Pharmacokinetics, systemic toxicity, thermoregulation and acute behavioural effects of 25CN-NBOMe
Abstract
N-(2-methoxybenzyl)phenethylamines (NBOMes) are a family of potent 5-HT2A agonists containing substances emerging on the illicit drug market as a replacement for N,N-diethyllysergamide (LSD). Despite the increasing use of NBOMes for diagnostic, research and recreational purposes, only a limited number of studies have focussed on their in vivo effect. Here, we investigated pharmacokinetics, systemic toxicity, thermoregulation in individually and group-housed animals, and acute behavioural effects after subcutaneous administration of 2,5-dimethoxy-4-(2-((2-methoxybenzyl)amino)ethyl)benzonitrile (25CN-NBOMe; 0.2, 1, and 5 mg/kg) in Wistar rats. Drug concentration peaked 1 h after the administration of 5 mg/kg in both blood serum and brain tissue with a half-life of 1.88 and 2.28 h, respectively. According to Organisation for Economic Co-operation and Development 423 toxicity assay, the drug is classified into category 3 with a lethal dose of 300 mg/kg and an estimated LD50 value of 200 mg/kg. Histological examination of organs collected from rats injected with the lethal dose revealed subtle pathological changes, highly suggestive of acute cardiovascular arrest due to malignant arrhythmia. Altered thermoregulation after 5 mg/kg was demonstrated by reduced body temperature in individually housed rats (p < 0.01). Behavioural effects assessed by the Open Field test and Prepulse Inhibition of Startle Response revealed that the two lower doses (0.2 and 1 mg/kg) caused a reduction in locomotor activity (p < 0.01), increased anxiety (p < 0.05) and 5 mg/kg additionally impaired sensorimotor gating (p < 0.001). In summary, 25CN-NBOMe readily passes the blood–brain barrier and exhibits a moderate level of toxicity and behavioural effect comparable with other NBOMes.
1 INTRODUCTION
2,5-Dimethoxy-4-(2-((2-methoxybenzyl)amino)ethyl)benzonitrile (25CN-NBOMe) belongs to a family of N-benzylphenethylamine derivatives (NBOMes). NBOMes, also known as N-Bombs or Smiles, are highly potent new psychoactive substances (NPS) introduced to the illicit drug market around 2010.1 Before NBOMes hit the street, they were initially used in academic laboratories and studied for their interactions with the central nervous system.2 Compared to classical psychedelics, NBOMes display high selectivity for 5-HT2 receptors, together with increased binding affinity at serotonergic 5-HT2A, 5-HT2C, dopaminergic D1–3, adrenergic α1, histaminergic H1 receptors, and monoamine transporters, and decreased binding to 5-HT1A receptors and trace amine-associated receptor 1.3 Activation of 5-HT2A receptors is currently thought to play a critical role in mediating hallucinogenic and therapeutic effects.4, 5
On the illicit market, NBOMes are typically distributed on preloaded paper doses (blotters) or in powder or liquid form due to their high potency.6 Interestingly, NBOMes show poor oral bioavailability,7 so their main absorption is via buccal mucosa, and they are almost inactive when ingested. Because of their high pharmacological activity, they are used in very low doses (50–1000 μg) with a long-lasting duration of action.1 Subjective effects are comparable to those of classical psychedelics such as N,N-diethyllysergamide (LSD) and psilocybin and include mental and physical stimulation, increased creativity, perceptual changes, vibrant colouring and strong sound distortion.8 Adverse effects, such as panic attacks, loss of position and time, paranoia, seizures, nausea and hyperthermia, have also been reported.1, 9 Many cases of severe and fatal intoxication have been connected to commonly used 2-(4-iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine (25I-NBOMe), N-(2-methoxybenzyl)-2,5-dimethoxy-4-chlorophenethylamine (25C-NBOMe) and N-(2-methoxybenzyl)-2,5-dimethoxy-4-bromophenethylamine (25B-NBOMe).10-12 However, more NBOMes are available on the drug market1 and potentially an almost infinite number of novel analogues can be discovered and disseminated among the user community; preclinical in vivo investigation is therefore urgently needed.
Regarding 25CN-NBOMe, except in vitro characterisation13, 14 demonstrating its high binding affinity for 5-HT2 receptors, particularly for the 2A subtype, and one metabolic study,15 no data describing the in vivo effects are currently available. 25CN-NBOMe is structurally related to one of the most selective 5-HT2A receptor agonists known to date,16 25CN-NBOH, which has been detected as one of its metabolites in the rat model.15 Taken together, 25CN-NBOMe has the potential to be misused as a recreational drug, whereas its behavioural effect and toxicity level remain unexplored. Therefore, we present a study investigating this compound's pharmacokinetics, thermodynamics, systemic toxicity and behavioural impact in Wistar rats.
A series of experiments intending to describe the effects of psychoactive substances were used.17-22 The drug's brain and serum levels were examined over 24 h according to the long-term effect reported by recreational NBOMes users.1 Because of poor oral bioavailability, the relevant doses were administered subcutaneously (s.c.) as a surrogate for standard oral administration in toxicity assay 423 defined by Organisation for Economic Co-operation and Development (OECD). Furthermore, we expected a thermodynamic effect supported by the data collected from fatal overdose cases of NBOMes.1 Given that environmental conditions can considerably modulate drug-induced thermodynamic changes,23, 24 body temperature (rectal measurement) was evaluated in both isolated and crowded conditions represented by rats housed individually and in a group of five. Prepulse inhibition of acoustic startle response (PPI ASR) and open field test (OFT) was used to assess sensorimotor gating and locomotor activity alterations. Different groups of rats were tested 15 and 60 min after drug application to determine the drug's early and late effects. Serotonergic agonists, including NBOMes, have already been documented to have the potential to induce inhibition of locomotor activity,25-28 increase anxious behaviour25, 28 and also disrupt sensorimotor gating.29
2 MATERIALS AND METHODS
2.1 Animals
Outbred Wistar rats (VELAZ, Prague, Czech Republic) were housed in pairs of the same sex at controlled temperature (22 ± 2°C), light (12/12 h light/dark) and humidity (30–70%) with ad libitum water and pellet diet access. All rats were habituated to laboratory conditions during the first 10 days after delivery, and all were regularly handled and weighted. At the beginning of the experiments, the rats weighed between 200 and 275 g. A total 200 male rats were used for behavioural tasks and thermoregulation measurement (n = 10 per group): OFT (N = 80), PPI ASR (N = 80) and thermoregulation (N = 40). Eight additional male rats were used for pharmacological sampling (n = 8 per time point) (for sampling at 30 min); the rest of the experimental group (n = 40) consisted of rats that had been used previously in behavioural tasks as the controls. Finally, nine more female rats were used for acute toxicity assessment.
All procedures were conducted following the principles of laboratory animal care of the National Committee for the Care and Use of Laboratory Animals (Czech Republic) and Guidelines of the European Union (86/609/EU). The protocol was approved by the National Committee for the Care and Use of Laboratory Animals (Czech Republic) under the number: MZDR 48237/2017 – 3/OVZ.
2.2 Drugs and chemicals
25CN-NBOMe and corresponding deuterium labelled internal standard 25CN-NBOMe-d3 were synthesised, purified and subsequently converted to hydrochloride salts by the University of Chemistry and Technology in Prague at the Forensic Laboratory of Biologically Active Compounds. The resulting hydrochlorides were certified to be of more than 97% purity (analysed by nuclear magnetic resonance and high-performance liquid chromatography ultraviolet). 25CN-NBOMe-d3 served as a reference standard for pharmacokinetic analyses using liquid chromatography/mass spectrometry (LC/MS). Chemicals used in a LC/MS analysis were of LC/MS grade and together with other necessary chemicals were purchased from Merck (Czech Republic). Other chemicals for sample preparation procedures were of p. a. grade supplied by Merck (Czech Republic). Ultrapure water, 18.2 MΩ/cm, was produced by the Smart2Pure 12 UV system (Thermo Scientific, Germany). Homogenisation beads, SiLibeads, type ZY 0.4–0.6 mm, were supplied by Ginzel (Czech Republic). Stock solutions of analytical standards were prepared in methanol and stored at −20°C. 25CN-NBOMe was held in a dry and dark place and dissolved in deionised water and 20 μL of Tween 80 immediately before experiments. The highest dose had to be briefly dissolved using an ultrasonic homogeniser.
2.3 Dosage
The doses of 25CN-NBOMe for behavioural studies and pharmacokinetics were selected according to the potency of related compounds, such as 2C-B and 25I-NBOMe.29, 30 They were 0.2, 1 and 5 mg/kg for behavioural experiments and 5 mg/kg for pharmacokinetics and thermoregulatory measurements only. Doses for acute toxicity reflected OECD 423 assay (see below) (300 and 50 mg/kg). In all cases, the compound was dissolved in saline and 20 μL of Tween 20 using ultrasonic homogeniser and administered subcutaneously at a volume of 2 mL/kg; an equivalent volume of physiological solution and 20 μL of Tween 20 was used as a vehicle in controls.
2.4 Pharmacokinetic sampling
Rats were administered 5 mg/kg 25CN-NBOMe s.c. and decapitated after 0.5, 1, 2, 4, 8 and 24 h (n = 8 per time point). Serum was obtained by enabling the blood to clot in a refrigerator for 1 h. The clotted blood was centrifuged for 10 min at 1500g, 10°C, and the serum was then collected. Serum and brains were stored at −20°C until pharmacological analysis. Drug concentrations in the serum and dissected brain tissue were calculated as ng/mL or ng/g, respectively. The brain/serum ratio was calculated as the median brain concentration/median serum concentration per sampling time point.
2.4.1 Determination of 25CN-NBOMe in serum and brain samples using LC-MS/MS
LC/MS analysis: the samples in this section were analysed using Ultra High Pressure Liquid Chromatography - Mass Spectrometry instrumentation (UltiMate 3000 Thermo Scientific Dionex – 6500+ QTrap AB Sciex with electrospray ionisation source). A column Zorbax Eclipse Plus C18 (50 × 2.1 mm, 1.8 μm) with a pre-column was used for a chromatographic separation at 35°C with gradient elution in the system of mobile phases 5-mM ammonium formate with 0.1% (v/v) formic acid (A) and acetonitrile with 0.1% (v/v) formic acid (B). The gradient started at 10% B, increased from 10% to 20% B in 0.5 min and from 20% to 100% B in 1.5 min, and held for 2 min at 100% B, decreased to 10% B in 0.5 min and held for 2 min at 10% B. Flow rate was 0.250 mL/min. Tandem mass spectrometry was operated in positive ion mode with the gas temperature at 350°C, curtain gas 20 and IonSpray voltage 5500 V. The protonated form of 25CN-NBOMe has a nominal mass of 327.2 m/z. For quantification, multiple reaction monitoring methods were used. The following transitions were monitored: 327.2 to 121.0 for quantification, 327.2 to 91.2 for confirmation, using collision energies of 21 V and 55 V, respectively. To track the internal standard 25CN-NBOMe-d3, the acquisition was set for transitions of 330.2 to 124.1 and 330.2 to 92.1 using collision energies of 21 V and 58 V, respectively. 25CN-NBOMe was quantified using an external matrix-matched calibration. The limit of quantification was 1 ng/mL (5 ng/g for brain tissue).
Serum pre-treatment: (1) dilution of 200 μL of serum with 195 μL of 1% NH4OH solution and spiked with 5 μL of internal standard (IS) (25CN-NBOMe-d3, concentration 240 ng/mL); (2) homogenisation in a BulletBlender for 5 min (speed 2); (3) addition of 400 μL ethyl acetate to the mixture and homogenisation (BulletBlender, speed 3, 5 min); (4) addition of 400 μL ethyl acetate to the mixture and homogenisation (BulletBlender, speed 3, 5 min); (5) centrifugation for 10 min at 25°C (12,000 RPM); (6) evaporation of 600 μL acidified supernatant to dryness (Centrivap Concentrator); and (7) reconstitution with 100 μL acetonitrile. Before analysis, the samples were diluted with water in the ratio of 1:1 (v/v), mixed and centrifuged.
Brain pre-treatment: (1) addition of 195 μL of 1% NH4OH solution and 5 μL of IS (25CN-NBOMe-d3, concentration 240 ng/mL) to 200 mg of brain tissue; (2) homogenisation with 200 mg of zirconium oxide beads in a BulletBlender for 10 min (speed 5); (3) addition of 400 μL ethyl acetate to the mixture and homogenisation (BulletBlender, speed 3, 5 min); (4) addition of 400 μL ethyl acetate to the mixture and homogenisation (BulletBlender, speed 3, 5 min); (5) centrifugation for 10 min at 25°C (12,000 RPM); (6) evaporation of 400 μL acidified supernatant to dryness (Centrivap Concentrator); and (7) reconstitution with 100 μL acetonitrile. Before analysis, the samples were diluted with water in the ratio of 1:1 (v/v), mixed and centrifuged (0.2 μm polyvinylidene difluoride filter).
2.5 Systemic toxicity
Modified Acute Toxic Class Method (OECD Guideline no. 423, 2001) was used to test the acute oral toxicity of chemicals. This method is not intended to assess the exact calculation of median lethal dose (LD50) but allows, using pre-defined doses, ranking and classification of a substance according to the Globally Harmonised System (GHS) for the classification of chemicals. Five GHS categories are defined, represented by the most severe Category 1 (LD50 ≤ 5 mg/kg), Category 2 (LD50 > 5 and ≤ 50 mg/kg), Category 3 (LD50 > 50 and ≤ 300 mg/kg), Category 4 (LD50 > 300 mg/kg and ≤ 2000 mg/kg) and Category 5 (LD50 > 2000 mg/kg and ≤ 5000 mg/kg). The principle of this test is a stepwise procedure using a minimum number of animals per step. Each step uses three animals, usually females, that are more sensitive to toxic compounds.29 The starting dose is selected from four fixed levels, 5, 50, 300 and 2000 mg/kg body weight. The starting dose represents the value most likely to cause death to some of the administered animals. If no information on the test substance is available, it is recommended to use the starting dose of 300 mg/kg. The absence or presence of compound-related mortality of animals dosed in one step determines the next step. After the single dose, the animals are observed individually at least twice an hour for the first 24 h and then daily for a total of 14 days. Animals found in moribund condition or showing severe pain or distress were humanely euthanised.
Collected organs (brain, heart, lung, liver, kidney and spleen) from three dead experimental animals fixed in 4% buffered formalin solution were submitted for further pathologic–anatomical analysis. The tissues were processed and embedded in paraffin blocks. Subsequently, 4-μm-thick sections were made and stained by the basic haematoxylin-eosin method. Two pathologists performed microscopic analysis independently.
2.6 Thermoregulation
Rectal temperature was measured over 10 h in group housed (five rats per cage) and individually housed (one rat per cage) setup. The rat was briefly (10 s) restrained in a Plexiglas tube during the measurement, and its rectal temperature was measured with a digital thermometer. Directly after the measurement, a rat was returned to the cage (43 × 27 × 15 cm). The first three measurements were taken at baseline time (7:00, 8:00, and 9:00). At 9:00, either 25CN-NBOMe (5 mg/kg) or vehicle was administered, followed by six measurements at a 30-min interval. Until noon and then continued hourly until 17:00, altogether, 14 measurements were taken. The procedure was conducted in a room with a controlled temperature (22 ± 2°C).
2.7 Behavioural experiments
All experiments were performed during the daytime (between 07:00 and 15:00 h) under standard laboratory conditions described previously. Two testing onsets were set up – starting either 15 or 60 min after compound administration. The design was chosen to (1) evaluate a wider temporal window in which drug effects might evolve differently and without possible habituation-caused bias and (2) obtain comparable data to our previous study of closely related substances.
2.7.1 Open field test
OFT was initiated by placing the rat in the central part of an empty black arena (80 × 80 cm) surrounded by walls (40 cm) placed in a soundproof and evenly dimly lit room (60 lx). A rat was allowed to move freely within the arena for 30 min, and its behaviour was recorded and quantified using the software EthoVision XT v. 14.0. (Noldus, Netherlands). The arena was virtually divided into a 5 × 5 grid of identical squares for data processing; 16 of which were placed around the arena walls (peripheral zones) and nine situated centrally (central zones). The following parameters were measured: trajectory length divided into 5-min intervals (centimetre; corrected for deviations of < 3 cm), time spent in the central zone (seconds; Tcentre = ∑time central zones) and probability of occurrence in the peripheral zone (thigmotaxis = Σfperipheral zones/Σfall zones, where f = frequency of occurrence in a zone). The described procedure is consistent with our previous studies.17-20
2.7.2 PPI of the ASR
PPI ASR was measured in the startle chambers (SR-LAB, San Diego Instruments, California, USA), each consisting of an evenly lit and soundproof enclosure, a high-frequency loudspeaker (producing all acoustic stimuli and background noise of 75 dB) and a Plexiglas stabilimeter (8.7 cm inner diameter). The amplitudes of the startle responses were detected with a piezoelectric accelerometer and were digitised.
Two days before the measurement, the rat was habituated to the startle boxes for 5 min during which five presentations of pulse stimulus alone (125 dB/40 ms) were delivered over white background noise (75 dB). PPI ASR measurement was initiated by a habituation period consisting of 5-min of background white noise (75 dB), followed by 72 trials with an inter-trial interval (ITI) of 15–30 s (mean ITI: 22.5 s). Six 125 dB/40 ms duration pulse alone trials were performed to establish baseline ASR. Then, 60 trials were conducted in a pseudo-random order: (A) pulse alone: 40 ms 125 dB; (B) prepulse alone: 20 ms 83 dB or 91 dB; (C) prepulse-pulse: 20 ms 83 dB or 91 dB prepulse, a variable (30, 60 or 120 ms) inter-stimulus interval (ISI: mean 70 ms), then 40 ms 125 dB pulse; and (D) 60 ms no stimulus. Finally, six pulse alone trials were performed. Habituation was calculated by the percentage reduction in ASR from the initial six baseline trials to the last six trials. Mean ASR was obtained from the pulse alone trials. Animals with a mean ASR response lower than 10 arbitrary units were excluded from analyses as non-responders. PPI was calculated as (100 − [mean response for the prepulse–pulse trial/startle response for the single pulse trials] * 100).
2.8 Statistical analysis
The analyses were conducted in software STATISTICA (version 13.3., StatSoft, Inc.), R software (version 4.0.5) and R Studio (version 1.4.1717). Alpha was set up at p = 0.05, two tailed.
The half-life of the drug elimination was calculated using Package PKNCA version 0.9.4 implemented in the R software.32
Thermoregulation and behavioural data were analysed using analysis of variance (ANOVA) with a factorial design. Significant main effects and inter-factor interactions were followed by the Tukey post hoc test. The thermoregulation data were analysed using a 3 × 2 × 14 mixed factorial design with drug treatment (25CN-NBOMe 5 mg/kg versus control) and home-cage condition (group versus individually housed) as between-subjects factors and time (14 time points) as a within-subjects factor. Locomotor activity in OFT (mean trajectory length divided into 5-min blocks) was evaluated using a 2 × 4 × 6 mixed factorial ANOVA with testing onset (15 min versus 60 min) and drug treatment (25CN-NBOMe at 0.2, 1, or 5 mg/kg versus control) as between-subjects factors, and time blocks (6 × 5 min intervals) as a within-subjects factor. Spatial occurrence of a rat in the arena (thigmotaxis and Tcentre) and PPI parameters (habituation, ASR and PPI) were each analysed using a 2 × 4 factorial ANOVA with testing onset (15 min versus 60 min) and drug treatment (25CN-NBOMe at 0.2, 1 or 5 mg/kg versus control) as between-subjects factors.
3 RESULTS
3.1 Pharmacokinetics
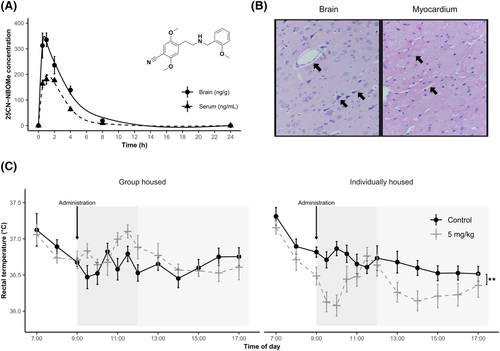
One hour after subcutaneous administration of 5 mg/kg, the mean concentration of 25CN-NBOMe peaked in both blood serum (201 ng/mL) and brain tissue (350 ng/g) with a half-life of the drug elimination of 1.88 and 2.28 h, respectively. The concentration gradually decreased until it was almost undetectable after 8 h in both serum and brain (Figure 1A). Further pharmacological parameters are displayed in Table 1.
| Parameters | Brain | Serum |
|---|---|---|
| F (%) | 0.195 ± 0.016 | 0.781 ± 0.095 |
| VD (mL) | 0.286 ± 0.024 | 8.457 ± 1.031 |
| Ka (h−1) | 0.960 ± 0.079 | 0.971 ± 0.118 |
| Ke (h−1) | 0.480 ± 0.039 | 0.708 ± 0.086 |
- Abbreviations: F, Bioavailability; VD, Volume of distribution; Ka, Absorption rate constant; Ke, Elimination rate constant.
3.2 Systemic toxicity
In the initial phase of the procedure, three rats were administered 300 mg/kg of 25CN-NBOMe. In all animals, hypolocomotion accompanied with hypothermia (minimum 35, 4°C) and mild tremor was observed. Two of the animals subsequently exhibited convulsions and died within 3 h after drug administration. The third animal recovered and was monitored for the following 2 weeks and then sacrificed. A lower dose (50 mg/kg) was applied to the other three rats in the next phase. None of the observed animals died or exhibited any severe distress after the dose. Therefore, according to the protocol, a single dose of 50 mg/kg was administered to the last three rats. In this phase, again, none of the observed animals died or suffered from severe distress. Based on these results, we determined the toxicity category of the compound to be Category 3 as defined by the Globally Harmonised Classification System with an estimated LD50 at a dose of 200 mg/kg. The dose of 50 mg/kg was proven safe for use in behavioural studies.
Organs collected from rats that died in toxicity assay were submitted to pathologic–anatomical analysis. Histological findings in the lungs, liver and spleen were utterly negative, whereas very subtle findings were observed in the brain, heart and kidneys. The brain showed diffuse mild pericellular oedema (Figure 1B), and the kidney focally exhibited desquamation of tubular epithelium, indicative of incipient tubular necrosis. In the myocardium, regressive changes of individual or groups of cardiomyocytes were detected, such as cytoplasmic hypereosinophilia accompanied by pyknotic nuclei (Figure 1B).
3.3 Body temperature
Body temperature was significantly affected by home-cage condition [F1, 36 = 5.926, p < 0.05], time [F13, 468 = 9.363, p < 0.001] and treatment × home-cage condition interaction [F1, 36 = 10.184, p < 0.01]. The baseline values (i.e. measurements before the compound administration at 7:00, 8:00 and 9:00) did not vary according to either treatment or home-cage condition. 25CN-NBOMe decreased body temperature of individually housed rats across the whole measurement (p < 0.01), while a similar effect was absent in group-housed ones (Figure 1C).
3.4 Behaviour
3.4.1 Open field test
The analysis of trajectory length revealed a significant main effect of drug treatment [F3, 72 = 26.578, p < 0.001], blocks [F5, 360 = 206.200, p < 0.001] and time × treatment and treatment × testing onset × blocks interactions [minimum F15, 360 = 2.400, p < 0.01]. Based on post hoc analyses, all tested groups displayed normal locomotor habituation (progressive decline of locomotor activity over the session), except for the highest dose (5 mg/kg; Figure 2A).
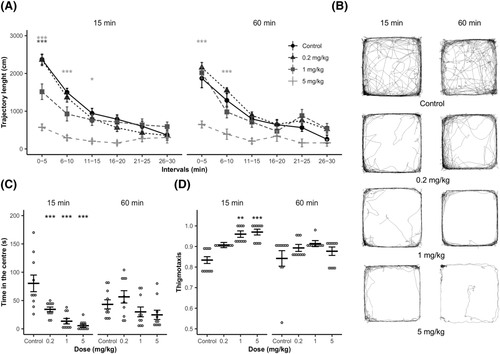
Compared to controls, the 0.2 mg/kg did not alter activity in any time blocks or testing onsets. On the other hand, 1 mg/kg and 5 mg/kg reduced activity in the first interval (0–5 min, p < 0.001), and the effect persisted in 5 mg/kg to the third interval (i.e. 11–15 min, maximum p < 0.01) in 15-min onset and to the second interval (i.e. 6–10 min, p < 0.001) in 60-min onset (Figure 2A).
The main effects of treatment and testing onset × treatment interaction were significant for Tcentre (minimum, F[3, 72] = 12.69, p < 0.001). Post hoc tests showed that at 15-min testing onset, all 25CN-NBOMe groups spent significantly less time in the centre compared to vehicle controls (p < 0.01 − p < 0.001). In contrast, a similar effect was not presented in 60-min testing onset(Figure 2C).
Thigmotaxis was significantly affected by both treatment and testing onset (minimum, F[1, 72] = 6.430, p < 0.05). Post hoc tests revealed that intermediate and high doses (1 and 5 mg/kg, respectively) caused thigmotaxis increase compared to controls (p < 0.05) in 15-min testing onset. At the same time, no effect was presented in the test initiated 60 min post-administration (Figure 2D).
3.4.2 PPI of ASR
The mean ASR of any of the tested rats was lower than 10 arbitrary units. Therefore, all individuals were used in the analyses. Habituation was not affected by any of the tested factors. The analysis of baseline ASR revealed a significant effect of treatment [F (3, 72) = 14.835, p < 0.001], whereas the rest of the factors did not have any effect. Compared to controls, 1 and 5 mg/kg reduced baseline ASR values (minimum p < 0.01, Figure 3A).
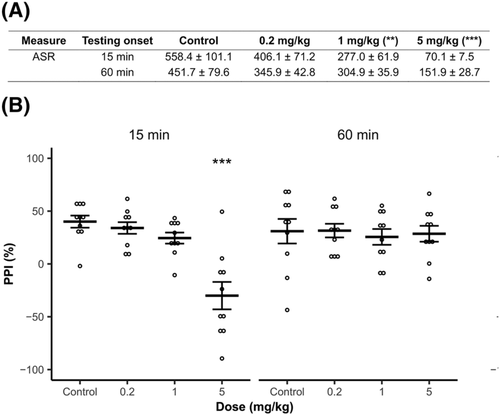
The percentage of PPI was significantly affected by treatment [F (3, 72) = 7.974, p < 0.001], testing onset [F (1, 72) = 4.231, p < 0.05] and their interaction [F (3, 72) = 7.160, p < 0.001]. While low and intermediate doses (0.2 and 1 mg/kg, respectively) had no effect, the highest of these (5 mg/kg) significantly impaired PPI (p < 0.001) at 15-min onset and similar effect was absent in 60-min onset (Figure 3B).
4 DISCUSSION
In summary, 25CN-NBOMe concentration levels in both serum and brain tissue peaked 1 h after administration of 5 mg/kg s.c., and then the substance was removed from the body with a prolonged rate. Based on the OECD 423 toxicity assay, 300 mg/kg was determined to be a lethal dose, whereas 50 mg/kg caused no deaths, and the LD50 can be estimated at 200 mg/kg. Histological changes suggest that malignant arrhythmia leading to an acute cardiovascular arrest most probably caused death in rats after injection of 300 mg/kg s.c. Further, 5 mg/kg induced hypothermia in individually housed rats but not in group-housed animals. Finally, we observed a dose-dependent inhibition of locomotor activity, increased anxiety and impaired sensorimotor gating in drug-treated rats. These behavioural changes were particularly presented at both time points of testing that is in line with the drug kinetics.
25CN-NBOMe, as a lipophilic substance, readily crosses the blood–brain barrier, resulting in considerably higher peak concentration detected in the brain tissue compared to blood serum 1 h after administration. The higher concentration in the brain compared to serum was maintained for several more hours, indicating significant retention of the drug in brain tissue. This contributes to the overall kinetics of the substance in the body. The drug remains at detectable levels for up to 8 h in both biological materials. The determined half-life of 1.88 h in blood serum and 2.28 h in the brain indicates a relatively slow turnover compared to related psychedelics such as 2C-B and mescaline, whose half-life has been determined to be approximately 1 h in rats.30, 33
25CN-NBOMe exhibits, according to OECD testing, moderate acute toxicity classifiable as Class 3, with an estimated LD50 of 200 mg/kg when administered subcutaneously. Comparison with related NBOMe substances is difficult due to the lack of information on their mean lethal doses in rats. As far as classical psychedelics are concerned, 25CN-NBOMe is more lethal than the least potent mescaline (LD50 404 mg/kg, s.c.).34 The histopathological findings are indistinct, and the precise cause of death remains inconclusive, however, highly suggestive of acute cardiovascular arrest because of malignant arrhythmia, secondarily leading to brain oedema and very subtle incipient tubular necrosis. Heart rhythm disturbances and predominantly tachycardia have also been reported in fatal human NBOMe overdoses.35
NBOMes are also known for rapid raising body temperature in humans, which in some cases can escalate to life-threatening hyperthermia.36, 37 Indeed, fatal cases of NBOMes overdose tend to be accompanied by hyperthermia.1 To mimic typical conditions associated with NPS use (i.e. social gatherings such as rave parties), we measured body temperature not only individually but also in group-housed rats. In our experiment, the only observed effect on body temperature was not hyper, but hypothermia shown by the individually housed rats. On the other hand, group-housed rats showed no significant change in body temperature. Preclinical investigations of congener 25I-NBOMe and 25C-NBOMe have revealed patterns consistent with the absence of the effect in group-housed male rats.29, 38 However, there is also evidence for hyperthermic action of NBOMes. Nevertheless, it is documented in females29 or under considerably elevated ambient temperature.23
25CN-NBOMe alters locomotor activity in a dose and time-dependent manner. Shortly after the application (15 min), 1 and 5 mg/kg induced inhibition, whereas 0.2 mg/kg was ineffective. Consistent with the drug kinetics, 5 mg/kg was still potent in a test initiated 60 min after the injection. Similarly, a robust inhibitory effect was observed in preclinical studies of phenethylamine hallucinogens39 and closely related NBOMes such as 25B-NBOMe, 25C-NBOMe and 25I-NBOMe.25-28 Interestingly, there is also evidence for the biphasic effect of 25I-NBOMe manifested by its both inhibitory (3 mg/kg, s.c.) and stimulatory potency (0.1 mg/kg, s.c.).40 Nevertheless, a similar pattern was not observed across our study.
The open-field data further indicate a dose-dependent reduction in the occurrence of rats in the central parts of the arena and a corresponding increase of thigmotaxis (i.e. the tendency to keep close to the walls), which is typical for compounds with the anxiogenic effect.41 Congruently, preclinical investigation of 25I-NBOMe and 25B-NBOMe has revealed anxiogenic-like properties of these compounds25, 28; anxious states related to NBOMes ingestion were also documented in humans.6, 42
Doses of 1 and 5 mg/kg reduced the ASR shortly after administration (15-min onset), and 5 mg/kg impaired PPI (i.e. the ability of prepulse to inhibit startle reflexes) the most. The effect was not significant in rats administered 0.2 mg/kg and in those tested 60 min after administration. The observed reduction of startle responses is mainly consistent with the inhibition of exploratory activity discussed above. Still, the changes induced by the 5 mg/kg were relatively short-lived in this case.
Disruption of sensory filtering is also typical for serotonergic psychedelics such as LSD,43, 44 mescaline,33 DOI,45 and 2C-B.46 In comparison, 25CN-NBOH reduced PPI at a lower dose (1 mg/kg, s.c.), whereas 4 mg/kg was ineffective.47 An intraperitoneal dose of 1 mg/kg of 25I-NBOMe29, 47, 31 was similarly effective.
To conclude, 25CN-NBOMe exhibited a pharmacological profile classifiable in the NBOMes family and showed a moderate level of toxicity. In contrast to humans, we observed rather hypothermia in rats. Based on the literature, 25CN-NBOMe induces behavioural effects comparable to other NBOMe compounds.
ACKNOWLEDGEMENTS
This work was supported by Safety Research under the Czech Ministry of the Interior (Project No. VI20172020056); Czech Science Foundation (Project No. 20-25349S); Czech Health Research Council (Project No. NU21-04-00307); Long-Term Conceptual Development of Research Organization (Project No. RVO 00023752); Specific University Research, Czech Ministry of Education, Youth and Sports (Project No. 260533/SVV/2021); and Operational Programme Research, Development and Education project (PharmaBrain EF16_025/0007444).
AUTHORS CONTRIBUTION
TP, MKu and KŠ were responsible for the study concept and design. KŠ drafted the manuscript. EK performed, analysed and substantially contributed to the interpretation of pharmacokinetic data. PP was responsible for the synthesis of the studied compound. LO assessed systemic toxicity and substantially contributed to the interpretation of the data. AŠ and BQH performed the histological examination and interpreted the findings. KŠ, KS, NP-L, ČV, LO, HD, KŠ-M and MKo substantially contributed to the acquisition, analysis and interpretation of behavioural data. PJ assisted the data analysis and interpretation of the findings. TP, ČV, NP-L, MKu, JN and PJ provided critical revision of the manuscript for important intellectual content. All of the authors made a significant contribution to this study, read, revised and gave final approval for the current version of the work to be published.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




