Inhibition of PSD95-nNOS protein–protein interactions decreases morphine reward and relapse vulnerability in rats
Funding information: This work is supported by DA047858 (to AGH) and DA042584 (to AGH and GVR) (National Institute on Drug Abuse), CA200417 (to AGH) (National Cancer Institute), and an Indiana Grand Challenge in Addictions research grant (to AGH, GVR, and JDC) (Indiana State Department of Health). VI was supported by the Harlan Scholars Research Program and as a Gill Graduate Research Fellow.
Abstract
Glutamate signalling through the N-methyl-d-aspartate receptor (NMDAR) activates the enzyme neuronal nitric oxide synthase (nNOS) to produce the signalling molecule nitric oxide (NO). We hypothesized that disruption of the protein–protein interaction between nNOS and the scaffolding protein postsynaptic density 95 kDa (PSD95) would block NMDAR-dependent NO signalling and represent a viable therapeutic route to decrease opioid reward and relapse-like behaviour without the unwanted side effects of NMDAR antagonists. We used a conditioned place preference (CPP) paradigm to evaluate the impact of two small-molecule PSD95-nNOS inhibitors, IC87201 and ZL006, on the rewarding effects of morphine. Both IC87201 and ZL006 blocked morphine-induced CPP at doses that lacked intrinsic rewarding or aversive properties. Furthermore, in vivo fast-scan cyclic voltammetry (FSCV) was used to ascertain the impact of ZL006 on morphine-induced increases in dopamine (DA) efflux in the nucleus accumbens shell (NAc shell) evoked by electrical stimulation of the medial forebrain bundle (MFB). ZL006 attenuated morphine-induced increases in DA efflux at a dose that did not have intrinsic effects on DA transmission. We also employed multiple intravenous drug self-administration approaches to examine the impact of ZL006 on the reinforcing effects of morphine. Interestingly, ZL006 did not alter acquisition or maintenance of morphine self-administration, but reduced lever pressing in a morphine relapse test after forced abstinence. Our results provide behavioural and neurochemical support for the hypothesis that inhibition of PSD95-nNOS protein–protein interactions decreases morphine reward and relapse-like behaviour, highlighting a previously unreported application for these novel therapeutics in the treatment of opioid addiction.
1 INTRODUCTION
Excessive glutamatergic activation of N-methyl-d-aspartate receptors (NMDARs) is implicated in altered forms of neural plasticity in opioid addiction.1, 2 Glutamatergic transmission is specifically implicated in forming associative memories, during both the opioid experience and opioid withdrawal.3 NMDARs and μ-opioid receptors (MORs) co-localize in various brain regions and exhibit bidirectional interactions.4, 5 The MOR agonist morphine potentiates NMDAR activity by increasing levels of the signalling molecule nitric oxide (NO).6 Excessive production of NO is involved in several pathological forms of neural plasticity, such as opioid tolerance, dependence, and reward.1, 7, 8 NMDAR antagonists such as MK-801 attenuate these negative effects of opioids.9-12 However, their clinical use is limited by adverse side effects (e.g., learning and memory impairment, motor ataxia, cognitive dissociation, and increased lethality).13-17 Neuronal nitric oxide synthase (nNOS) catalytic inhibitors also suppress opioid reward, tolerance, and dependence1, 5, 18-20 but lack of isoform selectivity similarly contributes to unwanted side effects.21-25 Thus, indirect mechanisms to disrupt NMDAR signalling without the negative side effects of NMDAR antagonists or non-selective NOS catalytic inhibitors are needed to leverage the clinical potential on NMDAR-dependent NO signalling for suppressing opioid addiction.
NO production via the NMDAR-nNOS signalling cascade is dependent upon the scaffolding protein postsynaptic density 95 kDa (PSD95), which tethers nNOS to NMDARs.26, 27 Upon excessive NMDAR activity, nNOS interacts with PSD95 to stimulate NO production.6, 28 Inhibitors of PSD95-nNOS protein–protein interactions attenuate pathophysiological conditions involving excess glutamatergic signalling in animal models of pathological pain,29-31 ischaemic injury,32 traumatic brain injury,33 and post-traumatic stress disorder.34 Small molecule inhibitors such as 2-((1H-benzo [d] [1,2,3] triazol-5-ylamino) methyl)-4,6-dichlorophenol (IC87201), the first-in-class disruptor of PSD95-nNOS interactions, inhibit the binding of nNOS to PSD95 in vitro without altering nNOS catalytic activity.29 A structurally related PSD95-nNOS inhibitor, 4-(3,5-dichloro-2-hydroxy-benzylamino)-2-hydroxybenzoic acid (ZL006), reduced infarct size and cerebral ischaemic injury following middle cerebral artery occlusion and reperfusion in wild-type but not nNOS−/− mice.32 We previously reported that IC87201 and ZL006 directly inhibited binding of purified PSD95 and nNOS proteins, suppressed NMDA-stimulated cGMP production (a marker of NO formation), and reduced glutamate-induced cell death at levels comparable with the NMDAR antagonist MK-801.31 We also showed that both IC87201 and ZL006, administered systemically, suppressed inflammatory and neuropathic pain and neurochemical markers of inflammation-evoked neuronal activation in rats.30, 31 Both PSD95-nNOS inhibitors are blood–brain barrier penetrant and, unlike MK-801, did not impair motor function, spatial memory, source memory, or basal nociceptive thresholds.29-32, 35 Accordingly, we hypothesized that inhibition of PSD95-nNOS interactions may disrupt NMDAR-dependent opioid reward without the unwanted effects of NMDAR antagonists of nNOS catalytic inhibitors.
In the present study, we asked whether small molecule PSD95-nNOS inhibitors, IC87201 and ZL006, are inherently reinforcing and whether they can effectively block morphine-induced reward using a conditioned place preference (CPP) paradigm. We used fast-scan cyclic voltammetry (FSCV) in combination with electrical stimulation of the medial forebrain bundle (MFB) to measure the impact of ZL006, the best characterized PSD95-nNOS interaction inhibitor to date, on changes in electrically evoked DA efflux in the nucleus accumbens shell (NAc shell) in the presence and absence of morphine. We also evaluated effects of ZL006 on the acquisition and maintenance of morphine self-administration and on the relapse of morphine seeking after forced abstinence.
2 METHODS
2.1 Drugs
IC87201 and ZL006 were synthesized by the laboratory of Ganesh A. Thakur in the Center for Drug Discovery at Northeastern University (by PMK and SG). IC87201 and ZL006 were dissolved in a vehicle containing 20% dimethyl sulfoxide (DMSO) and 80% of ethanol:emulphor:saline in a 1:1:8 ratio and administered i.p. at 10 mg/kg in a final volume of 2 ml/kg injection30 for CPP and voltammetry experiments. In the self-administration experiments, ZL006 was dissolved in a vehicle containing 3% of DMSO and 97% of ethanol:emulphor:saline in a 1:1:18 ratio and administered i.p. at 10 mg/kg dose in a final volume of 2 ml/kg injection. The dose selection was based upon our previous studies showing maximal efficacy in inhibiting pain behaviour,30 without producing motor ataxia,30 decreasing ambulatory activity,33 or impairing spatial or source memory.35 Morphine hydrochloride was obtained from the National Institute on Drug Abuse (NIDA), dissolved in a physiological saline solution and administered at 6 mg/kg i.p. for CPP and FSCV and at 100 μg/kg/infusion for self-administration.36
2.2 Subjects
All procedures were approved by the Institutional Animal Care and Use Committee at the Indiana University Bloomington. Male Sprague–Dawley rats (Envigo, IN) were singled-housed upon arrival and given ad libitum access to water and food and maintained on a 12-h reversed light/dark cycle. Rats were subjected to at least 48 h of acclimation after arrival before starting any experimental procedure.
2.3 Conditioned place preference (CPP)
We used a three-chamber CPP apparatus (Med-Associates Inc., Fairfax, VT, USA), which contained a central neutral chamber with grey walls and two side conditioning chambers with distinct visual cues on the walls: one chamber with black and white vertical stripes and one with black and white horizontal stripes. The floors were made of wire mesh in a grid pattern. The chambers were separated by computer-controlled guillotine doors that slid upward to allow access to all three chambers. Photobeams detected the location of the animal in all three chambers and were controlled by Med-PC software (Med-associates Inc., Fairfax, VT, USA). We used the same protocol described in our previous publications37-39 (Figure 1A). Briefly, rats were allowed to habituate for 20–30 min in the experimental room prior to placement in the CPP apparatus. All experiments were performed during their dark cycle. On days 1 to 3, rats were placed into the central chamber and allowed to habituate for 5 min. Then, the guillotine doors to the two flanking conditioning chambers automatically opened, and the animals were able to explore all three chambers for 30 min (1800 s). The time that the animals spent in each chamber was recorded. On day 3, an assessment of baseline chamber preference was conducted to confirm that the rats did not demonstrate a bias for any particular chamber prior to pharmacological manipulation (i.e., pre-test). Rats were excluded from the experiment if they spent less than 360 s (i.e., 20% of time) or more than 1440 s (i.e., 80% of the time) in either conditioning chamber.37-39 On days 4 to 11, rats received repeated pairings of drug (days 4, 6, 8, and 10) and vehicle (days 5, 7, 9, 11) on alternate days. We evaluated whether IC87201 (10 mg/kg i.p.) and ZL006 (10 mg/kg i.p.), administered to the drug-paired chamber, would produce reward or aversion when administered alone. Separate groups received morphine (6 mg/kg i.p.) in the drug-paired chamber or vehicle (i.p.) in both chambers. Finally, we examined the impact on morphine-induced CPP of IC87201 (10 mg/kg i.p.) and ZL006 (10 mg/kg i.p.) co-administered with morphine (6 mg/kg i.p.). The total injection volume (2 ml/kg) and number of injections were the same across all groups. Animals were placed into either the left or right chamber immediately after injection to achieve drug (or vehicle) pairings. On day 12, rats were again allowed to explore all three chambers for 30 min, and their chamber preference was evaluated in the drug-free state (i.e., post-test).
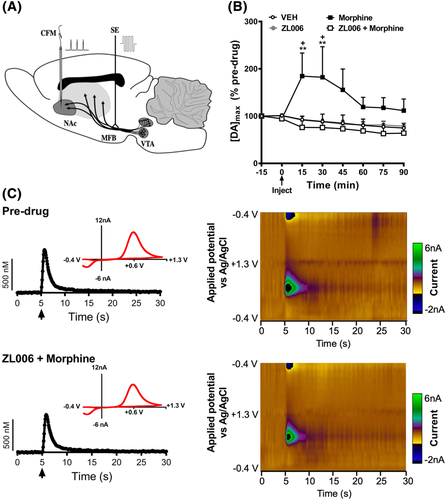
2.4 Fast-scan cyclic voltammetry
In vivo anaesthetised FSCV was used to ascertain whether disruption of PSD95-nNOS protein–protein interactions by ZL006 suppresses morphine-induced DA efflux in the NAc shell. We measured changes in extracellular DA in the NAc shell evoked by the application of biphasic stimulations into the MFB.40-43 Rats were anaesthetised with urethane (1.6 g/kg i.p.) and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA) with a heating pad underneath the subject to maintain body temperature throughout experiments. The skull surface was exposed, and 2 mm diameter craniotomies were made in the skull to position the Ag/AgCl reference electrode, a bipolar stimulating electrode, and a carbon-fibre microelectrode (Figure 2A). The coordinates anteroposterior (AP), mediolateral (ML), and dorsoventral (DV) were referenced to bregma. The stimulating electrode was positioned in the MFB (−4.6 AP, +1.3 ML, −7.5 DV), the carbon-fibre microelectrode was positioned in the NAc shell (+1.2 AP, +1.4 ML, −6.6 DV), and the reference electrode was placed in the contralateral cortex. Final DV coordinates for the carbon-fibre microelectrode and the stimulating electrode were based on optimizing the electrically evoked DA signal and were not changed for the duration of the experiment. Carbon-fibre microelectrodes were fabricated by inserting a single carbon fibre T650 (6.0 μm diameter, Cytec Engineering Materials, West Paterson, NJ, USA) into a borosilicate capillary tube (1.2 mm outer diameter; Sutter Instruments, Novato, CA, USA) and pulling a taper using a vertical micropipette puller (PF-2 Narishige, Tokyo, Japan). The carbon-fibre microelectrode was held at a potential of −0.4 V, and every 100 ms the potential was ramped to +1.3 V and back at a rate of 400 V/s. The currents were amplified and recorded using a potentiostat (EI-400 Cypress systems Lawrence, KS, USA), computer-controlled interface boards (National Instruments, Austin, TX, USA), and TarHeel CV software (ESA Bioscience, Chelmsford, MA, USA). Electrical stimulation was computer generated and passed through a constant-current generator (custom-made). Software-controlled biphasic stimulation pulses were applied to a twisted bipolar electrode (Plastics One, Roanoke, VA, USA), with tips separated ∼1 mm. Stimulus intensity was 300 μA, with a biphasic pulse duration of 4 ms (2 ms for each phase); trains were applied at a frequency of 60 Hz for 0.4 s (i.e., a total of 24 pulses). Recordings were sampled with at least 5-min intervals after a stimulating train. To determine changes in the amplitude of electrically evoked DA, signals were recorded under baseline conditions and were analysed to determine the average peak amplitude (i.e., maximal concentration of DA evoked by electrical stimulation [DA]max). Recordings from baseline (i.e., 15 min prior to i.p. injection) and following drug injections were analysed, and values were normalized to the subject's baseline [DA]max. Carbon-fibre microelectrodes were calibrated after experimental data collection, and currents recorded at peak oxidative potential for DA (~+0.6 V) were converted to DA concentration based on post-calibration peak currents using DA (1 μM) buffer solution containing (in mM) 12 Tris–HCl, 1.2 CaCl2, 1.25 NaH2PO4, 1.2 MgCl2, 2 Na2SO4. FSCV recordings were statistical analysed, relative to baseline, across the entire 90-min post-injection interval from animals receiving injections of vehicle, morphine (6 mg/kg i.p.), ZL006 (10 mg/kg i.p.), or ZL006 (10 mg/kg i.p.) co-administered with morphine (6 mg/kg i.p.).
2.5 Lever training
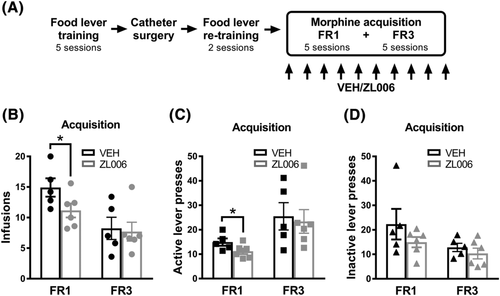
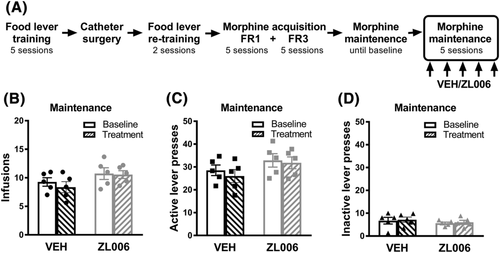
Prior to catheter surgery, rats were food restricted (15 g/day) and pre-trained to lever press for food in operant chambers (Med-Associates Inc., Fairfax, VT, USA) equipped with two levers (referred to as left and right levers). First, rats were exposed to a single magazine training session (30 min) where a food pellet was delivered every 60 s with both levers retracted. Next, rats were trained (four sessions) to lever press (fixed ratio 1; FR1) for food on the two levers. The session started by inserting one lever and rewarding each of 10 responses on that lever, then retracting the lever, and inserting the other lever and rewarding each of 10 responses on it. The system alternated between levers until 60 food pellets or 60 min elapsed.36, 44 Rats were returned to ad libitum food and the catheter surgeries were performed. After surgical recovery, rats were food restricted again and re-trained (two sessions) to lever press for food. Finally, all groups were returned to and maintained on ad libitum food during the entire self-administration experiments36 (Figure 3A).
2.6 Jugular catheter surgery
A backmount catheter was constructed and surgically implanted into the left jugular vein of rats (300–350 g) under isoflurane anaesthesia as described previously.44 Briefly, a silastic tubing was passed subcutaneously and inserted 3.5 cm into the jugular vein.45, 46 Rats were allowed to recover 1 week following catheter implantation before resuming food lever re-training. Catheters were flushed daily (200 μl) with a heparinized saline solution (100 units/ml) before and after the drug self-administration sessions.
2.7 Morphine i.v. self-administration
Animals were tested once daily in 50 min drug self-administration sessions as described in our previous work.36, 44 Operant chambers (Med Associates Inc., Fairfax, VT, USA) were equipped with grid floors, a house light, two retractable levers (referred to as left and right levers) and an infusion pump. Operant chambers used for self-administration were different and located in another room from those used for the lever training. Levers were randomly assigned as active or inactive for every animal. Each session started with the two levers extended, lever presses on the inactive lever resulted in no infusion while the required number of presses on the active lever resulted in delivery of one infusion paired with a light cue (i.e., discrete cue). Specifically, the infusion pump and light cue turned ON for approximately 6 s, and 6–8 μl were infused based on the rat's body weight. Each infusion was followed by a 5 s timeout period. Levers retracted during an infusion and time out. A morphine concentration of 100 μg/kg/infusion was used for all experiments based upon previous studies in our lab.36, 44
In the acquisition experiments, rats were allowed to self-administer morphine for 10 consecutive days: 5 days on FR1 and 5 days on FR3. This operant schedule training ensured that animals learned to distinguish between the reinforced and non-reinforced lever. Rats received ZL006 10 mg/kg or vehicle i.p. 30 min before each of the 10 acquisition sessions. The outcomes of once daily injection (i.p.) of ZL006 10 mg/kg or vehicle were examined over the last 5 days of acquisition. The numbers of infusions, and active/inactive lever presses were compared between groups.
In the maintenance experiments, a new group of rats was first allowed to self-administer morphine for 10 consecutive days as before, 5 days on FR1, and 5 days on FR3. Then, rats continued on FR3 until stable or baseline (pre-drug) infusion intake was attained. The criterion for stable (i.e., baseline) morphine intake was reached when the rats exhibited less than 20% variance in the number of infusions for three consecutive days.45 After meeting baseline criteria, the animals received ZL006 (10 mg/kg i.p.) or vehicle (i.p.) 30 min before the self-administration session for 5 days (i.e., treatment). We analysed the number of infusions, and active/inactive lever presses between groups. Comparisons were also made between baseline and treatment for each experimental group, within- and between-subjects, as appropriate.
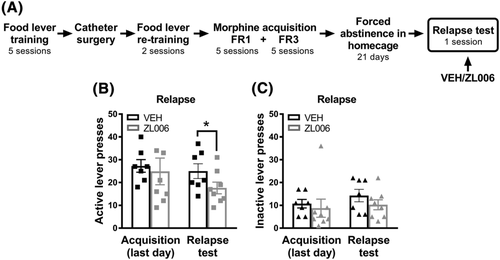
In the relapse test, a separate cohort of animals was trained to self-administer morphine for 10 consecutive days as before: 5 days on FR1 followed by 5 days on FR3. This group then experienced 21 days of forced abstinence (i.e., withdrawal) in their home cages followed by a single cue-induced drug seeking session (i.e., relapse test). In order to minimize the experimenter-induced stress on the relapse test day, animals were handled daily during the forced abstinence period. This daily handling was carried out in a different room from where the self-administration experiments were conducted to avoid previous environmental/context drug-associations. Rats were treated with ZL006 (10 mg/kg i.p.) or vehicle (i.p.) 30 min before the relapse test session. On the relapse test, subjects were placed back into to the drug self-administration environment/context (i.e., operant chambers) with an identical configuration; however, active lever pressing resulted in the delivery only of the discrete cue previously paired with morphine, but no morphine was infused. We analysed active/inactive lever presses and made comparisons between the last day of morphine self-administration (i.e., last day of morphine acquisition) and the relapse test day.
2.8 Statistical analysis
Data were analysed by analysis of variance (ANOVA) followed by Bonferroni post hoc tests. The Geisser–Greenhouse correction was applied to adjust the lack of sphericity in repeated measures ANOVA. Based upon our prior hypothesis and prior results in CPP and voltammetry studies, differences between groups in a specific direction in the self-administration experiments (i.e., treatment is effective) were determined using an unpaired Student's t test (one-tailed). All statistical analyses were performed using GraphPad Prism (GraphPad Software, La Jolla, CA, USA). Data are presented as mean ± SEM, and p < 0.05 was considered statistically significant.
3 RESULTS
3.1 Rats exhibit robust conditioned place preference to morphine
When rats received vehicle in both chambers (i.e., vehicle-vehicle pairings; Figure 1B), there was no difference in the time spent in each chamber. The interaction between conditioning phase and drug treatment was not significant (F1,12 = 1.535, p = 0.2390), and no main effects of vehicle treatment (F1,12 = 0.001873, p = 0.9662) or conditioning phase (F1,12 = 0.05027, p = 0.8264) were observed. Thus, vehicle–vehicle pairings did not produce either CPP or conditioned place aversion (CPA). By contrast, repeated pairings with morphine (6 mg/kg i.p.) produced robust CPP to the drug-paired chamber compared with the vehicle-paired chamber (Figure 1C). A significant main effect of drug treatment (F1,20 = 5.503, p = 0.0294) was observed, the interaction between conditioning phase and drug treatment was significant (F1,20 = 7.688, p = 0.0117), but no overall effect of the conditioning phase (F1,20 = 0.2862, p = 0.5985) was observed. Post hoc comparisons revealed that rats exhibited an increased time in the morphine-paired versus vehicle-paired chamber on the CPP test day (p = 0.0017) but no such difference was observed at baseline prior to conditioning, consistent with the development of CPP for morphine.
3.2 PSD95-nNOS interaction inhibitors alone do not cause reward or aversion
IC87201 (10 mg/kg i.p.) treatment alone did not alter time spent in the drug-paired versus the vehicle-paired chamber on the CPP test day (Figure 1D). The interaction between the conditioning phase and drug treatment was not significant (F1,12 = 0.2044, p = 0.6592), and no main effect of drug treatment (F1,12 = 2.125, p = 0.1706) or conditioning phase (F1,12 = 3.946, p = 0.0703) were observed. Similarly, in a separate group of rats, ZL006 (10 mg/kg i.p.) treatment alone did not alter time spent in the drug-paired chamber versus the vehicle-paired chamber on the CPP test day (Figure 1E). The interaction between the conditioning phase and drug treatment was not significant (F1,18 = 0.0008305, p = 0.9773), and no main effect of drug treatment (F1,18 = 0.5107, p = 0.4840) or conditioning phase (F1,18 = 0.04688, p = 0.8310) was observed. Thus, PSD95-nNOS inhibitors did not themselves produce reward or aversion.
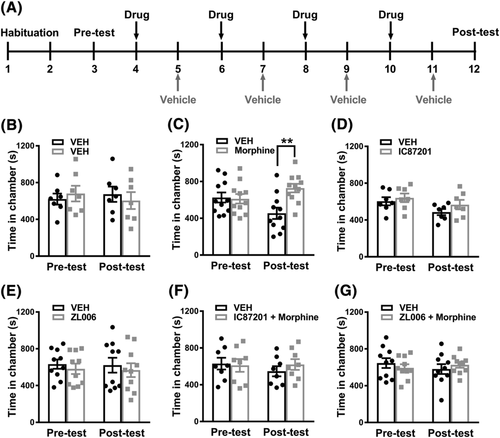
3.3 PSD95-nNOS interaction inhibitors block morphine-induced CPP
Combination treatment with morphine (6 mg/kg i.p.) + IC87201 (10 mg/kg i.p.) blocked morphine-induced CPP (Figure 1F). The interaction between the conditioning phase and drug treatment was not significant for the morphine + IC87201 combination treatment (F1,14 = 0.8627, p = 0.3687), and no main effect of drug treatment (F1,14 = 0.147, p = 0.7072) or conditioning phase (F1,14 = 0.5245, p = 0.4809) was observed. Similarly, in a separate group of rats, combination treatment with morphine (6 mg/kg i.p.) + ZL006 (10 mg/kg i.p.) blocked morphine-induced CPP (Figure 1G). The interaction between the conditioning phase and drug treatment was not significant for the morphine + ZL006 combination treatment (F1,18 = 1.059, p = 0.3170), and no main effect of drug treatment (F1,18 = 0.003592, p = 0.9529) or conditioning phase (F1,18 = 0.123, p = 0.7299) was observed. Thus, two structurally distinct PSD95-nNOS inhibitors blocked morphine-induced CPP.
3.4 ZL006 blocks morphine-induced increases in DA efflux in NAc shell evoked by electrical stimulation of the MFB
Morphine increased the extracellular [DA]max signal relative to the baseline DA signal at 15 (p = 0.0014), 30 (p < 0.0001), and 45 (p = 0.0457) min post-injection (Figure 2B). To ascertain whether disruption of PSD95-nNOS interaction has any effect on morphine-induced increases in DA efflux, we characterized the effects of ZL006 on [DA]max. The extracellular [DA]max signal subsequently declined from 60–90 min post injection compared with the maximal levels observed 15–30 min post-injection (p < 0.05 at each 60–90 min post-injection time point, respectively) (Figure 2B). ZL006 alone (10 mg/kg i.p.) did not alter the extracellular [DA]max signal relative to vehicle at any time point (p > 0.9999; Figure 2B). Neither vehicle, ZL006, nor ZL006 + morphine altered the extracellular [DA]max signal relative to baseline at any post-injection time point (p > 0.9999; Figure 2B). Pharmacological manipulations altered [DA]max in a time-dependent manner (interaction: F21,119 = 2.587, p = 0.0007; Figure 2B). [DA]max did not differ between groups overall and trended to decrease across the recording interval irrespective of drug treatment (Drug: F3,17 = 3.067, p = 0.0561; Time: F7,119 = 2.477, p = 0.0249). Post hoc comparisons revealed that morphine increased evoked [DA]max at 15–30 min post-injection relative to all other groups (p < 0.05 at each time point; Figure 2B). Notably, co-administration of ZL006 (10 mg/kg i.p.) with morphine (6 mg/kg i.p.) prevented the morphine-induced increase in evoked [DA]max at 15 (p = 0.0041), 30 (p < 0.0001), and 45 (p = 0.0245) min post-injection (Figure 2B). Representative voltammetry scans of electrically evoked DA signal (Figure 2C left panels) and colour plots (Figure 2C right panels) illustrate that the DA signal measured pre-drug and after the co-administration of ZL006 + morphine remained essentially unaltered.
3.5 ZL006 did not alter the acquisition of morphine self-administration
ZL006 (10 mg/kg i.p.) did not change the outcomes of the acquisition of morphine self-administration (Figure 3). Rats in this experiment received i.p. injections of ZL006 or vehicle during the 10 days of morphine self-administration that comprise the acquisition phase: 5 days in FR1 and 5 days in FR3 (Figure 3A). The interaction between treatments and FR was not significant for number of infusions (F1,9 = 1.57, p = 0.2417), active lever presses (F1,9 = 0.05735, p = 0.8161), or inactive lever presses (F1,9 = 0.466, p = 0.5120). Interestingly, we detected an initial descending trend (i.e., first five sessions, FR1) in the animals receiving ZL006 in the infusion number (FR1, VEH vs. ZL006, t(9) = 2.031, p = 0.364, unpaired t test, one-tailed; Figure 3B) and active lever presses (FR1, VEH vs. ZL006, t(9) = 2.031, p = 0.364, unpaired t test, one-tailed; Figure 3C). However, these differences disappeared when reaching the end of the acquisition phase (i.e., last five sessions, FR3) (Figure 3B,C). No differences were found in the inactive lever presses at any time in the acquisition phase (Figure 3D). Furthermore, no interaction effect was found in the overall acquisition between sessions and treatment in the infusion number (F9,81 = 1.972, p = 0.0532; Figure S1A), active lever presses (F9,81 = 0.8499, p = 0.5729; Figure S1B), or inactive lever presses (FF9,81 = 1.108, p = 0.3668; Figure S1C).
3.6 ZL006 did not alter morphine intake during the maintenance phase of morphine self-administration
ZL006 (10 mg/kg i.p.) did not alter morphine intake during the maintenance phase of morphine self-administration (Figure 4). Animals in this experiment completed the acquisition phase of morphine self-administration without receiving any (i.p.) drug treatment and continued self-administering morphine daily until a stable behaviour was reached (baseline), after which rats were injected with ZL006 or vehicle for the following five consecutive morphine self-administration sessions (Figure 4A). No interaction was found between drug treatment with ZL006 or vehicle and infusion intake (F1,8 = 1.138, p = 0.3172, Figure 4B), active lever presses (F1,8 = 0.4351, p = 0.5280, Figure 4C), or inactive lever presses (F1,8 = 0.0004363, p = 0.9838, Figure 4D). Furthermore, no differences in infusion intake across sessions were found for the vehicle group (F1.5,6.2 = 0.4503, p = 0.6109, Figure S2A) or the ZL006 group (F2.58,10.32 = 1.591, p = 0.2511, Figure S2B). In addition, no interaction was found between the sessions and lever presses in the vehicle group (F7, 28 = 0.702, p = 0.6701, Figure S2C) or ZL006 group (F7,28 = 1.64, p = 0.1653, Figure S2D), and the differences between active and inactive lever presses remained similar and significantly different (p < 0.001) in both groups during the baseline and treatment.
3.7 ZL006 reduced morphine seeking behaviours during relapse
ZL006 (10 mg/kg i.p.) reduced lever presses in the relapse test of morphine seeking behaviours (Figure 5). Rats were subjected to 21 days of forced abstinence in the home cage after completing the acquisition phase. On the relapse day, rats were injected with ZL006 or vehicle and then subjected to a single test session (Figure 5A). In this scenario, in which no morphine was infused, we evaluated whether ZL006 was effective in reducing drug seeking (i.e., lever pressing). No interaction was found in active lever presses, when comparing the last day of acquisition with the relapse test and the drug treatment (F1,13 = 0.6966, p = 0.4190). However, the number of active lever presses during the relapse test was lower in rats treated with ZL006 compared with vehicle (t13 = 1.803, p = 0.0473, unpaired t test, one-tailed; Figure 5B). No interaction was found in the inactive lever presses, when comparing the last day of acquisition with the relapse test and the drug treatment (F1,13 = 0.1505, p = 0.7043), and the number of inactive lever presses did not differ between groups (t13 = 1.62, p = 0.133, unpaired t test, one-tailed; Figure 5C).
4 DISCUSSION
We examined effects of disrupting PSD95-nNOS protein–protein interactions on the rewarding properties of morphine and DA neurotransmission. PSD95-nNOS inhibition decreased opioid reward and relapse-like behaviour after forced abstinence. Morphine-induced CPP was blocked by doses of IC87201 and ZL006 that did not produce preference or aversion when administered alone. Memory disrupting effects of PSD95-nNOS inhibitors cannot explain our data; we previously showed that IC87201 and ZL006 in rats do not impair spatial or source memory under conditions in which the NMDAR antagonist MK-801 produced marked memory impairment.35 Unlike MK-801, neither PSD95-nNOS inhibitors altered motor function in rotarod or open field tests.30, 34 Furthermore, ZL006 attenuated conditioned fear in rats without affecting social interaction or object recognition memory.34 We evaluated ZL006 in subsequent FSCV and morphine self-administration studies because it remains the best-characterized PSD95-nNOS inhibitor in the literature in terms of efficacy and side effects.
We used FSCV to measure the impact of ZL006 on extracellular DA in the NAc shell, a pivotal neurochemical substrate of reward,47 in the presence and absence of morphine. DA release was evoked by electrical stimulation of the MFB, which connects the ventral tegmental area (VTA) with the NAc and represents a critical part of brain reward circuitry.48 We recorded from anaesthetised rats to minimize potentially confounding influence of spontaneously occurring behaviour-related DA transients.40, 41, 43 Urethane was used as anaesthetic because it does not alter DA kinetics.49 We found that the electrically evoked DA signal was not modified by ZL006 alone, suggesting that ZL006 does not alter DA dynamics. This observation supports our hypothesis that ZL006 is not inherently rewarding. Strikingly, when morphine and ZL006 were co-administered, morphine-induced increase in [DA]max was blocked. This is consistent with the fact that glutamate receptors are implicated in the rewarding properties of morphine in the VTA50-52 and glutamatergic transmission is required for morphine-induced activation of DA neurons in the VTA.53
Finally, we examined the impact of ZL006 on reinforcing effects of morphine using an i.v. morphine self-administration paradigm. ZL006 did not alter the acquisition or maintenance of morphine self-administration, but reduced lever pressing after forced abstinence in a relapse test. Thus, effects of PSD95-nNOS inhibitors differed when measuring conditioned reward (i.e., non-contingent i.p. morphine administration) and conditioned reinforcing (i.e., contingent i.v. morphine self-administration). CPP measures associations between drug reward and a contextual environment whereas i.v. self-administration measures the direct reinforcing effectiveness of drugs. Our results document a dissociation between the actions of ZL006 on these two measurements of drug conditioning. ZL006 blocked morphine-induced CPP and morphine-induced increases in electrically evoked DA release, but did not attenuate morphine reinforcement of lever pressing behaviour when the drug was self-administered intravenously. These observations suggest that disrupting PSD95-nNOS protein–protein interaction attenuates opioid reward and opioid-induced increases in dopamine efflux, but is not sufficient to reduce opioid reinforcing effects in rats. This outcome may reflect in the fact that self-administration requires an operant schedule whereas behaviour in the CPP assay is not effort-based. Nevertheless, the DA hypothesis in opioid addiction remains controversial,54 and other neural substrates beyond the VTA-NAc pathway may sustain compulsive opioid use.
The relapse test after forced abstinence simulates key aspects of pharmacological and behavioural treatments for substance use disorder, which often start with a period of abstinence (forced or voluntary).55 In this model, drug-taking behaviour and drug-associated cues remain intact since they are not extinguished in repetitive drug-free sessions. Hence, the relapse after forced abstinence model is particularly important for (1) identifying therapeutic treatments that decrease relapse of drug-seeking behaviours and (2) studying withdrawal-associated neurobiological changes in the brain.55 Because heroin-paired cues increase extracellular glutamate in the NAc during reinstatement in animals trained on drug self-administration,56 we tested nNOS-PSD95 inhibitors in a relapse test. ZL006 given before the relapse test decreased responses on the lever previously associated with obtaining morphine infusions, but not the inactive lever, suggesting that ZL006 reduces opioid-seeking behaviours.
NMDARs appear to maintain the representation of opioid primary reward and facilitate relapse-like behaviour. The lack of effect of ZL006 on acquisition and maintenance of morphine self-administration may also be clinically beneficial; nNOS-PSD95 inhibitors suppress pain states that are treated by opioids in preclinical studies,29-31 suggesting that adjunctive analgesic therapies would attenuate pain without enhancing abuse liability and could facilitate opioid sparing effects. Nevertheless, NMDARs are not the only glutamate receptors implicated in opioid addiction since AMPAR and metabotropic glutamate receptors (mGluRs) are also involved (for detailed review, see Peters and De Vries57). Because PSD95-nNOS inhibitors were administered systemically in our studies, either direct or indirect mechanisms could mediate the effects on morphine reward and relapse-like behaviour described in this study. Likewise, our results do not preclude the possibility that distinct neuronal populations may be differentially impacted by PSD95-nNOS inhibitors to mediate effects observed herein. More work is necessary to elucidate the types of cells impacted by ZL006 and further characterize underlying circuit mechanisms. Prior food lever training has been proven to not influence maintenance, extinction, or reinstatement of drug self-administration,58 although its use could limit data interpretation in the acquisition phase. Hence, different experimental approaches could unmask additional effects of PSD95-nNOS inhibitors on the acquisition of morphine self-administration in animals not subjected to prior food lever training. Future studies are also required to determine if the results we observed in male rats generalize to female rats.
In conclusion, this is the first report of the impact of disrupting PSD95-nNOS protein–protein interactions in opioid addiction. Behavioural and electrochemical data suggest that disruption of PSD95-nNOS protein–protein interactions suppresses opioid-induced reward and attenuates opioid relapse-like behaviour while also supporting the premise that PSD95-nNOS inhibitors are unlikely to be inherently rewarding or addictive.
ACKNOWLEDGMENT
The authors are grateful to Tannia Gutierrez for conducting pilot studies for the conditioned place preference experiments.
AUTHOR CONTRIBUTIONS
IO performed the i.v. morphine self-administration experiments. SAS performed the conditioned place preference experiments with assistance from VI. CRB performed the in vivo voltammetry experiments with assistance from VI. KDB collected proof of concept in vivo voltammetry data. JDC wrote programs for data collection and analysis of CPP and drug self-administration studies and contributed to experimental design. AGH and GV designed experiments and supervised the project. IO, SAS, CRB, GVR, and AGH wrote the manuscript with assistance from JDC and YYL.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
n/a.




