19-22, 24 Our observation of higher intake in Sry− mice similarly suggests that female gonads promote EtOH consumption. This finding also replicates an earlier study in FCG mice that reported higher 30-min EtOH consumption in Sry− mice.32 It is interesting to note that in the current study, the largest effect size observed for consumption was for EtOH concentration and that concentration significantly interacted with Sry (gonad type). The effect of Sry on consumption was most pronounced at the lowest (5%) and highest (20%) concentrations of EtOH, which is reminiscent of a previous study from our lab that found concentration-dependent increases in responding for EtOH in female mice.12 Thus, increased consumption of EtOH in female mice, as driven by the Sry gene, may be concentration dependent.
Interestingly, the increased EtOH consumption observed in Sry− mice was not seen for EtOH preference. In fact, at some concentrations, preference was higher in Sry+ mice (vs. Sry−). Some prior studies have seen increased preference in female rodents16 while others have not.9, 11 We have observed a similar pattern of greater EtOH consumption but not preference in intact female versus male C57BL/6J mice using a drinking in the dark paradigm,9 and similar findings have been seen in rats.36, 37 In the current study, the absence of an increase in preference in females was driven by robust elevations in water intake in Sry− mice during EtOH drinking sessions relative to Sry+ mice. Indeed, among all the responses measured during the 24-h drinking sessions, Sry had the largest effect on water consumption (ω2 = 0.25). It is important to recognise that the EtOH and water consumption measures were influenced by body weight and that Sry also had a large effect on this measure (ω2 = 0.21). Higher water intake was also observed in a separate, EtOH-free session in Sry− versus Sry+ mice and in female versus male C57BL/6J mice. Although estrogens have been shown to influence water consumption in rodents,38-40 we did not find an influence of circulating ovarian hormones on water consumption in OVX versus SHAM female C57BL/6J mice, suggesting that sex differences in water consumption are not driven by ongoing (i.e. activational) influences of ovarian hormones. The influence of gonad type on water intake is an important consideration for studies of EtOH drinking behaviours, since variations in water intake could influence blood EtOH levels. It also raises the possibility that mice with female gonads may simply drink more fluids per kilogram of bodyweight than males. The observation of equal sucrose consumption across genotypes could argue against this conclusion, although we cannot rule out that consumption levels in that experiment reached a ceiling and obscured any effects. To be sure, studies of sex differences in alcohol drinking behaviours in rodents need to carefully control for the possibility that Sry− mice (likely via effects on body weight) have higher fluid intake.
In addition to gonadal influences on EtOH consumption, we report a novel role for sex chromosomes in influencing both consumption and preference, with similar effect sizes (ω2 = 0.19 and 0.16, respectively). In one other study of 30-min consumption of 10% EtOH in FCG mice, sex chromosome complement did not influence consumption.32 Here, as with consumption, we also saw that EtOH concentration had a large effect on preference and differences in preference between XX and XY mice were greatest at 15% concentration. Thus, the effects of chromosomes may be dependent on the drinking paradigm used or the concentration of EtOH presented. Importantly, sex chromosomes did not influence water or sucrose intake, suggesting that the observed effects are specific to EtOH. When considered alongside our findings regarding Sry influences on consumption but not preference, these results suggest that sex chromosome differences between males and females play a critical, and perhaps primary, role in driving sex differences in some EtOH drinking behaviours.
Another major finding of this work is that sex chromosomes and hormones influenced relapse-like behaviour. As in the 24-h continuous portion of the experiment, EtOH consumption during re-exposure sessions was higher for mice with XX (vs. XY) chromosomes and Sry− (vs. Sry+) mice with large effect sizes. Furthermore, escalations in drinking following deprivation (assessed by comparing consumption on re-exposure sessions to baseline consumption of 20% EtOH) were only observed in mice with XX chromosomes. The observation of the alcohol deprivation effect on re-exposure session 4 in XX/Sry− but not XX/Sry+ mice further suggests that the presence of female gonads may facilitate the effects of deprivation on drinking behaviour. One important consideration is that the alcohol deprivation effect was completely absent in XY/Sry+ mice, which are most similar to the male mice first used to establish this paradigm in the C57BL/6J line.34 Interestingly, delayed emergence of escalated drinking (during re-exposure session 6–7) was observed in a study by Melendez and colleagues (2006)34 when variants of the paradigm were used, including when a higher concentration of EtOH was available. It is possible that a similar delay occurred here in the XY/Sry+ mice because we used 20% EtOH, as opposed to 15%. Thus, while we cannot be certain, we think it likely that escalated drinking emerges more quickly (i.e., following fewer deprivation cycles) in vulnerable animals.
The alcohol deprivation effect has been demonstrated previously in male rats and mice34, 41, 42 and has also been observed in female rats and WSC-1 mice following a single two-week deprivation.43, 44 In the latter study, escalations in drinking following deprivation were similar in male and female mice.44 As such, our results are the first demonstration of greater female susceptibility in this model of relapse-like behaviour. One limitation of our study is that we did not assess the deprivation effect by comparing consumption to nondeprived animals, as has been done previously.34 Instead, we defined the deprivation effect as an increase in drinking compared with baseline. It is also important to note that prior EtOH exposure may have influenced the results of subsequent control experiments assessing water and sucrose consumption.
Another potential limitation of this study is that the estrous cycle was not assessed in Sry− mice. Estrous phase is typically unrelated to EtOH consumption levels in freely cycling rodents,10, 19, 24, 45, 46 supporting other work demonstrating that gonadal hormone effects on EtOH consumption in females occur during development (i.e., are organizational rather than activational).15, 16, 47, 48 Further, because we averaged intake and preference for each concentration of EtOH over five sessions, there are unlikely to be influences of estrous on the 24-h, continuous drinking results. We cannot, however, exclude the possibility that estrous cycle phase influenced results from the re-exposure sessions. Complicating the issue is the fact that extended EtOH exposure can alter the estrous cycle49-51 and XY mice with ovaries stop cycling earlier in life than XX mice.25 A related consideration is that we do not have data on circulating hormone levels in the FCG mice. However, FCG mice with XX chromosomes are fully feminized and XY mice fully masculinized on a number of traits,27 and gonadal hormones levels have consistently been found to be similar in XX and XY FCG mice with the same type of gonads.52, 53 Future studies in gonadectomized FCG mice will nevertheless be needed to fully resolve this issue as well as to confirm that the effects of gonadal hormones are organisational in nature.
A sex difference driven by sex chromosome complement may be due to genes on the Y chromosome, an extra dose of genes on the X chromosome in females or paternal imprinting of X-linked genes.54, 55 The mechanisms through which sex chromosomes influence EtOH drinking behaviours remain to be determined. Prior studies in the FCG mice have uncovered a role for sex chromosomes in the development of habit formation,32, 56 locomotor activation to cocaine,57 and in the density of tyrosine hydroxylase-expressing neurons in the midbrain54, 58 (though note that no effect on sucrose consumption was observed in the current study). It is thus possible that genes on the X and/or Y chromosomes produce sex differences in EtOH consumption, preference, relapse-like behaviour, and/or habit formation via influences on the development of brain reward systems.
In sum, the current results confirm an involvement of gonadal hormones and highlight a currently underappreciated contribution of sex chromosomes in EtOH drinking behaviours. In addition to continued exploration of the mechanisms by which sex hormones facilitate EtOH drinking, it is critical that future studies begin to explore sex-specific genetic influences on these behaviours.
ACKNOWLEDGEMENTS
This study was supported by the National Institute on Alcohol Abuse and Alcoholism (R15 AA027915; AKR), the National Institute of Neurological Disorders and Stroke (F99 NS118727; EAS), and the National Institute of Child Health and Human Development (R01 HD076125; APA) and by the College of Arts and Sciences, the Office of Research for Undergraduates, and the Department of Psychology's Broadening Undergraduate Research Participation in Behavioural Neuroscience program at Miami University. The authors would like to thank Kristen M. Schuh for assistance with behavioural experiments and Catherine Wasylyshyn for assistance with surgeries.
CONFLICT OF INTEREST
The authors declare no conflicting interests in this work.
AUTHOR CONTRIBUTIONS
AKR, EAS, LNR, NGC, ASJ and NJO designed the experiments. EAS, LNR, NGC, ASJ and NJO conducted the experiments. EAS and BLM conducted the surgeries. HH and APA provided the FCG breeding pairs. AKR, EAS, LNR, NGC, ASJ, NJO and MRH analysed the data. AKR, EAS and APA interpreted the findings. EAS and AKR drafted the manuscript. All authors edited, contributed to and approved the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




