Effects of chronic cocaine and ethanol self-administration on brain dopamine receptors in a rhesus monkey model of polysubstance abuse
Abstract
Most individuals with cocaine use disorder also use alcohol; however, little is known about the behavioural and pharmacological mechanisms that promote co-abuse. For example, although studies in humans and animals have documented that chronic use of either alcohol or cocaine alone decreases D2-like receptor (D2R) availability, effects of co-abuse of these substances on dopamine receptor function have not been characterized. These studies examined the effects of long-term cocaine self-administration in 12 male rhesus monkeys who also consumed either ethanol or an ethanol-free solution each day (n = 6 per group). Specifically, all monkeys self-administered cocaine (0.1 mg/kg per injection) 5 days per week in the morning. In the afternoon, six monkeys consumed 2.0 g/kg ethanol over 1 h to model binge drinking and six monkeys drank an ethanol-free solution. Assessment of D2R availability using positron emission tomography (PET) and [11C]raclopride occurred when monkeys were drug-naïve and again when monkeys had self-administered approximately 400-mg/kg cocaine. D3R function was assessed at the same time points by determining the potency of the D3R-preferring agonist quinpirole to elicit yawns. Chronic cocaine self-administration decreased D2R availability in subregions of the basal ganglia in control monkeys, but not those that also drank ethanol. In contrast, D3R sensitivity increased significantly after chronic cocaine self-administration in ethanol-drinking monkeys but not controls. These results suggest that co-use of ethanol substantially changes the effects of chronic cocaine self-administration on dopamine receptors, specifically implicating D3R as a target for medications in these individuals.
1 INTRODUCTION
Estimates indicate that up to 90% of people with cocaine use disorder (CUD) also use alcohol.1, 2 These individuals often have more severe CUD, are more adversely affected by their drug use and are less likely to remain in treatment.3-5 The neurobiological and behavioural mechanisms underlying polysubstance abuse are largely unknown. Research in laboratory animals has begun to study factors that may promote cocaine–ethanol polysubstance abuse. One possibility is that alcohol use prior to cocaine, as is the norm,6 increases an individual's sensitivity to cocaine when it is subsequently encountered. This hypothesis has not been supported by rodent and nonhuman primate (NHP) studies.7, 8 Another possibility is that alcohol and cocaine produce additive or synergistic euphoric effects when co-ingested. However, although both cocaine and alcohol independently increase extracellular dopamine concentrations,9-11 combinations are not reliably self-administered more than either drug alone, nor does ethanol increase cocaine-primed reinstatement of extinguished cocaine self-administration.12-15
The present studies addressed a third possibility, that the combined use of ethanol and cocaine produces different neurobiological adaptations in brain dopamine systems than those that occur during of the use of cocaine alone. This possibility was suggested by a study in rhesus monkeys in which low cocaine doses that lacked reinforcing effects prior to ethanol exposure were robustly self-administered after monkeys drank ethanol in a binge-like pattern for 8 weeks.14 Two caveats to that study were that monkeys had extensive cocaine experience prior to drinking ethanol and that cocaine self-administration sessions were suspended during the 8 weeks of ethanol drinking. Monkeys in the present study were exposed to the two drugs in a more ethologically relevant sequence in that they were first trained to drink ethanol and then self-administered cocaine and ethanol for more than 2 years.
Regarding mechanism, the present studies focused on dopamine D2-like receptors (D2R), which comprise D2, D3 and D4 subtypes [denoted with subscripts for clarity as D2R, D3R and D4R]. D2R and in particular D3R have been strongly implicated in the abuse-related effects of cocaine.16, 17 For example, brain imaging studies in humans with CUD show lower D2R availability in the striatum/basal ganglia compared to control subjects.18, 19 There is less agreement regarding alcohol use disorder (AUD), with some positron emission tomography (PET) studies demonstrating lower striatal D2R availability in AUD subjects versus controls,20-26 and others showing no difference.27-29 Discrepancies may involve differences in subjects' ages, alcohol-drinking histories and durations of abstinence at the time of the PET scan. A limitation of these human studies is that it is not possible to determine whether low D2R availability is a pre-existing determinant of vulnerability or represents a consequence of chronic drug use. Studies in laboratory animals, particularly NHPs, which can be studied longitudinally for years starting with drug-naïve subjects, can help bridge this gap. In this regard, PET studies that compared D2R availability when monkeys were cocaine-naïve and after 1 year of cocaine self-administration demonstrated that lower D2R availability can be both a pre-existing source of vulnerability to the reinforcing effects of cocaine and a consequence of chronic cocaine use.30
Compared to D2R, fewer D3R PET studies of individuals with CUD have been reported, but these confirm that individuals with CUD have lower D2R availability in the striatum and suggest higher D3R availability in non-striatal regions including the substantia nigra, hypothalamus and amygdala.31-34 Regarding AUD, only two PET studies of D3R have been published.35, 36 Although these showed no differences in D3R availability between individuals with AUD and control subjects, the duration for which participants had been abstinent varied widely. No longitudinal PET studies of D3R have been reported in NHPs. However, studies of yawning induced by the D3R-preferring agonist quinpirole, an unconditioned behavioural effect mediated by D3R,37, 38 have demonstrated that a history of ethanol but not cocaine self-administration increases sensitivity to D3R stimulation.14, 39-41 Thus, the sparse literature to date suggests the possibility that D3R function may be increased in individuals with CUD that also use alcohol.
The current study examined whether ethanol consumption modifies the effects of cocaine self-administration on brain D2R and D3R using [11C]raclopride PET imaging and quinpirole-induced yawning, respectively, in a NHP model of cocaine–alcohol co-abuse. Baseline data were collected in 12 drug-naïve male rhesus monkeys. Next, six monkeys consumed 2.0 g/kg ethanol over 1 h each morning, 5 days per week (the ethanol-drinking group). This regimen models binge drinking that results in blood ethanol concentrations ~80 mg/dl (equivalent to 0.08%, the legal limit to operate a motor vehicle in most states) but remains below the amount of exposure that produces physical dependence (2.6–8.0 g/kg/day).42-44 The remaining six monkeys drank a similar sweetened solution without ethanol (the cocaine-only control group). After monkeys had consumed the ethanol or control solution for 9 months, cocaine self-administration was initiated; groups did not differ in acquisition of cocaine self-administration.8 Next, monkeys self-administered injections of 0.1 mg/kg of cocaine (i.v.) 5 days per week until they reached a total intake of ~400 mg/kg of cocaine. Another PET scan was then acquired and quinpirole dose–effect curves were generated for comparison with baseline data. We hypothesized that dopamine D2R availability would decrease in both groups and that the effect would be larger in the ethanol-drinking group. Similarly, we hypothesized that ethanol-drinking monkeys would become more sensitive to quinpirole over time whereas the potency of quinpirole to produce yawns would not change in the cocaine-only group.
2 MATERIALS AND METHODS
2.1 Subjects
Twelve male adult (age 7.0 ± 0.5 years) rhesus monkeys (Macaca mulatta) served as subjects. Monkeys were pair-housed in stainless steel cages in which water was available ad libitum. Monkeys were weighed weekly and fed enough food (Purina LabDiet Chow, St. Louis, MO), fruit and vegetables daily to maintain healthy body weights without becoming obese as determined by daily inspection and periodic veterinary examinations. Each monkey was fitted with an aluminium collar (Primate Products, Redwood City, CA) and trained to sit in a standard primate chair (Primate Products). Each monkey had also been prepared with an indwelling venous catheter and subcutaneous vascular access port (VAP; Access Technologies, Skokie, IL) under aseptic surgical conditions as described previously.8 Animal housing, handling and experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011) and were approved by the Animal Care and Use Committee of Wake Forest University. Environmental enrichment was provided as outlined in the Institutional Animal Care and Use Committee's Non-Human Primate Environmental Enrichment Plan.
2.2 PET imaging
Prior to acquiring the baseline PET scan, T1-weighted magnetic resonance images were acquired with a 3.0-T Siemens SKYRA scanner. PMOD Biomedical Image Quantification Software (version 3.1; PMOD Technologies, Zurich, Switzerland) was used to define anatomical regions of interest (ROI), including the caudate nucleus, putamen, ventral striatum (VS), ventral pallidum (VP) and globus pallidis (GP) and cerebellum, for each subject. PET scans with the D2R radioligand [11C]raclopride were conducted to determine D2R availability using published procedures.45 Two scans were conducted in each monkey: one when monkeys were ethanol- and cocaine-naïve (“baseline”) and another when each monkey reached ~400 mg/kg of total cocaine intake (“400 mg/kg”). Monkeys had not self-administered cocaine or consumed ethanol for at least 24 h prior to each scan.
2.3 Quinpirole-induced yawning
Quinpirole-induced yawning was measured as described previously.41 Monkeys were seated in a primate chair and transported to a familiar room. The session was divided into three 30-min components, which were video-recorded. Saline, then two ascending doses of quinpirole (0.001–0.56 mg/kg, i.m.) were administered at the beginning of each component. Beginning immediately after injection, the number of yawns was recorded for 30 min. A yawn was defined as a full extension of the jaws, withdrawal of lips and exposure of teeth.46 Because quinpirole also induces hypothermia through activation of the D2R subtype of D2R, temperature was recorded via rectal thermometer at the start of the session and at the end of each 30-min interval. Videos were later scored by two reviewers. In the rare case of discrepancy (i.e., when the yawn count differed between observers), the time of the discrepancy was identified from written records and a third observer, blinded to the treatment the monkey received, made a final determination as to whether a yawn occurred. Monkeys did not self-administer cocaine or ethanol on the day of a yawning experiment.
2.4 Ethanol consumption
For six monkeys, ethanol was introduced in the home cage as described previously.14 Briefly, a solution of 4% (w/v) ethanol in 3.5% Tang was provided via a bottle attached to the monkey's cage for 1 h per day, 5 days per week in the afternoon. The monkey could consume a maximum of 2.0-g/kg ethanol each day. The monkeys in the age-matched control group were treated the same except that the Tang solution did not contain ethanol.
2.5 Cocaine self-administration
Cocaine self-administration was established as described.8 Monkeys self-administered injections of 0.1-mg/kg cocaine under a fixed ratio 30 schedule of reinforcement for 1 h, 5 days per week in the morning. Prior to the start of each session, the back of the monkey was scrubbed with 70% isopropyl alcohol and 10% povidone-iodine (Prevantics Swab, PDI Inc, Orangeburg, NY) and a 22-gauge Huber Point Needle (Access Technologies) and tubing connected to an infusion pump (Cole-Parmer Instrument Co., Niles, IL) was inserted into the vascular access port. The pump was then activated for approximately 3 s to fill the VAP and catheter with cocaine.
2.6 Data presentation and analysis
PMOD Software was used to co-register MRI and PET images and to calculate distribution volume ratios (DVRs) for each ROI using the cerebellum as the reference region by implementing the Logan method of analysis.47 DVRs for each region were not different between left and right sides, so data from each monkey was expressed as a mean of both sides. For each ROI, a two-way analysis of variance (ANOVA) with repeated measures was conducted using group (ethanol-drinking vs. control) and time (baseline vs. 400-mg/kg cocaine intake) as factors, followed by Sidak multiple comparisons testing. To quantify the potency of quinpirole to elicit yawns, an ED50 value was calculated for each monkey by interpolation of the linear portion of his yawning dose–effect curve. In all monkeys except R-1999, doses were available that bracketed 50%; these doses (expressed as log doses) were used to generate ED50 values, which were converted back to linear doses for presentation and analysis. In R-1999, quinpirole elicited no yawns at any dose tested up to 0.3 mg/kg. A conservative estimate of the ED50 was made by assuming that the next highest dose would have produced yawns. Group-averaged ED50s were compared using paired or unpaired t tests as appropriate. In all analyses, significance was accepted when p < 0.05. During the self-administration phase, one monkey in the control group was removed from the study due to extended health issues (R-2000). His baseline data were included in group comparisons at that time point. The data that support the findings of this study are available from the corresponding author upon reasonable request.
2.7 Drugs
Ethanol (95% ethyl alcohol; The Warner-Graham Company; Cockeysville, MD) was diluted each morning prior to mixing with Tang. (-)-Cocaine HCl was obtained from the National Institute on Drug Abuse (Bethesda, MD), and quinpirole HCl was purchased form Tocris Bioscience (Bristol, UK). These were dissolved in sterile 0.9% saline. Doses are expressed based on the salt form.
3 RESULTS
3.1 PET imaging of D2R
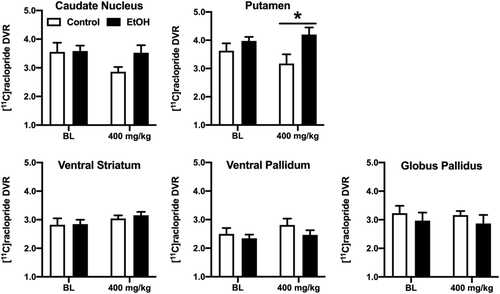
As hypothesized, no differences in D2R availability were observed between groups in any brain region at baseline (Table 1, Figure 1). When monkeys were re-scanned after self-administering ~400-mg/kg cocaine, average DVRs in the control group decreased in the caudate nucleus and putamen (Table 2). Although the effect did not reach statistical significance in the caudate nucleus (p = 0.11), in the putamen a two-way ANOVA revealed a significant main effect of group (F1,9 = 5.422, p < 0.05) and post-hoc testing demonstrated that the two groups differed significantly at the latter time point (p < 0.05). Neither group differences in D2R availability nor a significant effect of time was observed in the VS, VP or GP.
| Cd | Pt | VS | VP | GP | |
|---|---|---|---|---|---|
| Control group | |||||
| R-1995 | 3.90 | 3.95 | 3.49 | 3.06 | 3.55 |
| R-1999 | 3.45 | 3.17 | 2.39 | 2.35 | 3.13 |
| R-2000 | 3.89 | 4.65 | 3.20 | 2.18 | 2.43 |
| R-2001 | 3.02 | 3.96 | 3.24 | 2.86 | 4.04 |
| R-2002 | 4.59 | 4.22 | 2.43 | 1.91 | 2.57 |
| R-2003 | 2.81 | 2.85 | 2.56 | 2.31 | 2.86 |
| Mean | 3.61 | 3.80 | 2.88 | 2.45 | 3.10 |
| SEM | 0.29 | 0.30 | 0.21 | 0.19 | 0.28 |
| Ethanol-drinking group | |||||
| R-1992 | 3.62 | 4.49 | 2.64 | 1.95 | 2.73 |
| R-1993 | 3.62 | 3.96 | 3.32 | 2.84 | 3.50 |
| R-1994 | 2.75 | 3.47 | 2.32 | 2.49 | 3.13 |
| R-1996 | 3.53 | 3.91 | 3.18 | 2.28 | 1.70 |
| R-1997 | 3.92 | 4.21 | 2.67 | 2.06 | 3.13 |
| R-1998 | 4.08 | 3.81 | 2.95 | 2.45 | 3.61 |
| Mean | 3.59 | 3.97 | 2.85 | 2.35 | 2.97 |
| SEM | 0.21 | 0.16 | 0.17 | 0.14 | 0.31 |
| Cd | Pt | VS | VP | GP | |
|---|---|---|---|---|---|
| Control group | |||||
| R-1995 | 2.78 | 3.15 | 3.05 | 2.78 | 2.75 |
| R-1999 | 2.65 | 3.18 | 3.19 | 2.92 | 2.97 |
| R-2000 | n.d. | n.d. | n.d. | n.d. | n.d. |
| R-2001 | 3.43 | 4.34 | 2.97 | 2.05 | 3.12 |
| R-2002 | 2.99 | 2.84 | 3.32 | 3.43 | 3.43 |
| R-2003 | 2.45 | 2.36 | 2.68 | 2.88 | 3.53 |
| Mean | 2.86 | 3.17 | 3.04 | 2.81 | 3.16 |
| SEM | 0.17 | 0.33 | 0.11 | 0.22 | 0.14 |
| Ethanol-drinking group | |||||
| R-1992 | 3.63 | 4.56 | 2.91 | 2.48 | 3.03 |
| R-1993 | 2.80 | 2.47 | 3.22 | 2.59 | 3.02 |
| R-1994 | 3.19 | 4.36 | 3.30 | 2.86 | 3.19 |
| R-1996 | 3.60 | 4.24 | 2.81 | 1.87 | 1.43 |
| R-1997 | 4.69 | 5.06 | 3.06 | 2.13 | 2.97 |
| R-1998 | 3.24 | 3.51 | 3.63 | 2.86 | 3.57 |
| Mean | 3.52 | 4.20 | 3.16 | 2.47 | 2.87 |
| SEM | 0.29 | 0.28 | 0.13 | 0.18 | 0.33 |
- Note: n.d., not determined.
3.2 Quinpirole-induced yawning
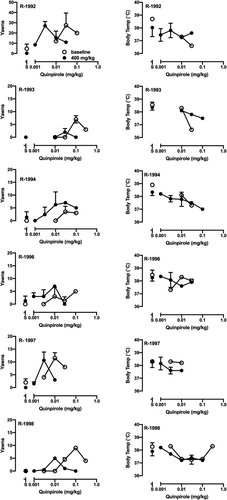
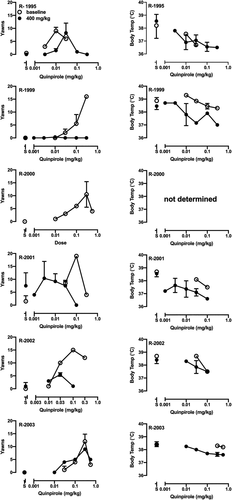
When animals were cocaine- and ethanol-naïve, administration of quinpirole resulted in a dose-dependent increase in yawns and, in most monkeys, an inverted U-shaped dose–effect curve (Figures 2 and 3, open symbols in left columns). In some monkeys, this dose range also lowered body temperature (Figures 2 and 3, open symbols in right columns). Following extensive cocaine self-administration, the ascending limb of the quinpirole dose–effect curve was shifted to the left in all monkeys in the ethanol-drinking group (Figure 2, filled symbols in left column). ED50 values decreased significantly from baseline (Table 3), indicating a greater sensitivity to D3R stimulation after self-administering cocaine and ethanol. In contrast, in the cocaine-only group the effect of long-term cocaine self-administration on the potency of quinpirole to elicit yawns was variable (Figure 3, filled symbols in left column). As shown in Table 3, the quinpirole ED50 increased in one monkey (R-1995), decreased in one (R-2001) and was unchanged in the other three (R-1999, R-2002, R-2003). In R-1999 and R-2002, the maximum number of yawns decreased compared to values collected when monkeys were drug-naïve (Figure 3). In most monkeys, long-term cocaine ± ethanol self-administration did not change the potency of quinpirole to produce hypothermia (Figures 2 and 3, right columns).
| Ethanol | Cocaine | Quin ED50 baseline | Quin ED50 400 mg/kg | |
|---|---|---|---|---|
| Control group | ||||
| R-1995 | 0 | 364.9 | 0.004 | 0.015 |
| R-1999 | 0 | 356.0 | 0.130 | 0.500 |
| R-2000 | 0 | n.d. | 0.074 | n.d. |
| R-2001 | 0 | 491.0 | 0.034 | 0.001 |
| R-2002 | 0 | 358.7 | 0.022 | 0.009 |
| R-2003 | 0 | 357.7 | 0.132 | 0.055 |
| Mean | 0 | 385.7 | 0.066 | 0.098 |
| SD | 0 | 59.0 | 0.055 | 0.175 |
| Ethanol-drinking group | ||||
| R-1992 | 981.7 | 385.4 | 0.011 | 0.001 |
| R-1993 | 1108.1 | 388.6 | 0.055 | 0.017 |
| R-1994 | 989.1 | 402.0 | 0.017 | 0.004 |
| R-1996 | 1039.2 | 364.8 | 0.044 | 0.004 |
| R-1997 | 1029.5 | 347.4 | 0.004 | 0.002 |
| R-1998 | 882.2 | 388.5 | 0.031 | 0.005 |
| Mean | 1005.0 | 380.8 | 0.027 | 0.006 |
| SD | 75.2 | 21.8 | 0.020 | 0.006 |
4 DISCUSSION
Although most individuals with CUD also use alcohol, little is known about the neurobiological changes that drive continued use. Identification of differences between individuals with CUD alone and those with comorbid AUD may lead to more effective pharmacotherapies for both groups. The present studies used a NHP model of cocaine–ethanol polysubstance abuse to characterize how the long-term self-administration of both drugs differs from the effects of self-administration of cocaine alone, with a focus on dopamine receptor function. The most consistently observed neurobiological effect of chronic cocaine self-administration in cross-sectional human brain imaging studies is lower D2R availability in the striatum/basal ganglia of cocaine-experienced subjects.18 Longitudinal studies in cocaine self-administering monkeys have confirmed that this difference in an effect of chronic cocaine.30 This observation was replicated and extended in the present study in the cocaine-only group. After self-administering approximately 400 mg/kg cocaine over 1.6 ± 0.3 years, D2R availability was lower in the caudate nucleus and putamen, subregions of the basal ganglia, with the difference reaching statistical significance in the putamen.
Lower D2R has also been observed in individuals with AUD compared to controls,21-25 which led to the hypothesis that monkeys who self-administered ethanol as well as cocaine would display even greater decreases in D2R availability than the group that only self-administered cocaine. However, D2R availability was unchanged from the drug-naïve baseline in monkeys who self-administered both drugs. This lack of effect was observed in all brain areas examined, including the caudate nucleus and putamen. Although this result might indicate that ethanol drinking prevented the cocaine-induced decrease in D2R, the well-documented inverse relationship between striatal D2R availability and sensitivity to multiple abused drugs23, 48 makes this unlikely. Alternatively, it is possible that differential changes in the number of available D2, D3 and D4 subunits cancel each other out, resulting in no net change in D2R availability. To assess this possibility, we characterized changes in function of the D3 subtype of the D2R receptor family by assessing the ability of the D3R-preferring agonist quinpirole to elicit yawning, an unconditioned behavioural effect of quinpirole that has been shown definitively to be mediated by D3R stimulation.37, 38
As hypothesized, the ability of quinpirole to elicit yawns did not differ when groups were drug-naïve. In the group that only self-administered cocaine, on average the potency of quinpirole to elicit yawns was unchanged. This result is consistent with a prior study, which found no difference in quinpirole yawning dose–effect curves between separate groups of cocaine-experienced and cocaine-naïve rhesus monkeys,40 and extends the result to a longitudinal, within-subject design. In all six monkeys that also drank ethanol, however, yawning dose–effect curves were shifted leftward and the average ED50 was significantly lower than that of the control group, indicating an increase in D3R sensitivity after chronic ethanol drinking. In a previous study in a different cohort, i.v. administration of ethanol itself produced yawns in monkeys experienced in self-administering ethanol + cocaine but not cocaine alone, and this effect was blocked by a D3R antagonist.41 Taken together these results suggest that acute and chronic ethanol administration increase sensitivity of D3R to stimulation by a D3R agonist.
At higher quinpirole doses, the shape and position of the yawning curve can also be affected by stimulation of D2R. Activation of the D2R subtype opposes the D3R-mediated increase in yawns, resulting in the descending limb of the yawning curve. The inflection point of the dose–effect curve coincides with the onset of quinpirole's hypothermic effects.38, 49 In the present study, in only one control monkey was a potential role for changes in D2R function observed. In R-1999 the potency of quinpirole to produce hypothermia increased after cocaine self-administration, and quinpirole produced no yawns at any dose. In the remaining 10 subjects, however, the data do not support the view that changes in quinpirole-induced yawning are explained by changes in sensitivity of D2R. The most parsimonious conclusion is that D3R sensitivity increased in monkeys that self-administered cocaine and ethanol, but not in those that self-administered only cocaine.
Taken together, the results of PET and yawning studies suggest that chronic ethanol consumption increases D3R densities and/or sensitivity to D3R stimulation. Although D3R levels in the caudate and putamen are relatively low compared to D2R,50 this conclusion is consistent with previous studies in rodents, which observed increased striatal D3R expression after ethanol self-administration.51-53 On the surface, this conclusion may seem inconsistent with that of two PET studies that used the D3R radiotracer [11C]-(+)-PHNO in humans with AUD and reported no difference from control subjects.35, 36 However, the extensive overlap in distribution of D3R and D2R coupled with the relatively low selectivity of PHNO for D3R versus D2R calls for caution in interpreting [11C]-(+)-PHNO binding as a selective measure of D3R availability.54, 55 Thus, D3R radiotracers with improved selectivity for D3R versus other subtypes are needed to provide greater resolution in determining D3R distribution in vivo. Furthermore, in one of the human studies,35 the time since subjects had last used alcohol was long and varied widely (mean, 415 days; range, 39–893 days), raising the possibility that alcohol-induced changes in D3R had recovered by the time of the PET scans. The more recent study, which constrained the duration of abstinence (7 ± 4 days), also reported no difference between AUD subjects and controls in the caudate nucleus or putamen. It should be noted, however, that the [11C]-(+)-PHNO binding in these regions predominantly (>80%) reflects D2R.56 Finally, monkeys in the current study consumed 18–24 h prior to PET or yawning studies. Thus, it is possible that the apparent discrepancy between the present study and previous PET studies in humans may reflect assessment of D3R function during ongoing drug use versus early/late abstinence and the suboptimal ability of PHNO to selectively measure D3R availability in some brain regions.
These studies have some limitations that are important to consider. First, each day, cocaine and alcohol consumption were separated in time. This design was implemented to ensure that monkeys were not intoxicated by ethanol during operant behavioural sessions, which could hinder cocaine self-administration. However, this represents a departure from the clinical condition in which the two drugs are typically consumed together. Concurrent intake of ethanol and cocaine results in generation of cocaethylene, which has pharmacodynamic, neurochemical and behavioural effects similar to cocaine.57-60 Separating cocaine and ethanol ingestion in the present study also ensured that behavioural effects of cocaethylene did not influence self-administration. Future studies of concurrent cocaine and ethanol use should better model clinical use patterns. In addition, very little is known about the potential alterations in D4R because the pharmacological tools used in the present and most previous studies lack sufficient pharmacological selectivity to distinguish them from D2R and D3R. Nonetheless, studies with D4R knockout mice and selective antagonists suggest it may play a role in substance use disorders.61 Finally, it should be noted monkeys in the current study self-administered 2.0 g/kg ethanol, 5 days per week, which models binge drinking but does not meet the definition of heavy use (>3.0 g/kg/day62). In this context it is interesting to note that the commonly reported decrease in D2R in individuals with AUD was not observed in a PET study in binge drinkers without AUD.63 Thus, additional studies are needed to assess the extent to which results of these studies generalize to subjects with higher (and lower) levels of alcohol and cocaine consumption.
In summary, chronic cocaine self-administration produced different effects on brain dopamine receptors depending on whether ethanol was also consumed during the same period. Specifically, D3R sensitivity increased only in monkeys who self-administered both drugs. Considering the well-documented involvement of D3R in cocaine reinforcement17 it will be important to determine whether this neurobiological effect of ethanol increases the reinforcing strength of cocaine to perpetuate ongoing use and increase the possibility of relapse. Another important clinical implication of this finding is that co-users may be more sensitive to putative pharmacotherapies compared to users of cocaine only. Although drugs that block D3R have been shown to decrease ethanol and cocaine self-administration in rodent models, selective reductions in cocaine self-administration are typically not observed in NHPs or in clinical trials.64, 65 However, the subjects of such studies lacked any/substantial alcohol exposure; the present results suggest that D3R may be more sensitive in individuals who also consume ethanol. Whether they are also more sensitive to D3R-acting medications remains to be tested. However, a precedent for differential effects according to alcohol-consuming status can be found in a clinical trial for the stimulant modafinil for CUD.66 Although modafinil did not reduce cocaine use in the total sample, post hoc analysis indicated that modafinil significantly increased non-use days in patients who did not have a history of AUD. In context of the present results, it is possible that drugs that block D3R may be more effective in decreasing cocaine use in individuals with a dual diagnosis of CUD and AUD.
ACKNOWLEDGEMENTS
This work was supported by the National Institute of Health, National Institute on Drug Abuse grant DA039953. The sponsor had no role in the study design; the collection, analysis or interpretation of data; writing of the report or the decision to submit the article for publication. The authors thank April Davenport, Stephanie Rideout and Miracle Collier for excellent technical assistance.
CONFLICT OF INTEREST
There are no conflicts of interests to disclose.
AUTHOR CONTRIBUTIONS
PWC, SHN and KKSS were responsible for study concept and design. FMS, AMT, PME, BEG, HAK and PWC contributed to collection and analysis of animal data. KKSS, SHN and PWC were responsible for PET imaging data collection, analysis and interpretation. FMS and PWC produced a draft of the manuscript, which was reviewed and approved by all authors.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




