Effects of varenicline and bupropion on laboratory smoking outcomes: Meta-analysis of randomized, placebo-controlled human laboratory studies
Abstract
Human laboratory studies are widely used to evaluate behavioural mechanisms of pharmacotherapy effects. Results from human laboratory studies examining smoking cessation pharmacotherapies have not been examined in aggregate. The current meta-analysis aimed to synthesize data from randomized, placebo-controlled human laboratory studies on the effects of non-nicotine pharmacotherapies on outcomes relevant for smoking cessation. Literature searches identified 15 human laboratory studies of varenicline (n = 697) and 9 studies of bupropion (n = 313) with sufficient data for inclusion. Studies involved acute or subacute pharmacotherapy treatment with administration durations ranging from a single dose to 8 weeks. Primary outcomes examined were craving, withdrawal and behavioural indices of smoking. Varenicline significantly reduced craving (Hedge's g = −0.36[−0.54,−0.17], p < 0.001), withdrawal (g = −0.25[−0.41,−0.09], p = 0.003) and behavioural indices of smoking (g = −0.36[−0.63,−0.08], p = 0.01) relative to placebo. In contrast, results were inconclusive regarding bupropion's effects on craving (g = −0.13[−0.32,0.05], p = 0.15), withdrawal (g = −0.15[−0.44,0.14], p = 0.31) and behavioural indices of smoking (g = −0.05[−0.35,0.24], p = 0.73) relative to placebo. Findings provide meta-analytic support that short-term varenicline treatment decreases craving, withdrawal symptoms and smoking behaviour under controlled laboratory conditions. However, findings also suggest the ability of human laboratory paradigms to detect pharmacotherapy effects may differ by treatment type. Pharmacotherapy discovery and evaluation efforts utilizing human laboratory methods should aim to align study designs and laboratory procedures with presumed therapeutic mechanisms when possible.
1 INTRODUCTION
Tobacco use remains a leading cause of preventable death globally, contributing to approximately eight million deaths each year.1 There is a clear need to develop effective smoking cessation treatments to combat tobacco's significant health harms. Nicotine replacement therapy (NRT) significantly improves cessation rates compared to placebo2 yet may have generally modest impacts on prolonged abstinence (e.g., 24% up to 6 months).3 Treatment guidelines4 and meta-analyses5 highlight a role for adjunctive pharmacotherapies in maximizing smoking cessation outcomes such that efforts to explore alternative or adjunctive pharmacotherapies, particularly non-nicotine pharmacotherapies, are needed.
Several established and emerging non-nicotine pharmacotherapies may aid in smoking cessation. Varenicline and bupropion are first-line, non-nicotine smoking cessation medications. Varenicline, a partial nicotinic acetylcholine α4β2 receptor agonist, has been shown to reduce nicotine's reinforcing effects6 and relieve withdrawal symptoms during smoking abstinence.7 Bupropion was initially developed as a non-tricyclic antidepressant.2 Bupropion's pharmacological mechanisms of action for smoking cessation remain largely unknown, and studies continue to characterize possible bupropion impacts on nicotine's reinforcing effects or withdrawal symptoms.8 Both varenicline and bupropion were associated with significantly increased odds of smoking abstinence sustained at least 6 months relative to placebo (odds ratio = 2.88[2.40,3.47] and 1.82[1.60,2.06], respectively) in a network meta-analysis of Cochrane reviews of randomized controlled trials (RCTs).2 Second-line pharmacotherapies for smoking cessation continue to emerge. Nortriptyline, a tricyclic antidepressant, and cytisine, a partial nicotinic receptor agonist with similar pharmacological properties to varenicline, both increased likelihood of prolonged smoking abstinence relative to placebo across RCTs.2 Such work from RCTs supports the efficacy of established and emerging non-nicotine pharmacotherapies in increasing likelihood of smoking abstinence among treatment-seeking smokers.
Human laboratory studies represent an additional, complementary piece of the pharmacotherapy discovery and evaluation process.9, 10 Specifically, human laboratory studies provide an efficient way to evaluate the potential efficacy of candidate therapies and delineate potential mechanisms of action. Laboratory studies can test causal effects of pharmacotherapies on smoking behaviour or related outcomes (e.g., craving and withdrawal) while often incorporating shorter treatment duration than in clinical trials. However, despite the relevance of laboratory research to pharmacotherapy development, there have been no attempts at quantitative synthesis of research on smoking pharmacotherapies from human laboratory studies. Meta-analysis of laboratory trials could help confirm the efficacy of established non-nicotine pharmacotherapies to reinforce the utility of laboratory-based screening for pharmacotherapy development. Further, meta-analytic efforts could probe efficacy of emerging or off-label pharmacotherapies within the controlled laboratory setting and establish effect sizes for future laboratory screening of these medications. Similar meta-analytic efforts have demonstrated support for laboratory paradigms in evaluating alcohol pharmacotherapies.11, 12
The current meta-analysis represents a quantitative synthesis of extant literature on non-nicotine pharmacotherapies in smoking cessation based on data from human laboratory studies. This meta-analysis aimed to estimate composite effect sizes for established and emerging non-nicotine smoking cessation medications in human laboratory settings as well as to examine methodological and sample characteristics that may moderate medication effects.
2 MATERIALS AND METHODS
2.1 Literature search
The current meta-analysis sought to identify randomized, placebo-controlled studies assessing the effects of non-nicotine medications on outcomes relevant to nicotine response or smoking cessation in controlled human laboratory settings. Based on initial literature screens, only two non-nicotine medications (varenicline and bupropion) were studied frequently enough to warrant inclusion in the meta-analysis. Several additional medications identified (e.g., naltrexone and galantamine) were examined only in isolated laboratory studies, resulting in an insufficient number of effect sizes for inclusion. Thus, the current meta-analysis was limited to focus on randomized human laboratory studies of varenicline and bupropion.
Systematic literature searches to identify relevant human laboratory studies on varenicline and bupropion were conducted until 1 January 2020. Database searches comprised Web of Science, Embase, PubMed/Medline and PsycINFO using the following sample search: (varenicline OR bupropion) AND (nicotine OR cigarettes OR smoking) AND (laboratory OR experiment* OR acute OR crav* OR subjective). Secondary searches comprised manual review of reference lists from eligible articles as well as from relevant reviews. This manuscript was prepared in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) criteria13; this study was not pre-registered.
2.2 Study eligibility criteria
Eligible studies comprised randomized, placebo-controlled studies assessing the effects of varenicline or bupropion on behavioural or subjective outcomes relevant to nicotine response or smoking cessation (e.g., nicotine craving, withdrawal, reinforcement, preference, motivation and cigarette smoking) in a controlled human laboratory setting. Randomized trials that did not include a human laboratory component, and studies with laboratory visits that focused solely on cognitive or questionnaire outcomes in the absence of a laboratory experimental manipulation or task, were excluded. For eligibility, experimental manipulation was defined here as any procedure designed to provoke outcomes relevant to nicotine responses or smoking cessation (e.g., overnight abstinence, smoking delay, laboratory cue reactivity paradigm and administration of an alcohol priming dose). Eligible studies provided sufficient data for effect size calculation, were published in a peer-reviewed English language journal and represented original data. Case studies, dissertations and conference abstracts were excluded.
2.3 Selection process
Figure 1 presents a flow diagram detailing study identification, screening, eligibility and selection. After removing duplicates, 1017 abstracts were screened for eligibility. Most abstracts excluded after title/abstract review were non-original research studies (e.g., review, meta-analysis, editorial, book chapter and trial protocol), non-human or tissue/molecular studies or conference abstracts. Following title/abstract reviews, 191 full texts were reviewed. Most articles were excluded for representing non-laboratory studies. Several studies reporting effects of varenicline and/or bupropion only when combined with another medication (e.g., naltrexone) were excluded. For publications reporting data derived from the same or an overlapping sample of participants as another publication, publications were retained if their effect sizes contributed to separate meta-analyses of different outcomes and, thus, did not violate the assumption of independence. For multiple publications reporting effect sizes of the same outcome from the same sample, publications reporting data on a smaller sample of participants and/or more limited information for effect size calculation were excluded.14-16 Finally, studies examining only infrequently studied outcomes (e.g., alcohol self-administration, attention and physiological measures of stress) were excluded due to an insufficient number of studies for meta-analysis. There also was considerable variability across examined ‘subjective effects’ constructs (e.g., positive or negative affect, sensory states and satisfaction) and most were examined in the context of related craving or withdrawal outcomes such that craving and withdrawal were prioritized for inclusion. Therefore, the current meta-analysis retained research on craving, withdrawal and behavioural indices of smoking as outcomes.
Both authors double coded a random subset of title/abstract reviews (10%) to verify agreement (87% agreement). Remaining title/abstract reviews were conducted by the first author and adopted a liberal approach to maximize detection of human laboratory research. Specifically, randomized placebo-controlled studies of varenicline and/or bupropion on smoking outcomes were retained for full text review even if the abstract did not mention a laboratory component. Full text review decisions involved both authors, and all discrepancies were resolved through discussions.
2.4 Data extraction
Data were extracted from eligible articles to code varenicline and/or bupropion effects on craving, withdrawal symptoms and behavioural indices of smoking. Effect size data were extracted from means and standard deviations (or standard errors that were converted to standard deviations)17 when reported. Means and standard errors were measured and extracted from graphs when necessary for effect size calculation. Effect sizes of the same outcome derived from multiple participant subgroups or similar measures/indices within a single study were averaged such that each study contributed only one effect size to each meta-analysis, including averaging across sex,18 multiple factors of craving or withdrawal,6, 19-22 smoking topography measures,6, 23-25 craving instruction sets26 and subgroups of participants defined based on intention to quit smoking23, 27 or psychiatric diagnosis.28
Data also were extracted to explore potential moderators of effect size estimates. Sample characteristics extracted included proportion of men (coded to assume binary classifications if not specified and to include data reported as ‘sex’ if descriptions were inconclusive) and mean participant age. Baseline mean cigarettes smoked per day, baseline mean Fagerström Nicotine Dependence Score (FTND), and a categorical variable representing whether participants were interested in/attempting to quit smoking (no, yes and mixed) also were coded. Data were derived from weighted means when necessary, rounding to the nearest whole number. Regarding pharmacotherapy treatment, duration of pharmacotherapy was extracted. Studies that included alternative doses to the standard maximum daily clinical dose of 2 mg for varenicline and 300 mg for bupropion were retained given the small number of eligible studies.
2.4.1 Craving
Phasic craving (e.g., change in craving scores following cue exposure or another laboratory manipulation) was prioritized over tonic craving when studies reported both, because tonic craving was often measured upon arrival to the laboratory before most experimental manipulations. Phasic craving comprised changes in smoking relative to neutral cue craving from before to after pharmacotherapy6, 26 and changes in craving from before to after smoking cue exposure.19 Phasic craving also represented post-treatment craving for a smoking relative to neutral cue following outpatient25, 27 and acute29 pharmacotherapy. Several varenicline studies examined phasic alcohol-induced cigarette craving, including change in cigarette craving from before to after a 0.03-g/dl target breath alcohol concentration prime following a laboratory smoking delay30 and cigarette craving following a 0.06-g/dl target breath alcohol concentration dose controlling for baseline craving.31 Tonic craving comprised change in craving from smoking as usual to mandatory abstinence7, 22, 32 and from before to after acute pharmacotherapy following brief abstinence.21, 33 Tonic craving also reflected post-treatment craving after overnight abstinence18, 34 and a laboratory smoking delay.20 The single study examining stress-induced craving was excluded for consistency.35 Finally, given that tonic and phasic craving are conceptualized as distinct processes relevant to smoking cessation, exploratory analyses were conducted to examine pharmacotherapy effects separately on phasic and tonic craving. For these exploratory analyses, studies providing sufficient data to code both phasic and tonic craving were allowed to contribute independently to both meta-analyses.
2.4.2 Withdrawal symptoms
Abstinence-induced withdrawal was prioritized over cue-induced withdrawal when both were available. Abstinence-induced withdrawal comprised changes in withdrawal from smoking as usual to overnight6 and multiple day abstinence22 after pharmacotherapy treatment. Abstinence-induced withdrawal also comprised changes in withdrawal following overnight abstinence from before to after acute pharmacotherapy29 and change in withdrawal from smoking as usual to a mandatory quit attempt during pharmacotherapy treatment.7 Abstinence-induced withdrawal also represented post-medication withdrawal during laboratory smoking abstinence phases.20, 27 Cue-induced withdrawal comprised change in withdrawal from before to after smoking cue exposure19 and withdrawal in response to smoking versus neutral cues25 following pharmacotherapy pretreatment. The single study examining stress-induced withdrawal was excluded for consistency.35
2.4.3 Behavioural indices of smoking
Behavioural indices included laboratory measures of smoking behaviour (e.g., number of cigarettes smoked, smoking lapse and smoking topography). Behavioural outcomes were examined in aggregate given the small number of effect sizes within each outcome. Measures of ad libitum smoking were prioritized if multiple behavioural indices were available within the same study. For studies with multiple alternative outcomes not including ad libitum smoking, the designated ‘primary’ outcome was selected. Ad libitum smoking comprised mean cigarettes smoked during laboratory smoking breaks36 and 1-,20, 30 3-37 or 4-h38 self-administration phases. Time to lapse data comprised one varenicline study using McKee and colleagues' smoking lapse paradigm20 comprising post-treatment mean time to lapse after overnight abstinence.28 Smoking topography data comprised average change in full cigarette puff count, time smoking and latency to begin smoking (reverse coded)6 and average change in puff count, puff volume and puff duration25 from pretreatment to end of pharmacotherapy. Smoking topography data also represented post-treatment average puff count, puff volume and carbon monoxide (CO) boost23 and average puff count, puff volume, puff duration, puff velocity and inter-puff interval (reverse coded).24 Cigarette choice task data comprised a number of puffs taken from a 0.6-mg nicotine cigarette compared to a 0.05-mg ‘denicotinized’ cigarette.33 Several studies included administration of a priming dose of alcohol.24, 30, 36
2.5 Study quality
Methodological indicators of study quality were coded using the Cochrane risk of bias tool for randomized trials (RoB 2).39 The RoB 2 estimates risk of bias across several categories (e.g., deviation from intended intervention/assignment, measurement of outcome data and reporting of multiple outcomes). These categories were supplemented with additional criteria to reflect methodological considerations that commonly relate to human laboratory pharmacotherapy studies (e.g., inclusion of a power analysis, specification of a primary outcome, assessing adherence to medication and direct observation of laboratory outcomes).
2.6 Data synthesis
Meta-analysis was conducted in Comprehensive Meta-Analysis, Version 3. Separate meta-analyses were conducted to examine varenicline and bupropion effects on craving, withdrawal and behavioural indices of smoking. Hedge's g was calculated as a bias-corrected effect size estimate, given its robustness to small sample sizes.40 Random effects models were specified due to the variability in outcomes and designs across included studies. Effect sizes were coded such that negative values represented reductions in craving, withdrawal and behavioural indices of smoking with varenicline/bupropion relative to placebo. Effect size calculations were standardized based on post score standard deviations to maintain a more consistent metric of standardization across the included between- and within-subjects effects.17 For within-subjects designs that did not report sufficient data on pre-post correlations of outcomes, a moderate correlation of 0.5 was imputed similar to prior meta-analyses of addiction pharmacotherapies.11, 41 For one study that did not report sample sizes within conditions, sample sizes were estimated based on other sample size information provided in the text, assuming random assignment to equal group sizes at baseline.
Heterogeneity in effect sizes between studies was estimated with Cochran's Q and I2. Cochran's Q represents a ratio of heterogeneity across studies to heterogeneity within studies (i.e., within-study error).40 I2 provides an estimate of inconsistency across effect size estimates adjusted for the number of included studies,42 and I2 values of 25%, 50% and 75% were interpreted to represent low, moderate and high heterogeneity.42 Moderation analyses were conducted for those effects with significant between-study heterogeneity. Continuous moderators (i.e., proportion of men, mean participant age, mean participant cigarettes smoked per day, mean participant FTND score and pharmacotherapy duration) were examined using mixed effects meta-regression. ‘One study removed’ analyses were conducted as an estimate of sensitivity to evaluate each study's impact on the combined effect size estimate. Further, ancillary analyses examined consistency of effect size estimates after removing studies involving acute (≤4 h) effects of pharmacotherapies or non-standard pharmacotherapy doses. Publication bias for meta-analysis results with at least 10 effect size estimates17 was assessed through the Trim and Fill method43 and Egger's regression test.44
3 RESULTS
3.1 Study characteristics
Systematic literature searches identified 15 qualifying laboratory-based examinations of varenicline (Table 1) and 9 examinations of bupropion (Table 2) effects on craving (k = 10 and 8, respectively), withdrawal (k = 5 and 4) and/or behavioural indices of smoking (k = 8 and 4). Articles were published between 2000 and 2019, representing predominantly adult samples (mean ages 25–45 years) of varied gender distribution (43%–88% men) and relevant sample size (ns = 8–124). The majority of studies were conducted in the United States. Varenicline doses included 0.5, 1 or 2 mg, with treatment duration ranging from acute administration to 21 days. Bupropion studies examined doses of 150 and/or 300 mg, with treatment duration ranging from acute administration to 8 weeks. Most studies (k = 17) reported assessing side effects, and the majority of these studies (k = 13) described at least one side effect, adverse event or serious adverse event. Most studies reported no serious adverse events and/or no discontinuations related to side effects, adverse events or serious adverse events (k = 8). Studies often reported side effects that were mild and/or did not differ from the placebo group (k = 4). Nevertheless, there were three studies describing discontinuations due to side effects.
| Sample characteristics | Pharmacotherapy | Study design | Relevant outcomes coded | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Men | Age | Quit? | Cig/day | FTND | Dose | Duration | |||
| Ashare and McKee (2012) (varenicline and placebo conditions only) | 37 | 62% | 37 | No | 19 | 6 | 2 mg | 7 days | Between-subjects design comparing varenicline and placebo after pharmacotherapy pretreatment | · Craving (tonic) |
| Austin et al. (2014) (abstinent smokers only) | 40 | 55% | 25 | No | 14 | 5 | 1 mg | <1 day | Between-subjects design comparing single dose varenicline and placebo after brief abstinence | · Craving (tonic) |
| Brandon et al. (2011) | 100 | 61% | 43 | No | 25 | 6 | 2 mg | 15 days | Between-subjects, multisession design comparing varenicline and placebo amid pharmacotherapy treatment |
· Craving (phasic) · Withdrawal (abstinence-induced) · Behavioural (smoking topography) |
| Franklin et al. (2011) | 22 | 73% | 36 | No | 18 | 5 | 2 mg | 21 days | Between-subjects design comparing varenicline and placebo after pharmacotherapy pretreatment |
· Craving (phasic) · Withdrawal (cue-induced) |
| Green and Ray (2018) | 37 | 68% | 37 | No | 15 | 5 | 2 mg | 10 days | Within-subjects, multisession design comparing varenicline and placebo after pharmacotherapy pretreatment | · Craving (tonic) |
| Hitsman et al. (2013) | 38 | – | 36 | No | 21 | 6 | 2 mg | <1 day | Within-subjects, multisession design comparing single dose varenicline and placebo during cue exposure paradigm |
· Craving (phasic) · Withdrawal (abstinence-induced) |
| Kozak et al. (2019) | 28 | 61% | 40 | No | 20 | 7 | 2 mg | 6 days | Within-subjects, multisession design comparing varenicline and placebo after pharmacotherapy pretreatment | · Behavioural (smoking lapse time) |
| McKee et al. (2009) | 20 | 80% | 35 | No | 21 | 5 | 2 mg | 7 days | Between-subjects design comparing varenicline and placebo following an alcohol priming dose after pharmacotherapy pretreatment | · Behavioural (ad lib smoking) |
| McKee et al. (2012)—Study 2 (varenicline and placebo conditions only) | 41 | 56% | 36 | No | 18 | 5 | 2 mg | 7 days | Between-subjects design comparing varenicline and placebo after pharmacotherapy pretreatment |
· Craving (tonic) · Withdrawal (abstinence-induced) · Behavioural (ad lib smoking) |
| Patterson et al. (2009) | 67 | 43% | 45 | Yes | 22 | – | 2 mg | 21 days | Within-subjects, multisession design comparing varenicline and placebo after pharmacotherapy pretreatment that included a mandatory abstinence phase |
· Craving (tonic) · Withdrawal (abstinence-induced) |
| Perkins et al. (2010) | 124 | 44% | 32 | Mixed | 16 | 5 | 2 mg | 14 days | Within-subjects, multisession design comparing varenicline and placebo after pharmacotherapy pretreatment that included a smoking quit attempt | · Behavioural (smoking topography) |
| Ray et al. (2014) (varenicline and placebo conditions only) | 60 | 68% | 36 | – | 15 | 4 | 2 mg | 9 days | Between-subjects design comparing varenicline and placebo following an alcohol priming dose after pharmacotherapy treatment | · Craving (alcohol-induced) |
| Roberts et al. (2018) (varenicline and placebo conditions only) | 19 | 74% | 35 | No | 16 | 6 | 2 mg | 7 days | Between-subjects design comparing varenicline and placebo following an alcohol priming dose after pharmacotherapy pretreatment |
· Craving (alcohol-induced) · Behavioural (ad lib smoking) |
| Roche et al. (2015) (varenicline and placebo conditions only) | 56 | 66% | 36 | No | 14 | 4 | 2 mg | 9 days | Between-subjects design comparing varenicline and placebo following an alcohol priming dose after pharmacotherapy pretreatment | · Behavioural (smoking topography) |
| Stoops et al. (2008) | 8 | 88% | – | No | 16 | – | 0.5 mg, 1 mg, 2 mg | <1 day | Within-subjects, multisession design comparing single dose varenicline and placebo | · Behavioural (ad lib smoking) |
- Note: Sample sizes reported are for the comparisons of interest. Values presented for age, cig/day and FTND represent sample means, or derived weighted means, rounded to the nearest whole number. Values for cig/day and FTND are presented pretreatment. ‘Quit’ indicates whether participants were currently considering or seeking treatment for smoking cessation. Dose represents the maximum daily dose throughout any multiday pharmacological intervention, often the final dose at the end of any titration period. FTND = Fagerström Nicotine Dependence Score.
| Sample characteristics | Pharmacotherapy | Study design | Relevant outcomes coded | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Men | Age | Quit? | Cig/day | FTND | Dose | Duration | |||
| Ashare and McKee (2012) (bupropion and placebo conditions only) | 40 | 63% | 36 | no | 20 | 6 | 300 mg | 7 days | Between-subjects design comparing bupropion and placebo after pharmacotherapy pretreatment | · Craving (tonic) |
| Cousins et al. (2001)—Study 1 | 12 | 58% | 31 | no | 21 | 6 | 150 mg, 300 mg | <1 day | Within-subjects, multisession design comparing single dose bupropion and placebo after overnight abstinence | · Behavioural (ad lib smoking) |
| Culbertson et al. (2011) | 30 | 70% | 42 | yes | 24 | 6 | 300 mg | 56 days | Between-subjects design comparing bupropion and placebo after pharmacotherapy pretreatment | · Craving (phasic) |
| Hussain et al. (2010) | 24 | 54% | 40 | no | 19 | – | 300 mg | 42 days | Between-subjects design comparing bupropion and placebo after pharmacotherapy pretreatment |
· Craving (phasic) · Withdrawal (cue-induced) · Behavioural (smoking topography) |
| McKee et al. (2012)—Study 2 (bupropion and placebo conditions only) | 42 | 60% | 36 | no | 18 | 6 | 300 mg | 7 days | Between-subjects design comparing bupropion and placebo after pharmacotherapy pretreatment |
· Craving (tonic) · Withdrawal (abstinence-induced) · Behavioural (ad lib smoking) |
| Rukstalis et al. (2005) | 26 | 58% | 44 | no | 18 | 4 | 300 mg | <1 day | Within-subjects, multisession design comparing single dose bupropion and placebo after brief abstinence |
· Craving (tonic) · Behavioural (cigarette choice task) |
| Shiffman et al. (2000) | 91 | 59% | 35 | no | 36 | 9 | 150 mg, 300 mg | 17 days | Between-subjects design comparing bupropion and placebo during abstinence on closed research ward after pharmacotherapy pretreatment |
· Craving (tonic) · Withdrawal (abstinence-induced) |
| Teneggi et al. (2005) | 23 | 52% | 34 | no | 23 | 8 | 300 mg | 17 days | Within-subjects, multisession design comparing bupropion and placebo after pharmacotherapy treatment that included an abstinence phase | · Craving (tonic) |
| Tidey and Rohsenow (2009) | 25 | 68% | 45 | mixed | 27 | 7 | 300 mg | 7 days | Within-subjects, multisession design comparing bupropion and placebo after pharmacotherapy pretreatment |
· Craving (phasic) · Withdrawal (abstinence-induced) |
- Note: Sample sizes reported are for the comparisons of interest. Values presented for age, cig/day and FTND represent sample means, or derived weighted means, rounded to the nearest whole number. Values for cig/day and FTND are presented pretreatment. ‘Quit’ indicates whether participants were currently considering or seeking treatment for smoking cessation. Dose represents the maximum daily dose throughout any multiday pharmacological intervention, often the final dose at the end of any titration period. FTND = Fagerström Nicotine Dependence Score.
3.2 Study quality
Thirty-two percent of studies were classified as having a low risk of bias, 18% were classified as high risk of bias and the remainder (50%) were classified as having ‘some concerns’ according to adapted RoB 2 criteria.39 Categories in which the RoB 2 was most frequently high or uncertain included measurement of study outcomes and selection of reported outcomes. Concerns in these areas typically reflected the inclusion of multiple outcomes, lack of prespecified primary outcomes and lack of prespecified analysis plans. Additionally, only four studies included information on a power analysis.
3.3 Varenicline
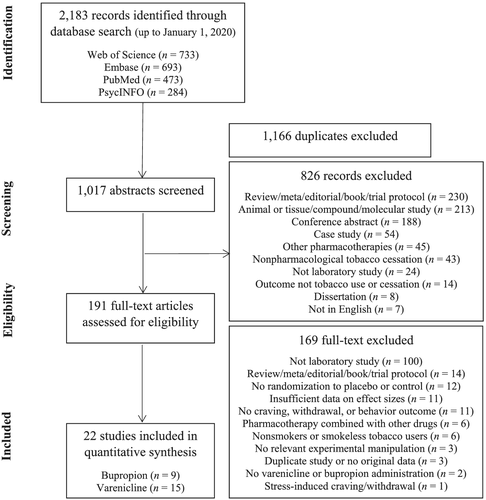
Forest plots depicting varenicline effects on craving, withdrawal and behavioural indices of smoking at the study level are shown in Figure 2. Relative to placebo treatment, varenicline was associated with significantly reduced craving (n = 461, k = 10, g = −0.36[−0.54,−0.17], p < 0.001), withdrawal (n = 268, k = 5, g = −0.25[−0.41,−0.09], p = 0.003) and behavioural indices of smoking (n = 396, k = 8, g = −0.36[−0.63,−0.08], p = 0.01; Table 3). One study removed analyses yielded relatively similar patterns of significance for craving (g = −0.41 to −0.31, ps < 0.01), withdrawal (g = −0.29 to −0.19, p = 0.002 to 0.095) and behavioural indices of smoking (g = −0.44 to −0.28, p = 0.003 to 0.04). Regarding craving type, exploratory analyses demonstrated significant effects of varenicline on tonic craving (n = 379, k = 8, g = −0.65[−0.84,−0.46], p < 0.001) and inconclusive effects on phasic craving (n = 239, k = 5, g = −0.13[−0.34,0.08], p = 0.22). There was significant, moderate heterogeneity between studies for behavioural indices of smoking (Q7 = 15.11, p = 0.04, I2 = 54%), but neither craving (Q9 = 13.44, p = 0.14, I2 = 33%) nor withdrawal (Q4 = 1.74, p = 0.78, I2 = 0%; Table 3). Regarding potential moderators for the behavioural meta-analysis, proportion of men (b = −0.76, p = 0.46), sample age (b = −0.01, p = 0.82), cigarettes per day (b = −0.04, p = 0.35), FTND score (b = −0.10, p = 0.58) and duration of pharmacotherapy (b = 0.01, p = 0.66) were inconclusive as moderators of the effect size estimate. Regarding publication bias, the Egger's regression test suggested no evidence of publication bias for the craving meta-analysis and, while the Trim and Fill method suggested imputation of one effect size estimate, the estimated summary statistic remained similar (g = −0.31).
| Varenicline | Bupropion | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k | Hedge's g [CI] | p | Q | df | p | I2 | k | Hedge's g [CI] | p | Q | df | p | I2 | |
| Craving | 10 | −0.36[−0.54,−0.17] | <0.001 | 13.44 | 9 | .14 | 33% | 8 | −0.13[−0.32,0.05] | 0.15 | 6.95 | 7 | 0.44 | 0% |
| Withdrawal | 5 | −0.25[−0.41,−0.09] | 0.003 | 1.74 | 4 | .78 | 0% | 4 | −0.15[−0.44,0.14] | 0.31 | 1.72 | 3 | 0.63 | 0% |
| Behavioural Indices | 8 | −0.36[−0.63,−0.08] | 0.01 | 15.11 | 7 | .04 | 54% | 4 | −0.05[−0.35,0.24] | 0.73 | 3.68 | 3 | 0.30 | 19% |
- Note: Ancillary meta-analysis removing studies on acute (≤4 h) effects of varenicline21, 29, 38 yielded comparable albeit somewhat stronger effect sizes for craving (g = −0.37[−0.53,−0.21], p < 0.001; Q7 = 6.37, p = 0.50, I2 = 0%), withdrawal symptoms (g = −0.29[−0.48,−0.10], p = 0.003; Q3 = 0.92, p = 0.82, I2 = 0%) and behavioural indices of smoking (g = −0.40[−0.71,−0.10], p = 0.009; Q6 = 14.77, p = 0.02, I2 = 59%). Ancillary meta-analysis removing doses other than 2 mg for varenicline21, 38 yielded similar results for craving (g = −0.31[−0.47,−0.14], p < 0.001; Q8 = 9.38, p = 0.31, I2 = 15%) and behavioural indices of smoking (g = −0.36[−0.63,−0.09], p = 0.009; Q7 = 15.02, p = 0.04, I2 = 53%); there were no non-standard doses in the varenicline withdrawal meta-analysis. Ancillary analyses removing studies on acute (≤4 h) effects of bupropion33, 37 yielded similar albeit somewhat stronger effect sizes for craving (g = −0.19[−0.40,0.02], p = 0.08, Q6 = 5.87, p = 0.44, I2 = 0%) and behavioural indices of smoking (g = −0.28[−0.76,0.20], p = 0.26; Q1 = 1.03, p = 0.31, I2 = 3%); there were no acute effects in the bupropion withdrawal meta-analysis. Ancillary meta-analysis removing doses other than 300 mg for bupropion22, 37 yielded similar results for craving (g = −0.14[−0.33,0.04], p = 0.13; Q7 = 6.74, p = 0.46, I2 = 0%), withdrawal (g = −0.17[−0.46,0.12], p = 0.26; Q3 = 1.86, p = 0.60, I2 = 0%) and behavioural indices of smoking (g = −0.01[−0.39,0.36], p = 0.94; Q3 = 5.41, p = 0.14, I2 = 44%). Finally, ancillary analyses examining varenicline effects on alcohol-induced craving only yielded inconclusive effects of varenicline relative to placebo (g = 0.05[−0.38,0.49], p = 0.81; Q1 = 0.20, I2 = 0%). CI = confidence interval.
3.4 Bupropion
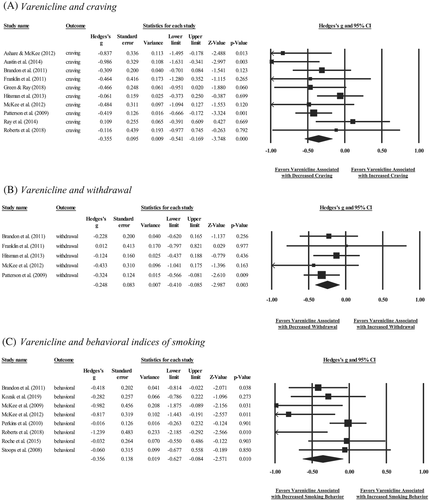
Forest plots depicting bupropion effects on craving, withdrawal and behavioural indices of smoking at the study level are shown in Figure 3. Bupropion was inconclusive in relation to craving (n = 301, k = 8, g = −0.13[−0.32,0.05], p = 0.15), withdrawal (n = 182, k = 4, g = −0.15[−0.44,0.14], p = 0.31) or behavioural indices of smoking (n = 104, k = 4, g = −0.05[−0.35,0.24], p = 0.73; Table 3). One study removed analyses yielded similar patterns of significance for craving (g = −0.19 to −0.09, p = 0.08 to 0.37), withdrawal (g = −0.24 to −0.06, p = 0.17 to 0.71) and behavioural indices of smoking (g = −0.16 to 0.04, p = 0.28 to 0.89). Regarding craving type, exploratory analyses demonstrated inconclusive effects of bupropion on both tonic (n = 222, k = 5, g = −0.12[−0.33,0.09], p = 0.25) and phasic (n = 79, k = 3, g = −0.19[−0.68,0.30], p = 0.44) craving. There was inconclusive heterogeneity in effect sizes for craving (Q7 = 6.95, p = 0.44, I2 = 0%), withdrawal (Q3 = 1.72, p = 0.63, I2 = 0%) or behavioural indices of smoking (Q3 = 3.68, p = 0.30, I2 = 19%; Table 3).
4 DISCUSSION
The current meta-analysis synthesized data from human laboratory research on two approved smoking cessation pharmacotherapies, varenicline and bupropion. Varenicline reduced nicotine craving, symptoms of withdrawal and behavioural indices of smoking in the laboratory relative to placebo. Findings support the utility of human laboratory work in efficiently capturing varenicline's effects on smoking outcomes in complement to controlled clinical trials with treatment-seeking participants. In contrast, bupropion's effects on laboratory craving, withdrawal or behavioural indices of smoking relative to placebo were inconclusive. Such findings are generally inconsistent with evidence that bupropion significantly improves abstinence rates in clinical trials. Future research is needed to better understand bupropion's mechanisms of action in relation to these apparently contradictory findings derived from human laboratory compared to clinical research.
Varenicline resulted in small to moderate reductions in craving, withdrawal and behavioural indices of smoking relative to placebo based on the magnitude of effect sizes across studies. These results complement support for small to moderate effects of varenicline on sustained smoking abstinence up to 6 months across a network meta-analysis of RCTs.2 Findings demonstrate the sensitivity of short-term laboratory paradigms in capturing clinically significant effects of varenicline on smoking indices that mirror those in larger clinical trials. Human laboratory studies may enable efficient screening of novel non-nicotine pharmacotherapies with comparable pharmacological profiles and/or mechanisms of action to varenicline. Future meta-analytic investigations could explore whether laboratory-based paradigms can similarly probe nicotine replacement therapies or emerging second-line pharmacotherapies resourcefully in complement to RCTs.
Varenicline's effects on craving and withdrawal were generally consistent in magnitude, although there was significant heterogeneity in varenicline's effects on behavioural smoking indices. Moderation analyses suggested that differences in varenicline's effects on behavioural indices as a function of proportion of men, sample age, cigarettes per day, FTND score and duration of pharmacotherapy were inconclusive, such that additional research is needed to identify contributions to such heterogeneity. Varenicline may have differential effects on varying types of behavioural outcomes (e.g., mean cigarettes smoked and time to lapse), although these could not be examined here due to insufficient group size and, as such, represent an important direction for future research. Future investigations also could explore the role of interest/motivation to quit smoking, which has been suggested to influence varenicline's effects on smoking behaviour in the laboratory23 yet could not be tested here given that most studies enrolled non-treatment-seeking smokers. Notably, despite overwhelmingly comprising non-treatment-seeking smokers, the current findings indicate fairly comparable effects of varenicline on laboratory outcomes. Results underscore the potential efficacy of varenicline for reducing smoking even in the absence of a long-term or sustained quit attempt, which might be informative for harm reduction interventions. Future efforts could explore whether varenicline's effects on smoking outcomes might be even stronger in laboratory studies that recruit samples with motivation for cessation. Efforts to probe the role of interest/motivation to quit smoking and any additional contributors to heterogeneity in varenicline's efficacy across populations may ultimately help to advance personalized approaches to smoking cessation treatment.
Bupropion's effects were inconclusive on craving, withdrawal or behavioural indices of smoking relative to placebo. Findings contrast support for bupropion improving smoking abstinence rates relative to placebo across meta-analyses of RCTs.2 Such differences in effects across laboratory and naturalistic trials could partly reflect that bupropion's mechanisms of action are not well understood, making it more challenging to design laboratory studies that can optimally detect medication effects. Bupropion may aid in naturalistic smoking cessation by helping to attenuate abstinence-induced withdrawal symptoms,8 particularly in light of its development as an antidepressant medication.2 Few of these laboratory studies probed negative reinforcement affective processes such that the current meta-analysis was unable to comprehensively examine outcomes relevant to negative affect components of withdrawal. Findings support earlier concerns that some common human laboratory paradigms (e.g., acute pharmacotherapy effects on craving and/or withdrawal during enforced abstinence; reductions in behavioural indices of smoking among unmotivated smokers) may lack sensitivity in capturing the efficacy of bupropion and other promising smoking cessation pharmacotherapies.45 Future investigations aimed at examining these negative affect processes might need to assess abstinence-induced withdrawal, recruit individuals in the process of quitting (i.e., clinical trials) and/or manipulate negative affect states within the laboratory.35 Findings highlight a broad need for more mechanistic research into extant as well as emerging smoking cessation pharmacotherapies. Laboratory research aimed at pharmacotherapy discovery and evaluation that does not account for contexts or processes critical to a pharmacotherapy's mechanism of action can miss potentially efficacious treatment options.
Several limitations of the identified research suggest important future directions. First, reporting of methodological details varied across studies, reflecting the general lack of systematic reporting standards for human laboratory studies. For example, few studies reported on the a priori determination of primary outcomes, analysis plans or power estimates. Efforts to increase systematic reporting within human laboratory pharmacotherapy investigations are needed to identify potential sources of bias and facilitate greater transparency in scientific reporting. Second, there were concerns with low power across identified studies. Sample sizes varied considerably, and only four studies reported power analyses. Power analyses that were reported also tended to assume medium to large effect sizes, which may be overly optimistic given the small effect sizes often identified in meta-analyses of clinical trials. Consistent with the possibility of inadequate power in some studies, many individual studies failed to demonstrate a statistically significant reduction in craving, withdrawal or smoking behaviour with varenicline administration, despite aggregate support for significant effects of varenicline on these outcomes. Underpowered investigations increase the likelihood of false negative findings and, thus, could obscure true effects of varenicline and bupropion on acute smoking outcomes as well as stifle early development of promising pharmacotherapies. Third, available literature was somewhat limited in scope such that the current meta-analysis was underpowered to explore additional non-nicotine pharmacotherapies as well as most differential effects of varenicline and bupropion on varied smoking outcomes. Exploratory analyses did suggest possibly stronger effects of varenicline on tonic compared to phasic craving, which would be important to test in future research to inform the need for supplemental cessation supports to manage cue-induced craving. Finally, identified research derived from a generally restricted range of samples comprising often unmotivated smokers recruited primarily within the United States, and thus, generalizability of the current findings remains to be demonstrated.
The current meta-analysis represents an important step towards characterizing efficacy of varenicline and bupropion for smoking cessation by synthesizing effects across human laboratory investigations. Findings for varenicline complement RCTs and support the importance of laboratory research in pharmacotherapy screening and development. Human laboratory paradigms can capture well-controlled, in-the-moment mechanistic processes and acute pharmacotherapy effects efficiently, thereby representing an important stage of pharmacotherapy evaluation. Following positive Phase II human laboratory trials, Phase III and Phase IV trials are critical for further evaluating clinical efficacy as well as unintended outcomes (e.g., side effects) to determine an overall therapeutic risk/benefit profile. Notably, findings from the bupropion meta-analyses point to the potential need to ensure that laboratory protocols are designed to model behavioural or contextual phenomena that might be critical for capturing medication effects. For example, laboratory procedures designed to measure smoking motivation while probing negative affect-related processes might be necessary to better understand bupropion's mechanisms of action. Similar efforts to align the design of pharmacotherapy screening paradigms with proposed mechanisms of action could help to increase sensitivity for screening emerging treatments and reconcile discrepancies between laboratory and naturalistic findings.
ACKNOWLEDGEMENTS
Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under award number T32 AA007583 to the University at Buffalo and a Canada Research Chairs Program award at the Centre for Addiction and Mental Health and University of Toronto (CH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CONFLICT OF INTEREST
The authors have no relevant conflicts of interest to declare.
AUTHOR CONTRIBUTION
M.J.Z. and C.S.H. were responsible for study concept and design. M.J.Z. performed the systematic literature search and data extraction. C.S.H. performed study quality coding. M.J.Z. drafted the manuscript, and C.S.H. provided critical revisions. Both authors approved the final version of the paper for submission.
Open Research
DATA AVAILABILITY STATEMENT
Data are available from the corresponding author upon reasonable request.




