Abnormal resting-state effective connectivity in reward network among long-term male smokers
Funding information: This work was funded by the Medical Science and Technology Research Project of Henan Province, Grant/Award Number: 201701011, and Natural Science Foundation of China, Grant/Award Numbers: 81871327, 81601467.
Abstract
Background
Tobacco addiction is a chronic, relapsing mental disorder characterized by compulsive tobacco seeking and smoking. Current evidence shows that tobacco addiction exerts their initial reinforcing effects by activating reward circuits in the brain, but the causal connectivity among reward circuits is still unclear. Therefore, it is vital to understand how the reward network works to lead to the compulsive smoking behaviour.
Method
We applied dynamic causal modelling (DCM) to resting-state functional magnetic resonance (rs-fMRI) to characterize changes in effective connectivity (EC) among eight major hubs from reward network between 76 long-term male smokers and 55 nonsmoking volunteers (matched with age, gender and education).
Results
Relative to the healthy controls, long-term smokers had stronger ECs from the right anterior insula to left ventral striatum, posterior cingulate cortex (PCC) to ventral tegmental area (VTA), PCC to left anterior insula, left anterior insula to VTA, and ventromedial prefrontal cortex (vmPFC) to PCC and weaker ECs from the VTA to left ventral striatum, right anterior insula to right ventral striatum, and anterior cingulate cortex (ACC) to right anterior insula.
Conclusions
Overall, our findings revealed disrupted neural causal interactions among parts of the reward network associated with tobacco addiction, expanding the growing evidence for the potential neurobiological mechanisms of tobacco addiction. We found abnormalities within the mesocorticolimbic system and a top-down regulation disorder in the dopamine-dependent process of response inhibition and salience attribution among long-term smokers, which may facilitate the development of effective therapies in tobacco addiction.
1 INTRODUCTION
Smoking is considered as the leading cause of preventable disease in the world, which has a negative influence on health, economic and society.1 Nearly 5 million deaths in the world are attributed to smoking. Males had a much higher prevalence of ever-smoking and current smoking (67.39% and 48.77%) than females (3.74% and 2.93%) in China.2 Nicotine, a main component of tobacco, is the principal reason for tobacco addiction. Tobacco addiction is a chronic, relapsing mental disorder characterized by compulsive tobacco seeking and smoking.3 A lot of studies found that smoking has an effect on brain function including mesocorticolimbic system, default mode network (DMN), and reward circuits.4, 5 Nonetheless, it is not yet clear which cerebrum areas play an important role in tobacco addiction and their relationship among these areas. Understanding the neural mechanism of tobacco addiction is helpful to guide smoking cessation scientifically.
The nature of addiction is frequently considered for either a voluntary behaviour or a biological vulnerability, which is associated with genetic vulnerabilities or comorbid psychiatric conditions.4
Traditional conception of tobacco addiction was that tobacco abuse predominantly exerts their initial reinforcing effects mediated by limbic circuits (especially ventral tegmental area and ventral striatum) in the brain, which was called reward deficiency syndrome.4, 6 Ventral tegmental area (VTA), located at midbrain, with dopamine neurons gathering, was reported closely related to the onset of substance addiction.7 Continuously, the initial voluntary action could turn into a chronic, relapsing mental disorder characterized by compulsive tobacco seeking and smoking.8 Such transition has been suggested that associated with a functional shift in subregions of striatum: Exaggerated responses of the ventral striatal reward system engaged in excessive substance using at an early stage, while dorsal part dominated after the formation of compulsive behaviours.9, 10 Although the VTA and striatum played an important role in describing the reaction-related features of tobacco addiction, results from recent neuroimaging studies have implicated the additional brain regions, especially the prefrontal cortex (PFC).10, 11 Goldstein's study found that PFC is involved in regulating cognitive and emotion process, which could result in the overvaluing of drug reinforcers, the undervaluing of alternative reinforcers, and impaired inhibitory control for substance response.12 Such changes in addiction were described as a model called impaired response inhibition and salience attribution (I-RISA).12 In order to respond to motivational salience and reward expectation, PFC participates in higher order executive functions such as self-directed (PCC/precuneus), salience attribution (anterior insula, ACC), and emotion (vmPFC) by regulating limbic system in different stages of tobacco addiction.13, 14 Moreover, the pattern of top-down regulation has been verified in other addictive diseases. Previous research has reported that individuals with Internet gaming disorder (IGD) showed lower RSFC between medial orbitofrontal cortex and VTA compared with healthy controls.15 Li's study suggested that decreased smoking craving via hypnotic treatment may arise from the top-down regulation of the prefrontal to insula.16 Combined, tobacco addiction is thought to be linked to the reward system and the top-down circuit plays a vital role in the reward system including PFC, ventral striatum, insula, and midbrain.17 Nevertheless, the interactions among these brain regions and any abnormalities in information flow in reward system remain unclear in long-term tobacco use.
In the past, we used functional connectivity to explore the intrinsic brain regions relationship in tobacco addiction.18, 19 Sweitzer's study indicated that for dorsal striatum seeds, smoking abstinence decreased whole-brain connectivity with several regions, including medial prefrontal cortex (mPFC), hippocampus, posterior cingulate cortex (PCC), and supplemental motor area (SMA).20, 21 However, such findings on reward network did not support sequitur about directed or causal connectivity between these brain regions implicated in smoking. This deficiency calls for a new method that can describe causal interactions and uncover brain information flows. Dynamic causal modelling (DCM), by calculating effective connectivity (EC) between coupled brain regions, could characterize the causal (directed) connections and information flow from one area to another.22 Although DCM was used to apply to task-based fMRI, recently, spectral DCM has been developed specifically to infer EC in rs-fMRI.23 Moreover, spectral DCM could not only estimate of DCM parameters more efficiently but also detect group differences of EC sensitively by using DCM parametric empirical Bayes (PEB) analysis. Several researches have demonstrated that EC is susceptive to describe brain connectivity—Tang's study found mPFC-PCC-IPL circuit alterations in tobacco addiction and Wei's study indicated enhanced EC from ventral tegmental area (VTA) to amygdala and reduced EC from amygdala to VTA in cocaine abuse.24-26 To sum up, it has been shown that spectral DCM is relatively efficient both theoretically and practically.23, 27
The exploration of causal or direct connectivity on reward network is also lacking, due to the deficiency of the traditional functional connection method. The aim of our study was to apply spectral DCM to rs-fMRI to characterize EC alterations over major nodes from the reward network. Besides, correlation analyses were performed to search links between EC within reward circuit and the degree of tobacco addiction. In this current study, we assumed that long-term smokers would display abnormal neural interactions in reward network including VTA, striatum, and prefrontal cortex, and such alterations would associate with the severity of tobacco addiction.
2 METHODS AND MATERIALS
2.1 Participants
The potential participants were recruited through online platforms and advertisements. A total of 131 male subjects were recruited for this study, including 76 long-term smokers and 55 nonsmokers. We used Fagerström Test for Nicotine Dependence (FTND) to assess smoking addiction and severity.28 Smokers were defined as individuals who smoked at least 10 cigarettes daily in the past 1 year, and met the DSM-IV criteria, and had no period of smoking abstinence longer than 3 months.3 Nonsmokers were included as those who smoked less than five cigarettes in their lifetime.29 Their age and years of education were matched with long-term smokers. Exclusion criteria for all participants were as follows: (1) Existence of a neuropsychiatric disease; (2) systemic diseases (such as diabetes, hypertension, and cerebrovascular disease); (3) current using psychotropic medications or concurrent other substance abuse, such as alcohol and cocaine; or (4) because the rates of smoking in Chinese males is much higher and the public health burden falls predominately on this group. The current study focused on male smokers and excluded female smokers.2 All subjects were adult male, aged 20–55 years, right-handed, and without contraindications for MRI examination. Smokers were required to smoke a cigarette in 30–45 min before entering the scanner to exclude withdrawal symptoms. All subjects were sated at the time of scanning. Relevant clinical information and smoking-related information were collected in the form of questionnaires, and the information obtained was used for scientific research only. This research was approved by the Medical Ethics Committee of First Affiliated Hospital of Zhengzhou University, and informed consent.
2.2 Image acquisition
MRI data were obtained using a 3.0T German Siemens Magnetom Skyra magnetic resonance imaging equipment with a 16-channel prototype quadrature birdcage head coil at First Affiliated Hospital of Zhengzhou University. Participants were instructed to rest with their eyes closed, keeping awake, not to think of anything, and to keep their head motionless during scanning. Earplugs were used to protect the hearing of subjects, and spongy pads were used to fix their heads to minimize head movement. No external stimuli were exerted during image acquisition. Resting-state functional images were collected using an echo-planar imaging sequence. The parameters were repetition time (TR)/echo time (TE) = 2000/30 ms, flip angle = 80°, matrix size = 64 × 64, field of view = 220 mm × 220 mm, voxel size = 3.4 mm × 3.4 mm × 4 mm, slices = 36, and slice thickness = 4 mm, a total of 180 volumes. All slices along the AC-PC line were acquired with a total scan time of 360 s.
2.3 Image preprocessing
The Data Processing and Analysis of Brain Imaging (DPABI v3.0) (http://rfmir.org/dpabi) toolbox was used to preprocess the functional imaging data. Preprocessing comprised format conversion (DICOM to NIFTI), discarding the first five volumes, slice-timing, realignment. Subjects were excluded by head motion of >2.5 mm in maximum displacement or >2.5° rotation in angular motion. No subjects were excluded in this step.30 The images were spatially normalized to the standard EPI template and resampled to 3 × 3 × 3 mm. Functional images were spatially smoothed with a Gaussian kernel of full-width at half-maximum of 6 mm. Finally, linear detrending and scrubbing further eliminated the influence of head motion and noise. After despiking, the outliners were replaced with the best estimate using a third-order spline fit to clean up the time course portions. (3dDespike).31
2.4 Dynamic causal modelling
Spectral DCM, as implemented in SPM12 (http://www.fil.ion.ucl.ac.uk/spm/), was used to perform effective connectivity analysis. First, a general linear model was built in SPM with cosine basis functions from 1/128 to 0.1 Hz as effects of interest and the movement parameters, and cerebrospinal fluid and white matter signals as nuisance regressors.32 Based on previous studies,33, 34 the VTA, bilateral ventral striatum, bilateral anterior insula, posterior cingulate cortex, anterior cingulate cortex, and ventromedial prefrontal cortex were selected as regions of interest (ROIs) (Figure 1). These regions are deemed to the hubs for the reward network, which have been reported that tobacco abuse exerts their initial reinforcing effects by activating reward circuits in the brain.4 ROIs were defined as spheres with radius of 6 mm centred at the MNI coordinate reported in the previous study (Table 1). When extracting the signal, the centre of the sphere was fixed. For each ROI, the first principal component of the time series from all voxels included in the sphere was calculated (corrected for confounds).
| Region | X | Y | Z |
|---|---|---|---|
| Ventral tegmental area | −2 | −22 | −12 |
| Posterior cingulate cortex | −4 | −30 | 36 |
| Anterior cingulate cortex | −2 | 28 | 28 |
| Ventromedial prefrontal cortex | 2 | 46 | −8 |
| Left ventral striatum | −12 | 12 | −6 |
| Right ventral striatum | 12 | 10 | −6 |
| Left anterior insula | −30 | 22 | −6 |
| Right anterior insula | 32 | 20 | −6 |
A DCM model was set up with the eight ROIs as key nodes. A fully connected eight-node DCM (including self-connection of each node, total 64 connections) was specified without any external stimuli for each subject and then inverted using spectral DCM. Then we assessed the model fit at this stage.
2.5 DCM parametric empirical Bayes (PEB) analyses
For each subject, a fully connected DCM had been specified and estimated. Then group level analysis of each ECs (a total of 64 tested connections) was performed using the PEB method.35 PEB is a between-subject hierarchical or empirical Bayesian model over parameters that models how individual connections relate to group or condition means. This parametric random effect modelling could use the full posterior density over the parameters from each subject's DCM to inform the group-level result.36 To evaluate how the connectivity of long-term smokers differs from HCs, we then used Bayesian model reduction (BMR) to search over PEB models with different combinations of connections and group differences. BMR was used to iteratively prune connection parameters from the full PEB model, until model-evidence started to decrease. The parameters of the best 256 pruned models were then averaged, weighted by their evidence (Figure S1). In PEB, group level analyses are conducted using Bayesian posterior inference, which does not need to contend with the multiple-comparison problem because of the lack of false positives.37 We use Bayesian posterior probability (Bayesian-PP) as an indicator of the confidence. The higher Bayesian-PP indicated the greater confidence. Here, the results were considered reliable if Bayesian-PP >0.95.38
2.6 Correlation analyses
According to previous study, FTND questionnaire was divided into two factors: (1) Assessed the urgency of restoring nicotine levels to a given threshold after nighttime abstinence and (2) reflected the persistence of nicotine levels keeping around the threshold while awake.39 Correlation analyses were conducted to search the relationship between EC with significant group difference based on the PEB analysis and clinical features such as FTND score (each factor as separate dependent variable) and pack-year.
3 RESULTS
3.1 Demographic and clinical data
Seventy-six male long-term smokers and 55 male healthy controls were included in this study. There were no significant differences in age and years of education between long-term smokers and nonsmokers. The detailed demographic information was displayed in Table 2.
| Long-term smokers (n = 76) | Healthy controls (n = 55) | t | p | |
|---|---|---|---|---|
| Age (year) | 32.1 ± 6.3 | 32.3 ± 7.4 | −0.155 | 0.877 |
| Education (year) | 15.1 ± 1.8 | 15.9 ± 3.9 | −1.683 | 0.096 |
| Age onset | 19.2 ± 3.4 | -- | -- | -- |
| Smoking years | 12.8 ± 6.1 | -- | -- | -- |
| Pack-year | 12.3 ± 7.9 | -- | -- | -- |
| Cigarettes/day | 18.0 ± 8.6 | -- | -- | -- |
| FTND | 3.7 ± 2.2 | -- | -- | -- |
- Note: Data represent mean ± standard deviation; FTND, Fagerström Test for Nicotine Dependence; Peak-year, years of smoking × cigarettes smoked per day/20.
3.2 Group comparison
Figure 2 displays the common effective connectivity of the reward network in long-term smokers and healthy controls (free energy, Bayesian-PP >0.95). Our main interest regarded differences between groups, so we will just make one observation about this result.40 It shows a complicated pattern of connectivity and all the ROIs in the reward system were engaged at the resting state. Among them, most regions show a negative self-connection but bilateral ventral striatum exhibit positive self-connection. Besides, the VTA establishes extensive connections with other brain regions, and connections entering the VTA were generally inhibitory, whereas outgoing connections were generally excitatory. Moreover, it is apparent that vmPFC and PCC (involved in DMN) have reciprocal negative effects with other brain regions.
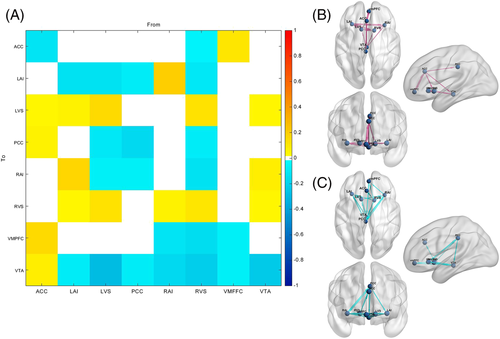
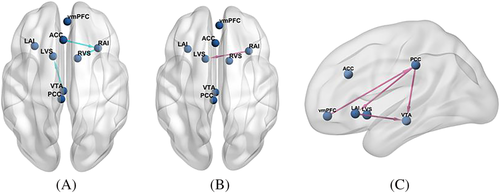
The group difference (long-term smokers-healthy controls) for each EC and the corresponding Bayesian-PP are shown in Table 3. Among the 64 ECs, five ECs in smokers including right anterior insula to left ventral striatum, posterior cingulate cortex (PCC) to ventral tegmental area (VTA), PCC to left anterior insula, left anterior insula to VTA, and ventromedial prefrontal cortex (vmPFC) to PCC ECs were greater than healthy controls and three ECs including VTA to left ventral striatum, right anterior insula to right ventral striatum, and anterior cingulate cortex (ACC) to right anterior insula were weaker (free energy, Bayesian-PP >0.95) (Figure 3).
| Group | Connectivity | EC (HZ) | Bayesian-PP |
|---|---|---|---|
| long-term smokers < healthy controls | VTA → LVS | −0.048 | 1.00 |
| RAI → RVS | −0.050 | 1.00 | |
| ACC → RAI | −0.054 | 0.99 | |
| long-term smokers > healthy controls | RAI → LVS | 0.056 | 1.00 |
| PCC → VTA | 0.120 | 1.00 | |
| PCC → LAI | 0.089 | 1.00 | |
| LAI → VTA | 0.142 | 1.00 | |
| VMPFC→PCC | 0.046 | 0.99 |
- Abbreviations: ACC, anterior cingulate cortex; EC, effective connectivity; LAI, left anterior insula; LVS, left ventral striatum; PCC, posterior cingulate cortex; PP, posterior probability; RAI, right anterior insula; RVS, right ventral striatum; vmPFC, ventromedial prefrontal cortex; VTA, ventral tegmental area.
3.3 Correlation analyses
The results presented that the EC from left anterior insula to VTA was positively correlated with the total score of FTND (r = 0.253, p = 0.033, uncorrected) (Figure S2). No significant linear correlations were found with each factor of FTND and pack-year.
4 DISCUSSION
This study focused on the effects of reward network on tobacco addiction and by means of spectral DCM. Compared to traditional resting-state functional connectivity (RSFC), effective connectivity calculated by spectral DCM can describe causal relationship that one brain region exerts to another, providing directed flow information underlying the observed correlations.22 We observed that reward circuit shared several common interaction patterns across long-term smokers and healthy controls. The group difference showed that long-term smokers relative to healthy controls displayed decreased EC mainly in VTA to left ventral striatum, right anterior insula to right ventral striatum, and anterior cingulate cortex (ACC) to right anterior insula. Besides, long-term smokers showed enhanced effective connectivity in a top-down (frontal lobe to midbrain) circuit including vmPFC, PCC, left anterior insula, and VTA comparing with healthy controls. These findings suggested alterations in functional integration among reward network between smokers and nonsmokers and revealed potential neurobiological mechanisms of tobacco addiction.
First, we observed that reward circuit shared several common interaction patterns across long-term smokers and healthy controls. Dopamine (DA) neurons located in VTA and projecting to the ventral striatum (nucleus accumbens) play an important role in the processing of reward-related stimuli.41 Since the self-connections in DCM are always inhibitory, the result of increased self-connection in bilateral ventral striatum means that the regions are more self-inhibited.24, 42 Besides, we found the connections entering the VTA were generally inhibitory, indicating cortical and subcortical brain regions had inhibitory effects on VTA at resting state. This inherent pattern of connection was partially consistent with the top-down control over the dopamine-dependent process of incentive salience attribution.12, 43 Moreover, we also identified that DMN (regions vmPFC and PCC) involved in self-awareness and introspection process at rest by interacting with subcortical brain regions, such as VTA, ventral striatum and insula.
Secondly, group difference indicated enhanced effective connectivity in a top-down (frontal lobe to midbrain) circuit encompassing vmPFC, PCC, left anterior insula, and VTA among long-term smokers. Previous study has suggested that vmPFC and PCC are key nodes of DMN5 and have distinct contributions to self-related evaluation and emotion. Insula is associated with interoceptive awareness and conscious awareness of substance craving, which facilitates rumination about substance abuse in addicted individuals.44, 45 For chronic smoking, posterior DMN dominated, PCC-insula functional connectivity could promote negative emotional states and rumination behaviours.46 Abnormality of such emotional and intersensory circuits leads to a stronger sensitivity to stressors and anxiety, which is known as the dark side of addiction: Dysfunction of normal reward network and constant recruitment of the brain stress systems.47, 48 The brain stress system in the PFC promotes the persistence of the “dark side,” which activates cyclic adenosine monophosphate (cAMP) and cAMP response element binding protein (CREB), regulate DA receptors, and act on VTA dopaminergic neurons.47 Moreover, the exposure of tobacco leads to the sensitization of glutamatergic projections from the vmPFC and leads to dopamine release in mesolimbic system.49, 50 For our results, disrupted function of the top-down regulation that PFC participates in (especially vmPFC-PCC-Insula-VTA loop) may be related to the dysfunction of emotional regulation and activation of negative reinforcement implicated by long-term smoking, which is consistent with a syndrome of impaired response inhibition and salience attribution (iRISA) in addiction.12 Thereinto, the effective connectivity from left anterior insula to VTA was stronger as the degree of tobacco dependence increase, similar to Claus's findings.51 It may prove the hypothesis that severely dependent individuals engage in more interoception awareness and show more negative physical experiences relative to craving because of their decreased sensitivity to rewards.52, 53
Furthermore, compared with healthy controls, we observed significant alterations of ACC-anterior insula-ventral striatum loop among long-term smokers, suggesting abnormalities in top-down regulation of the brain. Anterior insula and ACC are vital components of salience network (SN). The SN involves subjective affect processing, cognition, and motivation. It is assumed that SN dynamically allocate attentional and cognitive resources between the executive control network (ECN) and default mode network (DMN) to achieve the transformation of different status of the body.54, 55 Disruptions of the SN and malregulated connections with other cerebrum regions, such as striatum and DMN, have been found in variety neuropsychiatric disorders, including tobacco addiction.56, 57 It has been demonstrated that trends of decreased in strength RSFC were also observed in salience network and striatal reward network after e-cigarette use.58 Consistent with previous studies, our results suggested that compared with nonsmokers, long-term smokers represented reduced effective connectivity in ACC-anterior insula-ventral striatum loop, which might reflect the deteriorate capability of SN to integrate information in different status of the body and the imbalance in the dynamic association between cortical circuitry and the dopaminergic reward circuitry.59 Interestingly, the effective connectivity from anterior insula to ventral striatum showed asymmetric changes in bilateral cerebral hemispheres, which may be a compensation for reduced lateral effective connectivity to complete complex brain information transmission or related to the lateralization of the dopamine system.60
Different from the top-down pattern of connection, we also found abnormalities within the mesocorticolimbic system. As mentioned above, dopamine (DA) neurons gathered in the VTA and projected to the ventral striatum (nucleus accumbens, NAc) play an essential role in the reward and motivation circuit in tobacco addiction.41 Long-term smokers show decreased EC from VTA to left ventral striatum: A negative difference corresponds to a smaller excitatory, namely, a relative de-excitation in long-term smokers.36 Tobacco-induced DA increasing trigger various patterns of synaptic plasticity that can lead to strengthen or weaken the synaptic connectivity in different reward areas of the brain.61 Wiers et al. have demonstrated that current smokers showed lower striatal D2 receptor availability compared with healthy controls.62 In addition, neuroimaging studies have revealed reduced RSFC between VTA and ventral striatum in IGD subjects compared with healthy controls and decreased structural connectivity in bilateral VTA-ventral striatum tracts.15 It seems that both functional and structural changes in VTA-ventral striatum circuit manifest a similar pattern in addiction-related disorders (e.g., IGD, tobacco, and cocaine).4, 63, 64 Here, consistent with previous findings, we found aberrant effective connectivity in VTA-ventral striatum circuit in long-term smokers and demonstrated such alterations was derived from VTA, which extended the evidence for neurocircuitry disruptions in tobacco addiction.
There are a few limitations to our study. First, although this study would shed new light on the changes of causal connections among reward network in long-term smokers, the causal relationship between effective connectivity and development of tobacco addiction remains inexplicable through the integration of cross-sectional study. Second, spectral DCM could only calculate a relatively small number of nodes owing to computational reasons, so we only selected the key nodes in the reward network as ROI. The effective connections between the remaining brain regions need to be further explored. Third, the sample size is small and the subjects recruited in this study were male adults, while females were not included, so the results of this study are not applicable to all population.65 Forth, evidence showed that reliability and similarity of functional connectivity estimates can be greatly improved by increasing the scan lengths from 5 min up to 13 min, but this study only collected 6-min resting-state scanning, which did not meet recommendations for scan time.66
5 CONCLUSION
Overall, our findings indicated disrupted neural interactions among the reward network associated with tobacco addiction, expanding the growing evidence for the potential neurobiological mechanisms of tobacco addiction. In particular, long-term smokers showed abnormalities inside the mesocorticolimbic system, which was consistent with the traditional conception that tobacco addiction predominantly involved reward processes mediated by limbic circuits (e.g., the reward deficiency syndrome). More important, the effective connectivity alterations within vmPFC-PCC-anterior insula-VTA and ACC-anterior insula-ventral striatum crucial circuits could suggest a top-down regulation disorder in the dopamine-dependent process of response inhibition and salience attribution among long-term smokers. These current findings shed further light on causal interaction among the hubs of reward network and expand the traditional concepts of tobacco addiction, which may facilitate the development of effective treatments in tobacco addiction.
CONFLICTS OF INTEREST
The authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTION
All authors take responsibility for the content, gave approval of the submission, and made substantive intellectual contributions to the submitted work. Mengzhe Zhang, Xinyu Gao, and Bingqian Zhou conceived and designed the study. Xiaoyu Niu, Zhengui Yang, and Weijian Wang analyzed the data. Mengzhe Zhang and Xinyu Gao drafted the manuscript. Shaoqiang Han and Yarui Wei revised the manuscript. Yong Zhang and Jingliang Cheng supervised. Yong Zhang and Jingliang Cheng provided financial support.
Open Research
DATA AVAILABILITY STATEMENT
N/A.




